Measuring the Inter and Intraspecific Sexual Shape Dimorphism and Body Shape Variation in Generalist Ground Beetles in Russia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Sites and Data Acquisition
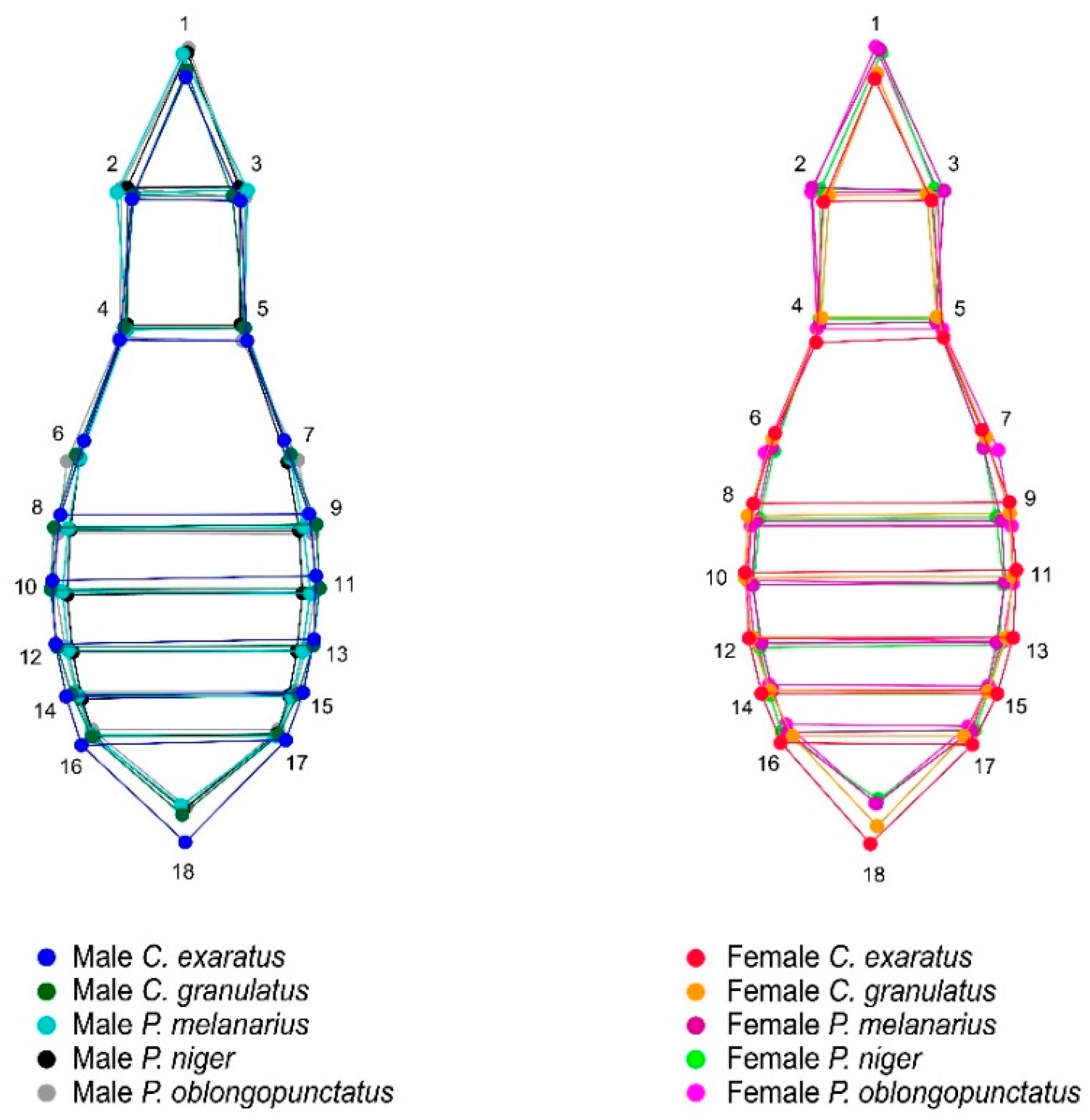
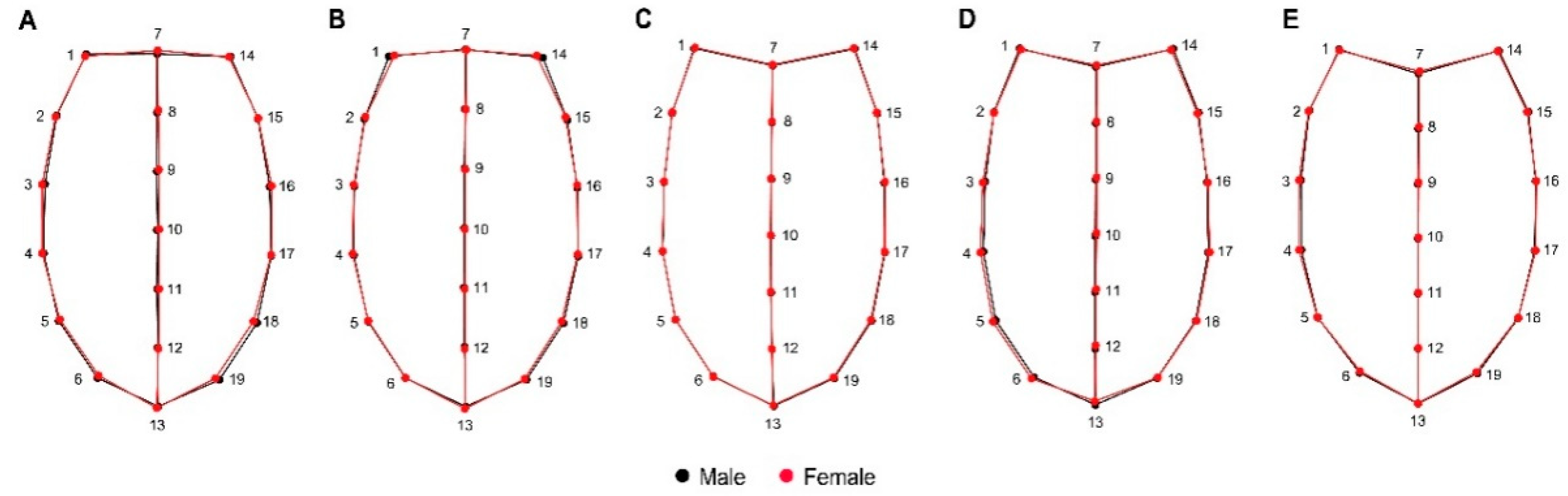
2.2. Shape Analysis
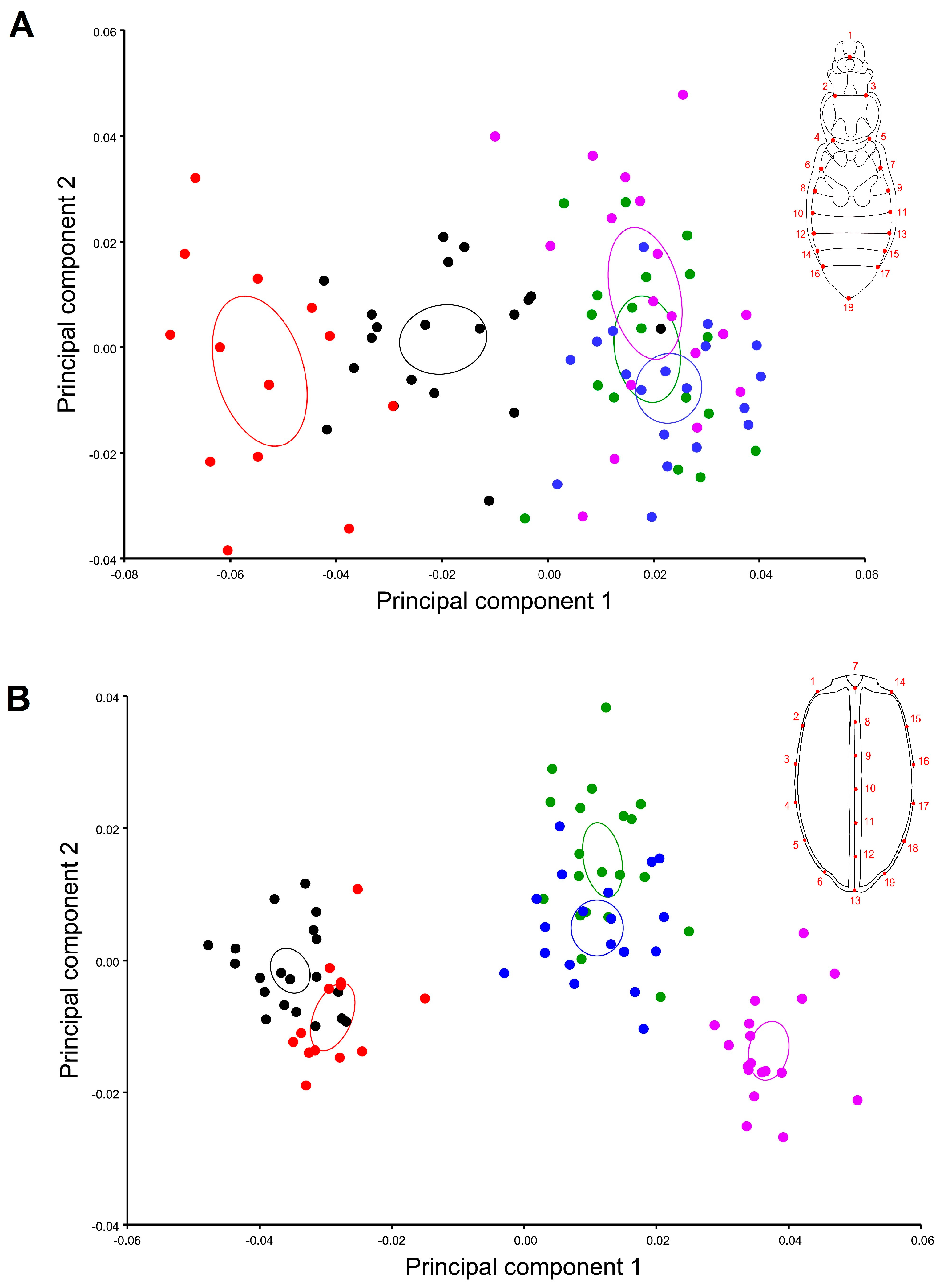
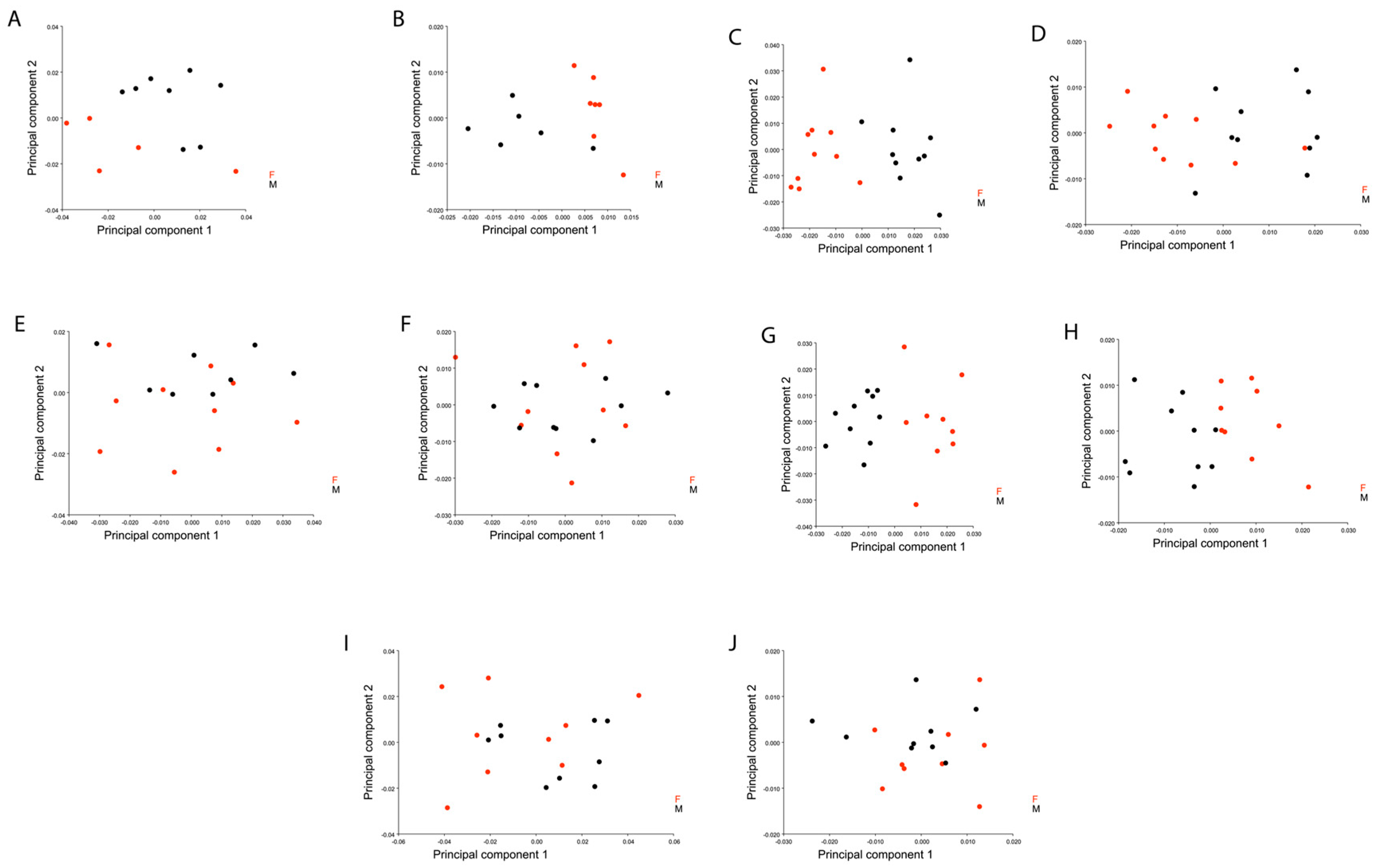
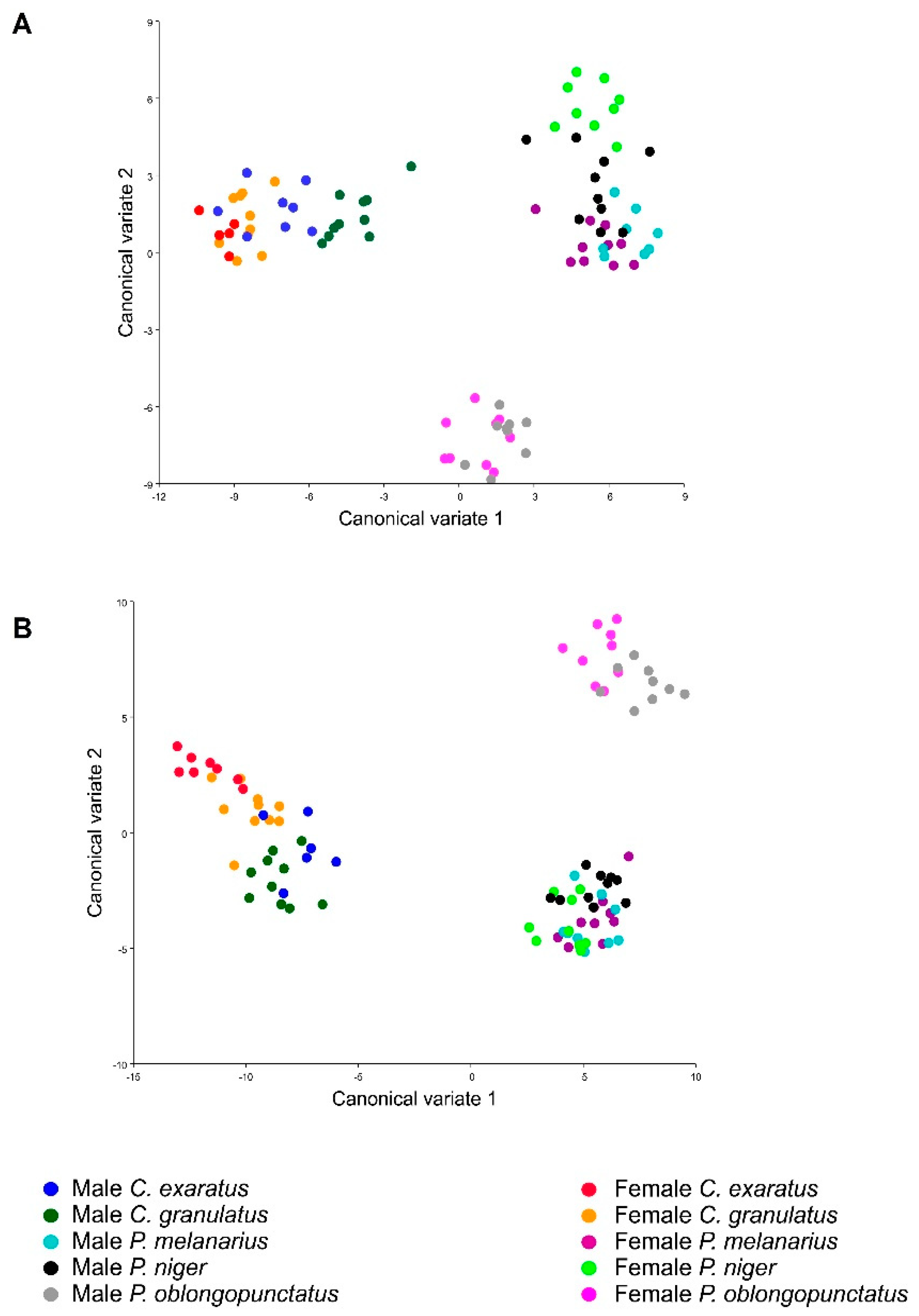
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Foelker, C.; Hofstetter, R. Heritability, fecundity, and sexual size dimorphism in four species of bark beetles (Coleoptera: Curculionidae: Scolytinae). Ann. Entomol. Soc. Am. 2014, 107, 143–151. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Zimmermann, M. Static, ontogenetic, and evolutionary allometry: A multivariate comparison in nine species of water striders. Am. Nat. 1992, 140, 601–620. [Google Scholar] [CrossRef]
- Bookstein, F.L. “Size and shape”: A comment on semantics. Syst. Biol. 1989, 38, 173–180. [Google Scholar] [CrossRef]
- Shingleton, A.W.; Frankino, W.A.; Flatt, T.; Nijhout, H.F.; Emlen, D.J. Size and shape: The developmental regulation of static allometry in insects. BioEssays 2007, 29, 536–548. [Google Scholar] [CrossRef] [Green Version]
