Suitability of European Trichogramma Species as Biocontrol Agents against the Tomato Leaf Miner Tuta absoluta
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Molecular and Morphological Identification
2.3. Host Acceptance and Host Preference
2.4. Host Searching Capacity
2.5. Statistical Analysis
3. Results
3.1. Molecular and Morphological Identification
3.2. Host Acceptance
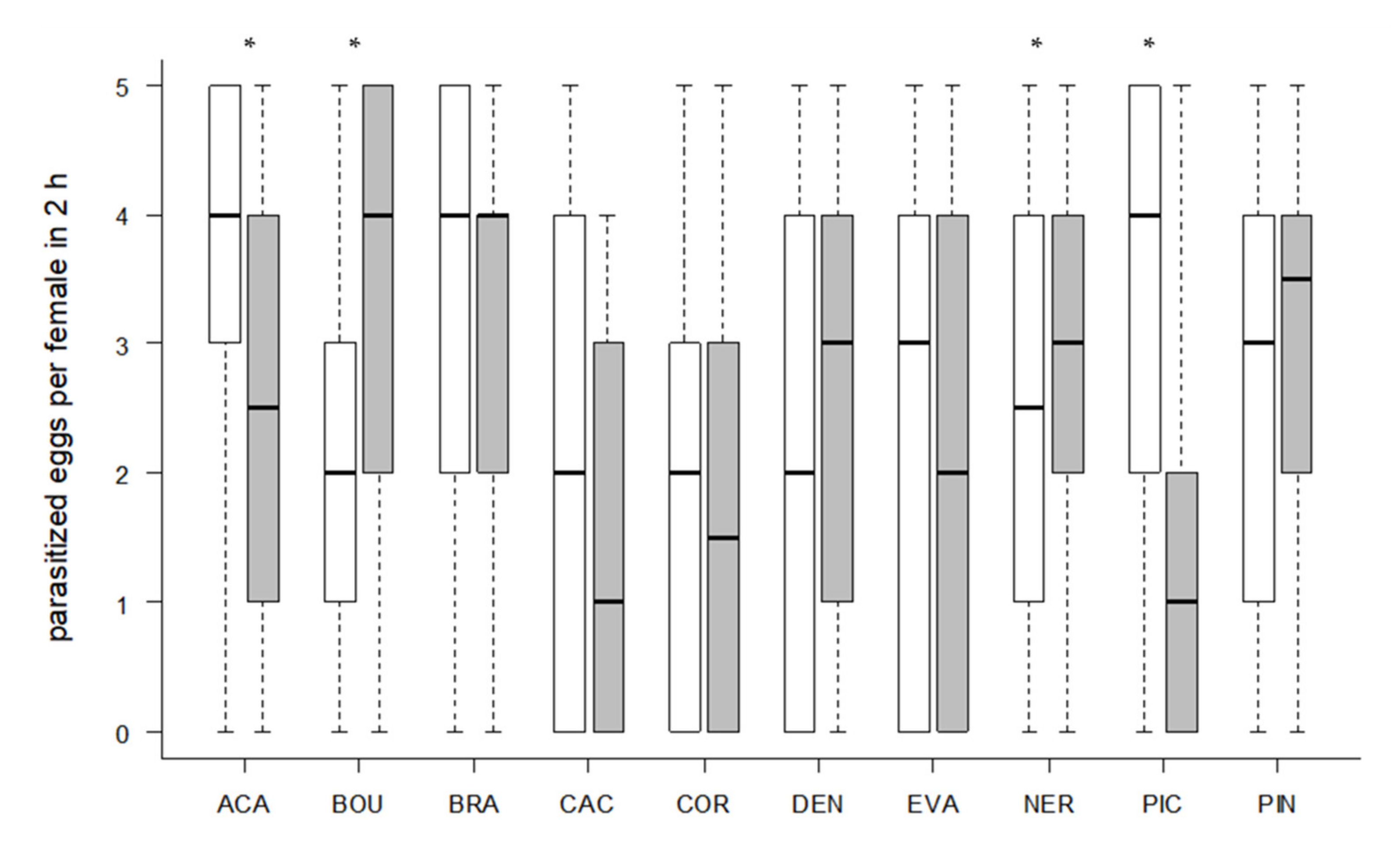
3.3. Host Preference
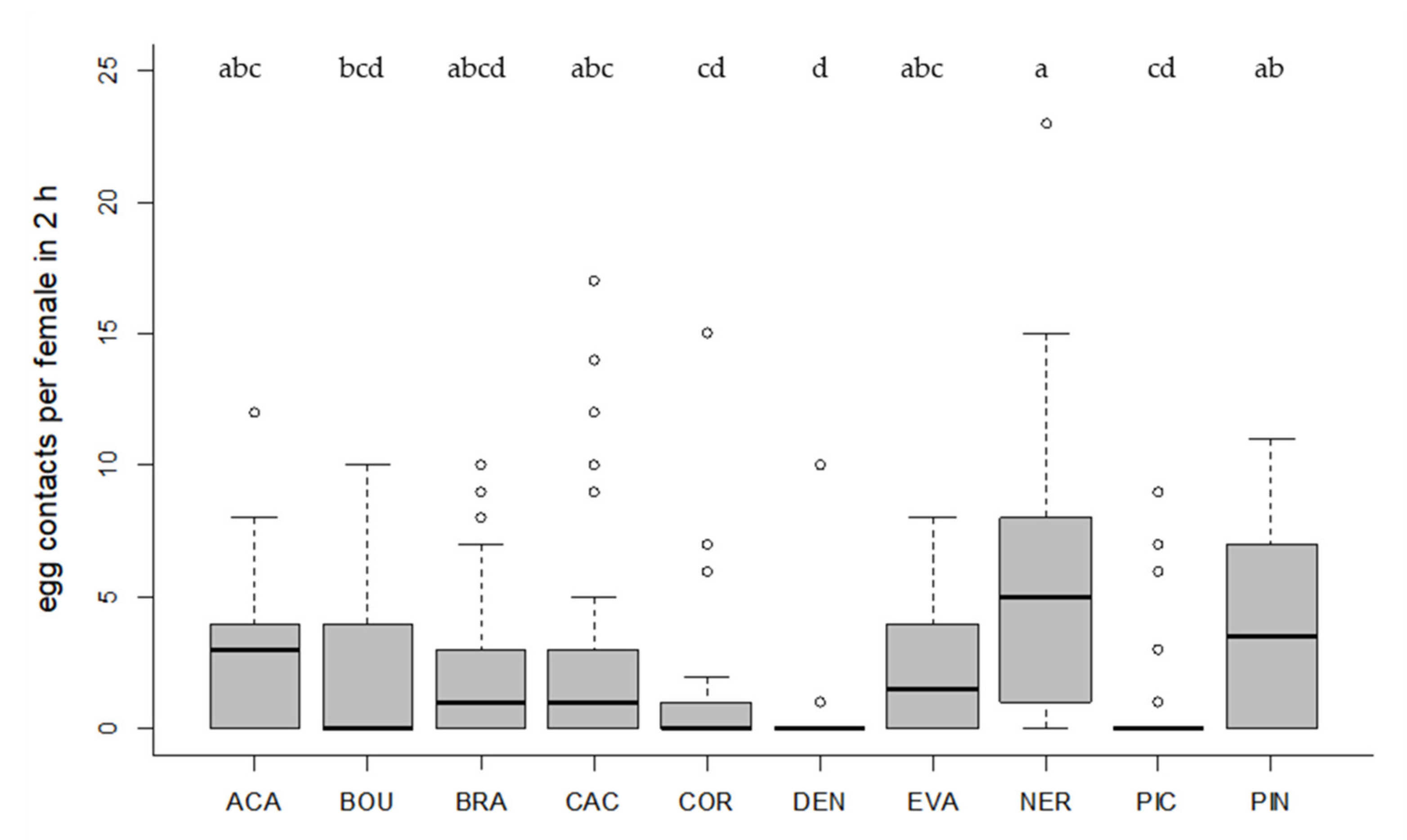
3.4. Host Searching Capacity
4. Discussion
4.1. Molecular and Morphological Identification
4.2. Host Acceptance and Host Preference
4.3. Host Searching Capacity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef]
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Desneux, N.; Luna, M.G.; Guillemaud, T.; Urbaneja, A. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: The new threat to tomato world production. J. Pest Sci. 2011, 84, 403–408. [Google Scholar] [CrossRef]
- Zimmermann, O.; Wührer, B. The South American tomato moth, Tuta absoluta, a new pest in Germany: An assessment of the biological control options. Tuta absoluta, eine neuer Schädling in Deutschland: Eine Einschätzung der Biologischen Bekämpfungsmöglichkeiten. DGaaE Nachr. 2010, 24, 22–23. [Google Scholar]
- Tropea Garzia, G.; Siscaro, G.; Biondi, A.; Zappalà, L. Tuta absoluta, a South American pest of tomato now in the EPPO region: Biology, distribution and damage. EPPO Bull. 2012, 42, 205–210. [Google Scholar] [CrossRef]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Ruescas, D.C.; Tabone, E.; Frandon, J. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- González-Cabrera, J.; Mollá, O.; Montón, H.; Urbaneja, A. Efficacy of Bacillus thuringiensis (Berliner) in controlling the tomato borer, Tuta absoluta (Meyrick)(Lepidoptera: Gelechiidae). BioControl 2011, 56, 71–80. [Google Scholar] [CrossRef]
- Arnó, J.; Gabarra, R. Side effects of selected insecticides on the Tuta absoluta (Lepidoptera: Gelechiidae) predators Macrolophus pygmaeus and Nesidiocoris tenuis (Hemiptera: Miridae). J. Pest Sci. 2011, 84, 513–520. [Google Scholar] [CrossRef]
- Lietti, M.M.; Botto, E.; Alzogaray, R.A. Insecticide resistance in argentine populations of Tuta absoluta (Meyrick)(Lepidoptera: Gelechiidae). Neotrop. Entomol. 2005, 34, 113–119. [Google Scholar] [CrossRef]
