The Effect of Rosmarinus officinalis Essential Oil Fumigation on Biochemical, Behavioral, and Physiological Parameters of Callosobruchus maculatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Used Substance
2.3. Chemical Analysis
2.4. Fumigation Mortality
2.5. Repellency
2.6. Open Field Test
2.7. Oxygen Consumption
2.8. Enzyme Assays
2.8.1. Acetylcholinesterase (AChE) Activity Assay
2.8.2. Catalase (CAT) Activity Assay
2.8.3. Glutathione S-Transferase (GST) Activity Assay
2.9. Statistical Analysis
3. Results
3.1. Chemical Analysis
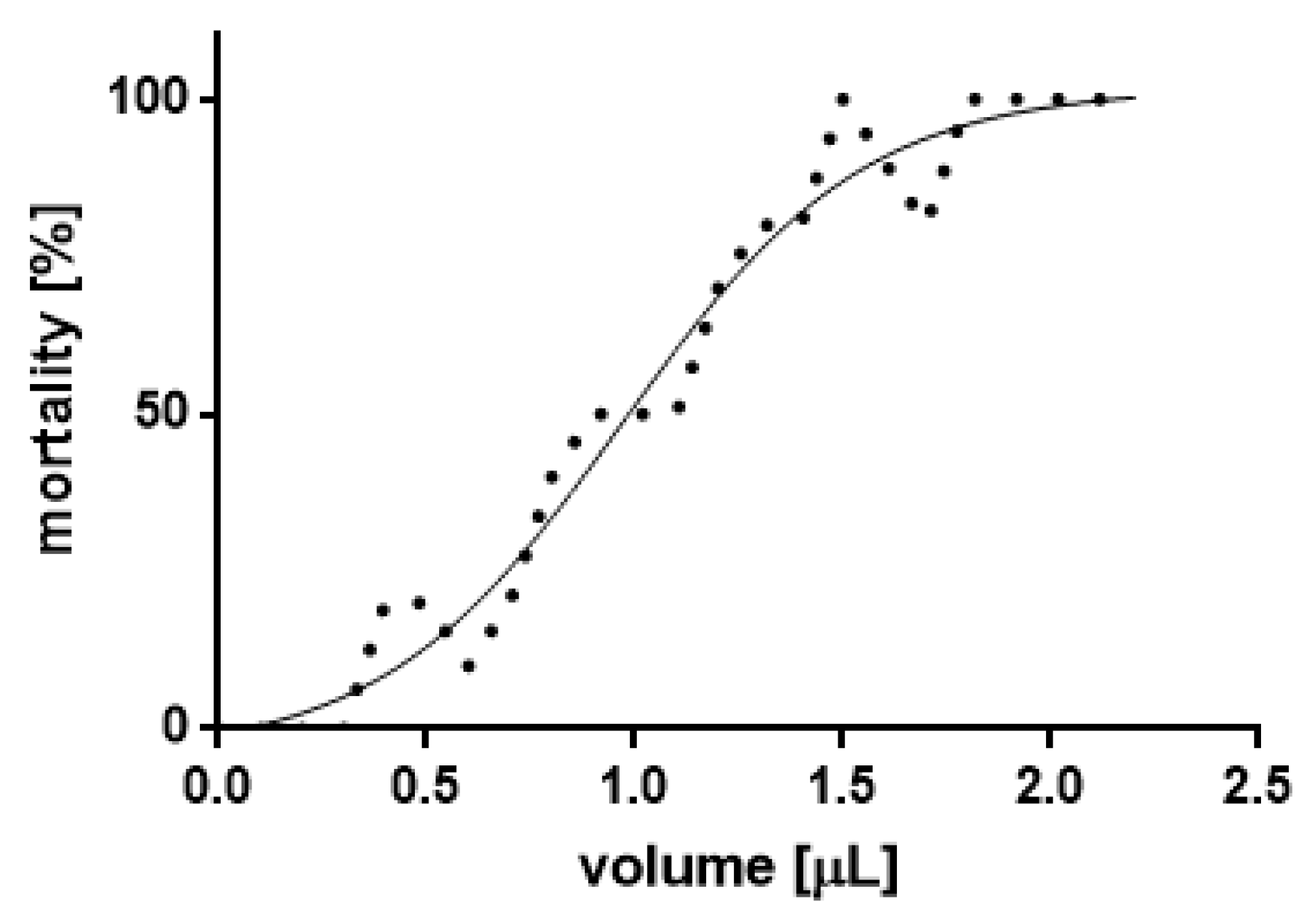
3.2. Mortality
3.3. Repellency
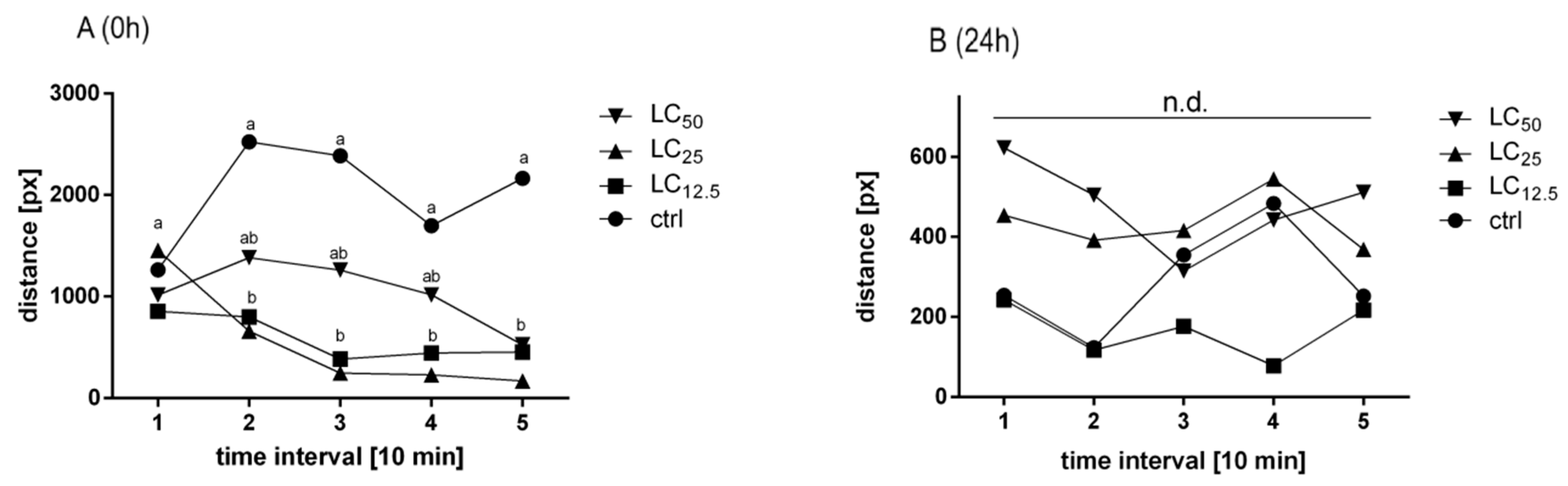
3.4. Open Field Test
3.5. Oxygen Consumption
3.6. Enzyme Assays
3.6.1. Acetylcholinesterase (AChE) Activity Assay
3.6.2. Catalase (CAT) Activity Assay
3.6.3. Glutathione S-Transferase (GST) Activity Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Heinrich, M.; Kufer, J.; Leonti, M.; Pardo-de-Santayana, M. Ethnobotany and ethnopharmacology-Interdisciplinary links with the historical sciences. J. Ethnopharmacol. 2006, 107, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Isikber, A.A.; Alma, M.H.; Kanat, M.; Karci, A. Fumigant toxicity of essential oils from Laurus nobilis and Rosmarinus officinalis against all life stages of Tribolium confusum. Phytoparasitica 2006, 34, 167–177. [Google Scholar] [CrossRef]
- Lee, B.H.; Lee, S.E.; Annis, P.C.; Pratt, S.J.; Park, B.S.; Tumaalii, F. Fumigant Toxicity of Essential Oils and Monoterpenes Against the Red Flour Beetle, Tribolium castaneum Herbst. J. Asia Pac. Entomol. 2002, 5, 237–240. [Google Scholar] [CrossRef]
- Sim, M.-J.; Choi, D.-R.; Ahn, Y.-J. Vapor Phase Toxicity of Plant Essential Oils to Cadra cautella (Lepidoptera: Pyralidae). J. Econ. Entomol. 2009, 99, 593–598. [Google Scholar] [CrossRef]
- Trivedi, N. Kumar Fumigant toxicity study of different essential oils against stored grain pest Callosobruchus chinensis. J. Pharmacogn. Phytochem. 2017, 6, 1708–1711. [Google Scholar]
- Athanassiou, C.G.; Arthur, F.H. Recent Advances in Stored Product Protection; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9783662561256. [Google Scholar]