- Rohlf, F.J.; Marcus, L.F. A revolution in morphometrics. Trends Ecol. Evol. 1993, 8, 129–132. [Google Scholar] [CrossRef]
- Andersson, M. Sexual Selection; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Fairbairn, D.J.; Blanckenhorn, W.U.; Székely, T. Sex, Size and Gender Roles: Evolutionary Studies of Sexual Size Dimorphism; Oxford University Press: London, UK, 2007. [Google Scholar]
- Cox, R.M.; Skelly, S.L.; John-Alder, H.B. A comparative test of adaptive hypotheses for sexual size dimorphism in lizards. Evolution 2003, 57, 1653–1669. [Google Scholar] [CrossRef]
- Lindenfors, P.; Gittleman, J.L.; Jones, K.E. Sexual size dimorphism in mammals. In Sex, Size and Gender Roles: Evolutionary Studies of Sexual Dimorphism; Fairbairn, D.J., Blanckenhorn, W.U., Székely, T., Eds.; Oxford University Press: London, UK, 2007; pp. 16–26. [Google Scholar]
- Stillwell, R.C.; Blanckenhorn, W.U.; Teder, T.; Davidowitz, G.; Fox, C.W. Sex differences in phenotypic plasticity affect variation in sexual size dimorphism in insects: From physiology to evolution. Annu. Rev. Entomol. 2010, 55, 227–245. [Google Scholar] [CrossRef] [Green Version]