- Biondi, A.; Desneux, N.; Siscaro, G.; Zappalà, L. Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: Selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere 2012, 87, 803–812. [Google Scholar] [CrossRef]
- Biondi, A.; Zappalà, L.; Stark, J.D.; Desneux, N. Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS ONE 2013, 8, e76548. [Google Scholar] [CrossRef]
- Cocco, A.; Deliperi, S.; Delrio, G. Control of Tuta absoluta (Meyrick)(Lepidoptera: Gelechiidae) in greenhouse tomato crops using the mating disruption technique. J. Appl. Entomol. 2013, 137, 16–28. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A. Understanding benefits and risks of pesticide use. Sci. Res. Essays 2009, 4, 945–949. [Google Scholar]
- Hamilton, D.; Crossley, S. Pesticide Residues in Food and Drinking Water: Human Exposure and Risks; John Wiley & Sons Ltd.: Chichester, UK, 2004. [Google Scholar]
- Luna, M.; Sánchez, N.E.; Pereyra, P.C.; Nieves, E.; Savino, V.; Luft, E.; Virla, E.; Speranza, S. Biological control of Tuta absoluta in Argentina and Italy: Evaluation of indigenous insects as natural enemies. EPPO Bull. 2012, 42, 260–267. [Google Scholar] [CrossRef]
- Reyes, M.; Rocha, K.; Alarcón, L.; Siegwart, M.; Sauphanor, B. Metabolic mechanisms involved in the resistance of field populations of Tuta absoluta (Meyrick)(Lepidoptera: Gelechiidae) to spinosad. Pestic. Biochem. Physiol. 2012, 102, 45–50. [Google Scholar] [CrossRef]
- Siqueira, H.; Guedes, R.; Fragoso, D.d.B.; Magalhaes, L. Abamectin resistance and synergism in Brazilian populations of Tuta absoluta (Meyrick)(Lepidoptera: Gelechiidae). Int. J. Pest Manag. 2001, 47, 247–251. [Google Scholar] [CrossRef]
- Siqueira, H.Á.A.; Guedes, R.N.C.; Picanço, M.C. Insecticide resistance in populations of Tuta absoluta (Lepidoptera: Gelechiidae). Agric. For. Entomol. 2000, 2, 147–153. [Google Scholar] [CrossRef]
- Scholz-Döbelin, H.; Brinza, M. Tuta absoluta: A new approach in biological pest control by granulovirus „Tutavir“ and Steinernema-nematodes in an operational tomato greenhouse. DGaaE Nachr. 2019, 33, 73–74. [Google Scholar]
- Directive 2009/128/EC, Sustainable Use of Pesticides. Available online: https://ec.europa.eu/food/plant/pesticides/sustainable_use_pesticides_en (accessed on 8 May 2020).