- Güdek, M.; Çetin, H. Fumigant Toxicity on Adults of Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae) of Essential Oil from Rosmarinus officinalis L. and its Side Effects on Chickpea Grains. J. Essent. Oil Bear. Plants 2017, 20, 272–281. [Google Scholar] [CrossRef]
- Erler, F.; Erdemir, T.; Ceylan, F.O.; Toker, C. Fumigant toxicity of three essential oils and their binary and tertiary mixtures against the pulse beetle, Callosobruchus macuiatus F. (Coleoptera: Bruchidae). Fresenius Environ. Bull. 2009, 18, 975–981. [Google Scholar]
- Dayaram, L.; Khan, A. Repellent, Fumigant and Contact Toxicity of Salvia Officinalis, Rosmarinus Officinalis and Coriandrum Sativum Against Callosobruchus Maculatus ( Fab.) ( Coleoptera: Bruchidae). Int. J. Trop. Agric. 2016, 34, 893–902. [Google Scholar]
- Lang, G.-J.; Yan Zhu, K.; Zhang, C.-X. Can Acetylcholinesterase Serve as a Target for Developing More Selective Insecticides? Curr. Drug Targets 2012, 13, 495–501. [Google Scholar] [CrossRef]
- Thany, S.H.; Tricoire-Leignel, H.; Lapied, B. Identification of cholinergic synaptic transmission in the insect nervous system. Adv. Exp. Med. Biol. 2010, 683, 1–10. [Google Scholar] [CrossRef]
- Bajalan, I.; Rouzbahani, R.; Pirbalouti, A.G.; Maggi, F. Antioxidant and antibacterial activities of the essential oils obtained from seven Iranian populations of Rosmarinus officinalis. Ind. Crops Prod. 2017, 107, 305–311. [Google Scholar] [CrossRef]
- Francikowski, J.; Baran, B.; Cup, M.; Janiec, J.; Krzyżowski, M. Commercially available essential oil formulas as repellents against the stored-product pest Alphitobius diaperinus. Insects 2019, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Baran, B.; Krzyżowski, M.; Cup, M.; Janiec, J.; Grabowski, M.; Francikowski, J. Repellent effect of volatile fatty acids on lesser mealworm (Alphitobius diaperinus). Insects 2018, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Lochmatter, T.; Roduit, P.; Cianci, C.; Correll, N.; Jacot, J.; Martinoli, A. SwisTrack—A flexible open source tracking software for multi-agent systems. 2008 IEEE/RSJ Int. Conf. Intell. Robot Syst. IROS 2008, 4004–4010. [Google Scholar] [CrossRef]