- Szekely, T.; Lislevand, T.; Figuerola, J. Sexual size dimorphism in birds. In Sex, Size and Gender Roles: Evolutionary Studies of Sexual Dimorphism; Fairbairn, D.J., Blanckenhorn, W.U., Székely, T., Eds.; Oxford University Press: London, UK; pp. 27–37.
- Teder, T. Sexual size dimorphism requires a corresponding sex difference in development time: A meta-analysis in insects. Funct. Ecol. 2014, 28, 479–486. [Google Scholar] [CrossRef]
- Tubaro, P.L.; Bertelli, S. Female-biased sexual size dimorphism in tinamous: A comparative test fails to support Rensch’s rule. Biol. J. Linn. Soc. 2003, 80, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Cooper, M.I. Sexual dimorphism in pill millipedes (Diplopoda). J. Entomol. Zool. Stud. 2018, 6, 613–616. [Google Scholar] [CrossRef] [Green Version]
- West-Eberhard, M.J. Sexual selection, social competition, and evolution. Proc. Am. Philos. Soc. 1979, 123, 222–234. [Google Scholar]
- Eberhard, W.G. Beetle horn dimorphism: Making the best of a bad lot. Am. Nat. 1982, 119, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Clutton-Brock, T. Size, sexual dimorphism, and polygyny in primates. In Size and Scaling in Primate Biology; Springer: Berlin/Heidelberg, Germany, 1985; pp. 51–60. [Google Scholar]