- Pratissoli, D.; Thuler, R.T.; Andrade, G.S.; Zanotti, L.C.M.; Silva, A.F. Estimate of Trichogramma pretiosum to control Tuta absoluta in stalked tomato. Pesqui. Agropecuária Bras. 2005, 40, 715–718. [Google Scholar] [CrossRef]
- Smith, S.M. Biological control with Trichogramma: Advances, successes, and potential of their use. Annu. Rev. Entomol. 1996, 41, 375–406. [Google Scholar] [CrossRef] [PubMed]
- Suckling, D.; Brockerhoff, E. Invasion biology, ecology, and management of the light brown apple moth (Tortricidae). Annu. Rev. Entomol. 2010, 55, 285–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zimmermann, O. Der Einsatz von Trichogramma-Schlupfwespen in Deutschland. Gesunde Pflanz. 2004, 56, 157–166. [Google Scholar] [CrossRef]
- Do Thi Khanh, H.; Chailleux, A.; Tiradon, M.; Desneux, N.; Colombel, E.; Tabone, E. Using new egg parasitoids (Trichogramma spp.) to improve integrated management against Tuta absoluta. EPPO Bull. 2012, 42, 249–254. [Google Scholar] [CrossRef]
- Desneux, N.; Pizzol, P.; Thomas, C.; Pautrat, E.; Bearez, P.; Poncet, C.; Tabone, E.; Kabiri, F.; Frandon, J. Potential for direct interference between natural enemies of Tuta absoluta on tomato. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010), International Symposium on Plant 917, Lisbon, Portugal, 22–28 August 2010; pp. 31–37. [Google Scholar]
- Chailleux, A.; Desneux, N.; Seguret, J.; Khanh, H.D.T.; Maignet, P.; Tabone, E. Assessing European egg parasitoids as a mean of controlling the invasive South American tomato pinworm Tuta absoluta. PLoS ONE 2012, 7, e48068. [Google Scholar] [CrossRef] [PubMed]
- Polaszek, A. Species diversity and host associations of Trichogramma in Eurasia. In Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma; Consoli, F.L., Parra, J.R.P., Zuchhi, R.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 237–266. [Google Scholar]
- Federal Ministry for the Environment. Nature Conservation and Nuclear Safety. Act on Nature Conservation and Landscape Management (Federal Nature Conservation Act—BNatSchG) of 29 July 2009. Federal Ministry for the Environment, Nature Conservation and Nuclear Safety Web site. 2019. Available online: www.bmu.de/GE142-1 (accessed on 3 May 2020).