- McLean, D.J.; Skowron Volponi, M.A. trajr: An R package for characterisation of animal trajectories. Ethology 2018, 124, 440–448. [Google Scholar] [CrossRef]
- Jones, C.G.; Daniel Hare, J.; Compton, S.J. Measuring plant protein with the Bradford assay—1. Evaluation and standard method. J. Chem. Ecol. 1989, 15, 979–992. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Orr, C.W.M. The Inhibition of Catalase (Hydrogen-peroxide: Hydrogen Peroxide Oxireductase, Ec 1.11.1.6) by Ascorbate. Methods Enzymol. 1970, 18, 59–62. [Google Scholar] [CrossRef]
- Yu, S.J. Host plant induction of glutathione S-transferase in the fall armyworm. Pestic. Biochem. Physiol. 1982, 18, 101–106. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of essential oil components by gas chromatography/mass spectrometry, 4th ed. Biochem. Syst. Ecol. 1995, 24, 594. [Google Scholar]
- Craven, A. Pesticide Residues in Coastal Tropical Ecosystems: Distribution, Fate and Effects; Taylor, M.D., Klaine, S.J., Carvalho, F.P., Barcelo, D., Everaarts, J., Eds.; Taylor & Francis: London, UK, 2003; Volume 541, p. 1360. ISBN 0 415 239176. [Google Scholar] [CrossRef]
- Vokou, D.; Liotiri, S. Stimulation of soil microbial activity by essential oils. Chemoecology 1999, 9, 41–45. [Google Scholar] [CrossRef]
- Tworkoski, T. Herbicide effects of essential oils. Weed Sci. 2002, 50, 425–431. [Google Scholar] [CrossRef]
- Dohi, S.; Terasaki, M.; Makino, M. Acetylcholinesterase inhibitory activity and chemical composition of commercial essential oils. J. Agric. Food Chem. 2009, 57, 4313–4318. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Mohamed, M.I.E.; Badawy, M.E.I.; El-Arami, S.A.A. Fumigant and contact toxicities of monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. J. Chem. Ecol. 2009, 35, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kang, J.; Park, I.K. Fumigant toxicity of Apiaceae essential oils and their constituents against Sitophilus oryzae and their acetylcholinesterase inhibitory activity. J. Asia Pac. Entomol. 2013, 16, 443–448. [Google Scholar] [CrossRef]