- Fincke, O.M.; Waage, J.K.; Koenig, W.D. Natural and sexual selection components of odonate mating patterns. In The Evolution of Mating Systems in Insects and Arachnids; Choe, J.C., Crespi, B.J., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 58–74. [Google Scholar]
- Benítez, H.A.; Avaria-Llautureo, J.; Canales-Aguirre, C.B.; Jerez, V.; Parra, L.E.; Hernandez, C.E. Evolution of sexual size dimorphism and its relationship with sex ratio in carabid beetles of Genus Ceroglossus Solier. Curr. Zool. 2013, 59, 769–777. [Google Scholar] [CrossRef]
- Shreeves, G.; Field, J. Parental care and sexual size dimorphism in wasps and bees. Behav. Ecol. Sociobiol. 2008, 62, 843–852. [Google Scholar] [CrossRef]
- Teder, T.; Tammaru, T. Sexual size dimorphism within species increases with body size in insects. Oikos 2005, 108, 321–334. [Google Scholar] [CrossRef]
- Hurlbutt, B. Sexual size dimorphism in parasitoid wasps. Biol. J. Linn. Soc. 1987, 30, 63–89. [Google Scholar] [CrossRef]
- Lande, R.; Arnold, S.J. Evolution of mating preference and sexual dimorphism. J. Theor. Biol. 1985, 117, 651–664. [Google Scholar] [CrossRef]