- Polaszek, A.; Rugman-Jones, P.F.; Stouthamer, R.; Hernandez-Suarez, E.; Cabello, T.; del Pino Pérez, M. Molecular and morphological diagnoses of five species of Trichogramma: Biological control agents of Chrysodeixis chalcites (Lepidoptera: Noctuidae) and Tuta absoluta (Lepidoptera: Gelechiidae) in the Canary Islands. BioControl 2012, 57, 21–35. [Google Scholar] [CrossRef]
- Candolfi, M.; Blümel, S.; Forster, R.; Bakker, F.; Grimm, C.; Hassan, S.; Heimbach, U.; Mead, M.; Reber, B.; Schmuck, R.; et al. Guidelines to Evaluate Side-Effects of Plant Protection Products to Non-Target Arthropods; Dreier Druck Reinheim: Hessen, Germany, 2000. [Google Scholar]
- Sumer, F.; Tuncbilek, A.S.; Oztemiz, S.; Pintureau, B.; Rugman-Jones, P.; Stouthamer, R. A molecular key to the common species of Trichogramma of the Mediterranean region. BioControl 2009, 54, 617–624. [Google Scholar] [CrossRef]
- Silva, I.M.; Honda, J.; van Kan, F.; Hu, J.; Neto, L.; Pintureau, B.; Stouthamer, R. Molecular differentiation of five Trichogramma species occurring in Portugal. Biol. Control 1999, 16, 177–184. [Google Scholar] [CrossRef]
- Stouthamer, R.; Hu, J.; van Kan, F.J.; Platner, G.R.; Pinto, J.D. The utility of internally transcribed spacer 2 DNA sequences of the nuclear ribosomal gene for distinguishing sibling species of Trichogramma. BioControl 1999, 43, 421–440. [Google Scholar] [CrossRef]
- Pinto, J.; Stouthamer, R. Systematics of the Trichogrammatidae with emphasis on Trichogramma. In Biological Control with Egg Parasitoids; Wajnberg, E., Hassan, S.A., Eds.; CABI: Wallingford, UK, 1994; pp. 1–36. [Google Scholar]
- Nagarkatti, S.; Nagaraja, H. Redescriptions of some known species of Trichogramma (Hym., Trichogrammatidae), showing the importance of the male genitalia as a diagnostic character. Bull. Entomol. Res. 1971, 61, 13–31. [Google Scholar] [CrossRef]
- Pintureau, B. Les Espèces Européennes de Trichogrammes, ILV ed.; InLibroVeritas; INRA: Villeurbanne, France, 2008. [Google Scholar]
- Pinto, J.D. Systematics of the North American Species of Trichogramma Westwood (Hymenoptera: Trichogrammatidae); Entomological Society of Washington: Washington, DC, USA, 1998. [Google Scholar]
- Herz, A.; Hassan, S.A.; Hegazi, E.; Khafagi, W.E.; Nasr, F.N.; Youssef, A.I.; Agamy, E.; Blibech, I.; Ksentini, I.; Ksantini, M. Egg parasitoids of the genus Trichogramma (Hymenoptera, Trichogrammatidae) in olive groves of the Mediterranean region. Biol. Control 2007, 40, 48–56. [Google Scholar] [CrossRef]
- Romani, R.; Isidoro, N.; Bin, F. Antennal structures used in communication by egg parasitoids. In Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma; Springer: Berlin/Heidelberg, Germany, 2009; pp. 57–96. [Google Scholar]
- Steidle, J.L.; Rees, D.; Wright, E.J. Assessment of Australian Trichogramma species (Hymenoptera: Trichogrammatidae) as control agents of stored product moths. J. Stored Prod. Res. 2001, 37, 263–275. [Google Scholar] [CrossRef]
- International Organization for Biological Control. IOBC Quality Control Guidelines. Available online: http://www.mrqa.unibo.it/guidelines.htm (accessed on 8 March 2020).