- Kiran, S.; Prakash, B. Assessment of Toxicity, Antifeedant Activity, and Biochemical Responses in Stored-Grain Insects Exposed to Lethal and Sublethal Doses of Gaultheria procumbens L. Essential Oil. J. Agric. Food Chem. 2015, 63, 10518–10524. [Google Scholar] [CrossRef]
- Park, C.G.; Jang, M.; Yoon, K.A.; Kim, J. Insecticidal and acetylcholinesterase inhibitory activities of Lamiaceae plant essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae). Ind. Crops Prod. 2016, 89, 507–513. [Google Scholar] [CrossRef]
- Saad, M.M.G.; Abou-Taleb, H.K.; Abdelgaleil, S.A.M. Insecticidal activities of monoterpenes and phenylpropenes against sitophilus oryzae and their inhibitory effects on acetylcholinesterase and adenosine triphosphatases. Appl. Entomol. Zool. 2018, 53, 173–181. [Google Scholar] [CrossRef]
- Tak, J.H.; Isman, M.B. Enhanced cuticular penetration as the mechanism for synergy of insecticidal constituents of rosemary essential oil in Trichoplusia ni. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Boyer, S.; Zhang, H.; Lempérière, G. A review of control methods and resistance mechanisms in stored-product insects. Bull. Entomol. Res. 2012, 102, 213–229. [Google Scholar] [CrossRef]
- Kolawole, A.O.; Olajuyigbe, F.M.; Ajele, J.O.; Adedire, C.O. Activity of the Antioxidant Defense System in a Typical Bioinsecticide- and Synthetic Insecticide-treated Cowpea Storage Beetle Callosobrochus maculatus F. (Coleoptera: Chrysomelidae). Int. J. Insect Sci. 2014, 6. [Google Scholar] [CrossRef]
- Oni, M.O.; Ogungbite, O.C.; Oguntuase, S.O.; Bamidele, O.S.; Ofuya, T.I. Inhibitory effects of oil extract of green Acalypha (Acalypha wilkesiana) on antioxidant and neurotransmitter enzymes in Callosobruchus maculatus. J. Basic Appl. Zool. 2019, 80. [Google Scholar] [CrossRef]
- Enayati, A.A.; Ranson, H.; Hemingway, J. Insect glutathione transferases and insecticide resistance. Insect Mol. Biol. 2005, 14, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.; Kujur, A.; Patel, L.; Ramalakshmi, K.; Prakash, B. Assessment of toxicity and biochemical mechanisms underlying the insecticidal activity of chemically characterized Boswellia carterii essential oil against insect pest of legume seeds. Pestic. Biochem. Physiol. 2017, 139, 17–23. [Google Scholar] [CrossRef]