- Fairbairn, D.J. Allometry for sexual size dimorphism: Pattern and process in the coevolution of body size in males and females. Annu. Rev. Ecol. Syst. 1997, 28, 659–687. [Google Scholar] [CrossRef]
- Benítez, H.A.; Vidal, M.; Briones, R.; Jerez, V. Sexual Dimorphism and Morphological Variation in Populations of Ceroglossus chilensis (Eschscholtz, 1829) (Coleoptera: Carabidae). J. Entomol. Res. Soc. 2010, 12, 87–95. [Google Scholar]
- Gidaszewski, N.A.; Baylac, M.; Klingenberg, C.P. Evolution of sexual dimorphism of wing shape in the Drosophila melanogaster subgroup. BMC Evol. Biol. 2009, 9, 110. [Google Scholar] [CrossRef] [Green Version]
- Bonduriansky, R. Convergent evolution of sexual shape dimorphism in diptera. J. Morphol. 2006, 267, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Benítez, H.A.; Bravi, R.; Parra, L.E.; Sanzana, M.-J.; Sepulveda-Zuniga, E. Allometric and non-allometric patterns in sexual dimorphism discrimination of wing shape in Ophion intricatus: Might two male morphotypes coexist? J. Insect Sci. 2013, 13, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairn, E.R.; Alarie, Y.; Schulte-Hostedde, A.I. Sexual size and shape dimorphism in Dineutus nigrior (Coleoptera: Gyrinidae). Coleopt. Bull. 2007, 61, 113–120. [Google Scholar] [CrossRef]