- Vargas, P.; Cabello, T. A new species of Trichogramma [T. cordubensis n. sp.][Hym.: Trichogrammatidae], parasitoid of Heliothis eggs in cotton crops in the sw of spain. Entomophaga 1985, 30, 225–230. [Google Scholar] [CrossRef]
- Cônsoli, F.L.; Parra, J.R.; Zucchi, R.A. Egg parasitoids in Agroecosystems with Emphasis on Trichogramma; Springer: Dordrecht, The Netherlands, 2010; Volume 9. [Google Scholar]
- Fursov, V. Discovery of four species of Trichogramma (Hymenoptera, Trichogrammatidae), new for the fauna of England. Вестник зоологии Vestn. Zool. 2000, 34, 107–113. [Google Scholar]
- Pinto, J.D.; Platner, G.R.; Stouthamer, R. The systematics of the Trichogramma minutum species complex (Hymenoptera: Trichogrammatidae), a group of important North American biological control agents: The evidence from reproductive compatibility and allozymes. Biol. Control 2003, 27, 167–180. [Google Scholar] [CrossRef]
- Stouthamer, R. The use of unisexual wasps in biological control. In Quality Control and Production of Biological Control Agents-Theory and Testing Procedures; CABI: Wallingford, UK, 2003; pp. 93–114. [Google Scholar]
- Borghuis, A.; Pinto, J.D.; Platner, G.R.; Stouthamer, R. Partial cytochrome oxidase II sequences distinguish the sibling species Trichogramma minutum Riley and Trichogramma platneri Nagarkatti. Biol. Control 2004, 30, 90–94. [Google Scholar] [CrossRef]
- Pintureau, B. Indices d’isolement reproductif entre espèces proches de Trichogrammes (Hym.: Trichogrammatidae). Annales de la Société Entomologique de France (Nouvelle Série) 1991, 27, 379–392. [Google Scholar]
- Pinto, J.D.; Stouthamer, R.; Platner, G.R.; Oatman, E.R. Variation in reproductive compatibility in Trichogramma and its taxonomic significance (Hymenoptera: Trichogrammatidae). Ann. Entomol. Soc. Am. 1991, 84, 37–46. [Google Scholar] [CrossRef]
- Stouthamer, R.; Jochemsen, P.; Platner, G.R.; Pinto, J.D. Crossing incompatibility between Trichogramma minutum and T. platneri (Hymenoptera: Trichogrammatidae): Implications for application in biological control. Environ. Entomol. 2000, 29, 832–837. [Google Scholar] [CrossRef]
- Hassan, S.; Guo, M.; Bigler, F. A simple method to control the quality of mass reared egg parasites of the genus Trichogramma. In Proceedings of the 5th International Workshop on Quality Control of Mass Reared Arthropods, IOBC, Wageningen, The Netherlands, 25–28 March 1991; pp. 127–137. [Google Scholar]
- Wäckers, F.; De Groot, I.; Noldus, L.; Hassan, S. Measuring host preference of Trichogramma egg parasites: An evaluation of direct and indirect methods. Meas. Host Prefer. Trichogramma Egg Parasites Eval. Direct Indirect. Methods 1987, 52, 339–348. [Google Scholar]
- Taylor, T.A.; Stern, V.M. Host-preference studies with the egg parasite Trichogramma semifumatum (Hymenoptera: Trichogrammatidae). Ann. Entomol. Soc. Am. 1971, 64, 1381–1390. [Google Scholar] [CrossRef][Green Version]
- Pak, G.; Van Dalen, A.; Kaashoek, N.; Dijkman, H. Host egg chorion structure influencing host suitability for the egg parasitoid Trichogramma Westwood. J. Insect Physiol. 1990, 36, 869–875. [Google Scholar] [CrossRef]
- Abdel-Latief, M.; Hilker, M. Innate immunity: Eggs of Manduca sexta are able to respond to parasitism by Trichogramma evanescens. Insect Biochem. Mol. Biol. 2008, 38, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.A.; Luhring, K.A.; Stafford, C.A.; Hansen, A.K.; Millar, J.G.; Hanks, L.M.; Paine, T.D. Host defensive response against an egg parasitoid involves cellular encapsulation and melanization. Biol. Control 2007, 41, 214–222. [Google Scholar] [CrossRef]
- van Lenteren, J.; Hale, A.; Klapwijk, J.; Van Schelt, J.; Steinberg, S. Guidelines for quality control of commercially produced natural enemies. In Quality Control and Production of Biological Control Agents: Theory and Testing Procedures; CABI: London, UK, 2003; pp. 278–316. [Google Scholar]
- Roriz, V.; Oliveira, L.; Garcia, P. Host suitability and preference studies of Trichogramma cordubensis (Hymenoptera: Trichogrammatidae). Biol. Control 2006, 36, 331–336. [Google Scholar] [CrossRef]
- Keller, M.A. Influence of leaf surfaces on movements by the hymenopterous parasitoid Trichogramma exiguum. Entomol. Exp. Appl. 1987, 43, 55–59. [Google Scholar] [CrossRef]