| Component | RI | RI Lit. * | % |
|---|---|---|---|
| Camphene | 943 | 954 | 9.18 |
| β-Pinene | 943 | 979 | 5.53 |
| α-Pinene | 948 | 936 | 22.64 |
| β-Myrcene | 958 | 990 | 1.27 |
| D-Limonene | 1018 | 1029 | 4.91 |
| o-Cymene | 1042 | 1026 | 2.76 |
| 1,8-Cineole | 1059 | 1031 | 21.53 |
| Linalool | 1082 | 1096 | 0.87 |
| Camphor | 1121 | 1146 | 21.84 |
| Isoborneol | 1138 | 1160 | 1.17 |
| endo-Borneol | 1138 | 1169 | 2.15 |
| α-Terpineol | 1143 | 1188 | 1.92 |
| Bornyl acetate | 1277 | 1288 | 2.45 |
| α-Terpinyl acetate | 1333 | 1349 | 0.40 |
| Caryophyllene | 1494 | 1419 | 1.32 |
| Humulene | 1579 | 1608 | 0.07 |
| LC (µL/50 mL) Values for Imago | |||
|---|---|---|---|
| 24 h | Goodness of Fit | ||
| LC5 | 0.1157 | Df | 65 |
| LC12.5 | 0.4132 | R2 | 0.9614 |
| LC25 | 0.6518 | Square error | 7.779 |
| LC50 | 0.9709 | Sum of Squares | 3933 |
| LC75 | 1.29 | ||
| LC95 | 1.826 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzyżowski, M.; Baran, B.; Łozowski, B.; Francikowski, J. The Effect of Rosmarinus officinalis Essential Oil Fumigation on Biochemical, Behavioral, and Physiological Parameters of Callosobruchus maculatus. Insects 2020, 11, 344. https://doi.org/10.3390/insects11060344
Krzyżowski M, Baran B, Łozowski B, Francikowski J. The Effect of Rosmarinus officinalis Essential Oil Fumigation on Biochemical, Behavioral, and Physiological Parameters of Callosobruchus maculatus. Insects. 2020; 11(6):344. https://doi.org/10.3390/insects11060344
Chicago/Turabian StyleKrzyżowski, Michał, Bartosz Baran, Bartosz Łozowski, and Jacek Francikowski. 2020. "The Effect of Rosmarinus officinalis Essential Oil Fumigation on Biochemical, Behavioral, and Physiological Parameters of Callosobruchus maculatus" Insects 11, no. 6: 344. https://doi.org/10.3390/insects11060344
APA StyleKrzyżowski, M., Baran, B., Łozowski, B., & Francikowski, J. (2020). The Effect of Rosmarinus officinalis Essential Oil Fumigation on Biochemical, Behavioral, and Physiological Parameters of Callosobruchus maculatus. Insects, 11(6), 344. https://doi.org/10.3390/insects11060344