- Lukianchuk, J.; Smith, S. Influence of plant structural complexity on the foraging success of Trichogramma minutum: A comparison of search on artificial and foliage models. Entomol. Exp. Appl. 1997, 84, 221–228. [Google Scholar] [CrossRef]
- Romeis, J.; Shanower, T.; Zebitz, C. Why Trichogramma (Hymenoptera: Trichogrammatidae) egg parasitoids of Helicoverpa armigera (Lepidoptera: Noctuidae) fail on chickpea. Bull. Entomol. Res. 1999, 89, 89–95. [Google Scholar] [CrossRef]
- Kauffman, W.C.; Kennedy, G.G. Relationship between trichome density in tomato and parasitism of Heliothis spp. (Lepidoptera: Noctuidae) eggs by Trichogramma spp. (Hymenoptera: Trichogrammatidae). Environ. Entomol. 1989, 18, 698–704. [Google Scholar] [CrossRef]
- Kashyap, R.; Kennedy, G.; Farrar, R. Behavioral response of Trichogramma pretiosum Riley and Telenomus sphingis (Ashmead) to trichome/methyl ketone mediated resistance in tomato. J. Chem. Ecol. 1991, 17, 543–556. [Google Scholar] [CrossRef]
- Kashyap, R.; Kennedy, G.; Farrar, R. Mortality and inhibition of Helicoverpa zea egg parasitism rates by Trichogramma in relation to trichome/methyl ketone-mediated insect resistance of Lycopersicon hirsutum f. glabratum, accession PI 134417. J. Chem. Ecol. 1991, 17, 2381–2395. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Durão, A.C.; Fontes, J.; Roja, I.S.; Tavares, J. Potential of Trichogramma achaeae (Hymenoptera: Trichogrammatidae) in biological control of Tuta absoluta (Lepidoptera: Gelechiidae) in Azorean greenhouse tomato crops. J. Econ. Entomol. 2017, 110, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Mills, N. Egg parasitoids in biological control and integrated pest management. In Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma; Springer: Berlin/Heidelberg, Germany, 2009; pp. 389–411. [Google Scholar]
- Chailleux, A.; Biondi, A.; Han, P.; Tabone, E.; Desneux, N. Suitability of the pest–plant system Tuta absoluta (Lepidoptera: Gelechiidae)–tomato for Trichogramma (Hymenoptera: Trichogrammatidae) parasitoids and insights for biological control. J. Econ. Entomol. 2013, 106, 2310–2321. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, F.; Bigler, F. Quality assessment of Trichogramma brassicae in the laboratory. Entomol. Exp. Appl. 1995, 75, 19–26. [Google Scholar] [CrossRef]
- Charnov, E.L. The Theory of Sex Allocation; Princeton University Press: Princeton, NJ, USA, 1982; Volume 18. [Google Scholar]
- Cherif, A.; Kaouthar, L.G. Trichogramma cacoeciae as a biological control agent of the tomato pinworm Tuta absoluta in Northeastern Tunisia. Entomol. Hell. 2013, 22, 35–42. [Google Scholar] [CrossRef]
- Ueno, T. Host-size-dependent sex ratio in a parasitoid wasp. Popul. Ecol. 1999, 41, 47–57. [Google Scholar] [CrossRef]
- Werren, J.H.; Simbolotti, G. Combined effects of host quality and local mate competition on sex allocation in Lariophagus distinguendus. Evol. Ecol. 1989, 3, 203–213. [Google Scholar] [CrossRef]
- Stouthamer, R.; Tilborg, M.v.; De Jong, J.; Nunney, L.; Luck, R. Selfish element maintains sex in natural populations of a parasitoid wasp. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 617–622. [Google Scholar] [CrossRef]
- van Vugt, J.F.; Salverda, M.; de Jong, J.H.; Stouthamer, R. The paternal sex ratio chromosome in the parasitic wasp Trichogramma kaykai condenses the paternal chromosomes into a dense chromatin mass. Genome 2003, 46, 580–587. [Google Scholar] [CrossRef]
- Werren, J.H.; Stouthamer, R. PSR (paternal sex ratio) chromosomes: The ultimate selfish genetic elements. Genetica 2003, 117, 85–101. [Google Scholar] [CrossRef]
- Gonçalves, C.I.; Huigens, M.E.; Verbaarschot, P.; Duarte, S.; Mexia, A.; Tavares, J. Natural occurrence of Wolbachia-infected and uninfected Trichogramma species in tomato fields in Portugal. Biol. Control 2006, 37, 375–381. [Google Scholar] [CrossRef]
- Jervis, M.; Kidd, N. Host-feeding strategies in hymenopteran parasitoids. Biol. Rev. 1986, 61, 395–434. [Google Scholar] [CrossRef]
- Hansen, L.S.; Jensen, K.-M. Effect of temperature on parasitism and host-feeding of Trichogramma turkestanica (Hymenoptera: Trichogrammatidae) on Ephestia kuehniella (Lepidoptera: Pyralidae). J. Econ. Entomol. 2002, 95, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.S.; Jensen, K.-M.V. Trichogramma turkestanica against Ephestia kuehniella in flour mills: Extent of host-feeding and initial results of a field trial. IOBC WPRS Bull. 2002, 25, 105–108. [Google Scholar]
- Ruberson, J.; Kring, T. Parasitism of developing eggs by Trichogramma pretiosum (Hymenoptera: Trichogrammatidae): Host age preference and suitability. Biol. Control 1993, 3, 39–46. [Google Scholar] [CrossRef]
- Mansfield, S.; Mills, N. A comparison of methodologies for the assessment of host preference of the gregarious egg parasitoid Trichogramma platneri. Biol. Control 2004, 29, 332–340. [Google Scholar] [CrossRef]
- Kivan, M.; Kilic, N. Host preference: Parasitism, emergence and development of Trissolcus semistriatus (Hym., Scelonidae) in various host eggs. J. Appl. Entomol. 2002, 126, 395–399. [Google Scholar] [CrossRef]
- Makee, H. Factors influencing the parasitism of codling moth eggs by Trichogramma cacoeciae March. and T. principium Sug. et Sor. (Hymen. Trichogrammatidae). J. Pest Sci. 2005, 78, 31–39. [Google Scholar] [CrossRef]
- Brotodjojo, R.R.; Walter, G.H. Oviposition and reproductive performance of a generalist parasitoid (Trichogramma pretiosum) exposed to host species that differ in their physical characteristics. Biol. Control 2006, 39, 300–312. [Google Scholar] [CrossRef]
- Klomp, H.; Teerink, B.J. Host selection and number of eggs per oviposition in the egg-parasite Trichogramma embryophagum Htg. Nature 1962, 195, 1020–1021. [Google Scholar] [CrossRef]
- Pak, G.A. Selection of Trichogramma for Inundative Biological Control. Ph.D. Thesis, Wageningen Agricultural University, Wageningen, The Netherlands, 1988. [Google Scholar]
- Hassan, S. Selection of suitable Trichogramma strains to control the codling moth Cydia pomonella and the two summer fruit tortrix moths Adoxophyes orana, Pandemis heparana [Lep.: Tortricidae]. Entomophaga 1989, 34, 19–27. [Google Scholar] [CrossRef]
- Babendreier, D.; Kuske, S.; Bigler, F. Parasitism of non-target butterflies by Trichogramma brassicae Bezdenko (Hymenoptera: Trichogrammatidae) under field cage and field conditions. Biol. Control 2003, 26, 139–145. [Google Scholar] [CrossRef]
- Gingras, D.; Dutilleul, P.; Boivin, G. Effect of plant structure on host finding capacity of lepidopterous pests of crucifers by two Trichogramma parasitoids. Biol. Control 2003, 27, 25–31. [Google Scholar] [CrossRef]
- Romeis, J.; Shanower, T.; Zebitz, C. Physical and chemical plant characters inhibiting the searching behaviour of Trichogramma chilonis. Entomol. Exp. Appl. 1998, 87, 275–284. [Google Scholar] [CrossRef]
- Baniameri, V.; Cheraghian, A. The first report and control strategies of Tuta absoluta in Iran. EPPO Bull. 2012, 42, 322–324. [Google Scholar] [CrossRef]
- Zucchi, R.; Querino, R. Towards a database for the Trichogramma species, their hosts and plant associations in the South America. In Proceedings of the XXI International Congress of Entomology, Iguassu Falls, Brazil, 25–27 August 2000; Abstract Book I, session 05; Wiley: Hoboken, NJ, USA, 2000; p. 201. [Google Scholar]
- Cagnotti, C.L.; Hernández, C.M.; Andormo, A.V.; Viscarret, M.; Riquelme, M.; Botto, E.N.; López, S.N. Acceptability and suitability of Tuta absoluta eggs from irradiated parents to parasitism by Trichogramma nerudai and Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Agric. For. Entomol. 2016, 18, 198–205. [Google Scholar] [CrossRef]
- Pizzol, J.; Desneux, N.; Wajnberg, E.; Thiéry, D. Parasitoid and host egg ages have independent impact on various biological traits in a Trichogramma species. J. Pest Sci. 2012, 85, 489–496. [Google Scholar] [CrossRef]
- Pizzol, J.; Pintureau, B. Effect of photoperiod experienced by parents on diapause induction in Trichogramma cacoeciae. Entomol. Exp. Appl. 2008, 127, 72–77. [Google Scholar] [CrossRef]
- Bueno, R.; Parra, J.; Haddad, M. Performance of trichogrammatids as biocontrol agents of Pseudoplusia includens Walker (Lepidoptera: Noctuidae). Neotrop. Entomol. 2009, 38, 389–394. [Google Scholar] [CrossRef]
- Pizzol, J.; Pintureau, B.; Khoualdia, O.; Desneux, N. Temperature-dependent differences in biological traits between two strains of Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). J. Pest Sci. 2010, 83, 447–452. [Google Scholar] [CrossRef]
- Tabone, E.; Bardon, C.; Desneux, N.; Wajnberg, E. Parasitism of different Trichogramma species and strains on Plutella xylostella L. on greenhouse cauliflower. J. Pest Sci. 2010, 83, 251–256. [Google Scholar] [CrossRef]
- Cascone, P.; Carpenito, S.; Slotsbo, S.; Iodice, L.; Sørensen, J.G.; Holmstrup, M.; Guerrieri, E. Improving the efficiency of Trichogramma achaeae to control Tuta absoluta. BioControl 2015, 60, 761–771. [Google Scholar] [CrossRef]





| Species | Rearing Strain 1 | Origin | Year | Reference |
|---|---|---|---|---|
| T. achaeae | ACA BC14 | Company 1 2 | unk. 3 | unk. |
| T. bourarachae | BOU EG02 | Egypt, olive | 2002 | [40] |
| T. brassicae | BRA DA | Company 2 2 | unk. | unk. |
| T. cacoeciae | CAC 1DE05 | Germany | 2005 | BBA 4 |
| T. cordubensis | COR PT93 | Portugal, tomato, Noctuidae | 1993 | [34] |
| T. dendrolimi | DEN D90 | Germany, apple, Cydia pomonella | 1990 | BBA |
| T. evanescens | EVA DE97K | Germany, cabbage, Pieris sp. | 1997 | BBA |
| T. nerudai | NER PT02 | Portugal, olive | 2002 | [40] |
| T. piceum | PIC MD91 | Moldova | 1991 | BBA |
| T. pintoi | PIN 3SY06 | Syria | 2006 | BBA |
| Strain | Estimated Size PCR Product [bp] | Size Consensus Sequence [bp] | Species 1 | Consensus [%] | Accession Number 2 |
|---|---|---|---|---|---|
| ACA | 630 | 317 | T. achaeae | 100.0 | JF415936 |
| BOU | 650 | * 78 | T. bourarachae | 96.2 | DQ389072 |
| BRA | 520 | 431 | T. brassicae | 100.0 | DQ314611 |
| CAC | 580 | 386 | T. cacoeciae | 99.5 | EU547668 |
| COR | 530 | 349 | T. sp.3 | 100.0 | AY146636 |
| DEN | 520 | 423 | T. dendrolimi | 99.8 | AF517576 |
| EVA | 550 | 395 | T. evanescens | 99.5 | JF9204591 |
| NER | 750 | 346 | T. nerudai | 100.0 | AY182756 |
| PIC | 850 | 441 | T. lingulatum4 | 99.8 | AY244466 |
| PIN | 690 | 511 | T. pintoi | 99.8 | JF920460 |
| Strain | Active Females [%] | Emergence Rate [%] | Females F1 [%] |
|---|---|---|---|
| (means ± SE) | (means ± SE) | (means ± SE) | |
| ACA | 63.3 ± 11.9 | 95.4 ± 2.6 | 63.6 ± 5.1 |
| BOU | 36.8 ± 2.7 | 100.0 ± 0.0 | 68.3 ± 4.2 |
| BRA | 46.7 ± 9.8 | 48.2 ± 13.0 | 66.8 ± 10.5 |
| CAC | 40.0 ± 8.2 | 100.0 ± 0.0 | 100.0 ± 0.0 |
| COR | 20.0 ± 8.2 | 66.7 ± 19.3 | 70.2 ± 5.2 |
| DEN | 13.3 ± 7.2 | 75.0 ± 21.7 | 38.9 ± 16.4 |
| EVA | 53.3 ± 9.8 | 100.0 ± 0.0 | 29.5 ± 8.4 |
| NER | 66.7 ± 7.2 | 98.1 ± 1.9 | 53.2 ± 7.3 |
| PIC | 20.0 ± 0.0 | 63.3 ± 18.5 | 77.2 ± 7.0 |
| PIN | 63.3 ± 13.6 | 88.8 ± 7.0 | 49.8 ± 6.2 |
| Strain | Active Females [%] | Active Females [%] |
|---|---|---|
| Host: T. absoluta | Host: S. cerealella | |
| (means ± SE) | (means ± SE) | |
| ACA | 86.7 ± 7.2 | 86.7 ± 7.2 |
| BOU | 80.0 ± 0.0 | 73.3 ± 2.7 |
| BRA | 86.7 ± 2.7 | 80.0 ± 4.7 |
| CAC | 56.7 ± 2.7 | 73.3 ± 7.2 |
| COR | 66.7 ± 7.2 | 63.3 ± 14.4 |
| DEN | 76.7 ± 2.7 | 66.7 ± 11.9 |
| EVA | 70.0 ± 8.2 | 70.0 ± 8.2 |
| NER | 80.0 ± 0.0 | 76.7 ± 2.7 |
| PIC | 63.3 ± 5.4 | 80.0 ± 4.7 |
| PIN | 76.7 ± 11.9 | 80.0 ± 9.4 |
| Species | Egg Length [µm] | Egg Width [µm] |
|---|---|---|
| (means ± SE) | (means ± SE) | |
| Tuta absoluta | 398.9 ± 3.8 b | 260.6 ± 2.9 a |
| Sitotroga cerealella | 622.1 ± 5.9 a | 265.7 ± 2.9 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, L.; Herz, A. Suitability of European Trichogramma Species as Biocontrol Agents against the Tomato Leaf Miner Tuta absoluta. Insects 2020, 11, 357. https://doi.org/10.3390/insects11060357
Schäfer L, Herz A. Suitability of European Trichogramma Species as Biocontrol Agents against the Tomato Leaf Miner Tuta absoluta. Insects. 2020; 11(6):357. https://doi.org/10.3390/insects11060357
Chicago/Turabian StyleSchäfer, Lea, and Annette Herz. 2020. "Suitability of European Trichogramma Species as Biocontrol Agents against the Tomato Leaf Miner Tuta absoluta" Insects 11, no. 6: 357. https://doi.org/10.3390/insects11060357
APA StyleSchäfer, L., & Herz, A. (2020). Suitability of European Trichogramma Species as Biocontrol Agents against the Tomato Leaf Miner Tuta absoluta. Insects, 11(6), 357. https://doi.org/10.3390/insects11060357