- Hernández-L, N.; Barragán, Á.; Dupas, S.; Silvain, J.-F.; Dangles, O. Wing shape variations in an invasive moth are related to sexual dimorphism and altitude. Bull. Entomol. Res. 2010, 100, 529–541. [Google Scholar] [CrossRef]
- Benítez, H.A. Sexual dimorphism using geometric morphometric approach. Sex. Dimorphism 2013, 3, 35–50. [Google Scholar]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. Geometric morphometrics: Ten years of progress following the ‘revolution’. Ital. J. Zool. 2004, 71, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. A field comes of age: Geometric morphometrics in the 21st century. Hystrix Ital. J. Mammal. 2013, 24, 7–14. [Google Scholar] [CrossRef]
- Palestrini, C.; Roggero, A.; Hernández Nova, L.K.; Giachino, P.M.; Rolando, A. On the evolution of shape and size divergence in Nebria (Nebriola) ground beetles (Coleoptera, Carabidae). Syst. Biodivers. 2012, 10, 147–157. [Google Scholar] [CrossRef]
- Faille, A.; Déliot, P.; Quéinnec, E. A new cryptic species of Aphaenops (Coleoptera: Carabidae: Trechinae) from a French Pyrenean cave: Congruence between morphometrical and geographical data confirm species isolation. Ann. Soc. Entomol. 2007, 43, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Zúniga-Reinoso, Á.; Benítez, H.A. The overrated use of the morphological cryptic species concept: An example with Nyctelia darkbeetles (Coleoptera: Tenebrionidae) using geometric morphometrics. Zool. Anz. A J. Comp. Zool. 2015, 255, 47–53. [Google Scholar] [CrossRef]
- Alibert, P.; Moureau, B.; Dommergues, J.L.; David, B. Differentiation at a microgeographical scale within two species of ground beetle, Carabus auronitens and C-nemoralis (Coleoptera, Carabidae): A geometrical morphometric approach. Zool. Scr. 2001, 30, 299–311. [Google Scholar] [CrossRef]
- Benítez, H.A.; Püschel, T.; Lemic, D.; Čačija, M.; Kozina, A.; Bažok, R. Ecomorphological variation of the wireworm cephalic capsule: Studying the interaction of environment and geometric shape. PLoS ONE 2014, 9, e102059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikac, K.M.; Lemic, D.; Bažok, R.; Benítez, H.A. Wing shape changes: A morphological view of the Diabrotica virgifera virgifera European invasion. Biol. Invasions 2016, 18, 3401–3407. [Google Scholar] [CrossRef]
- Lemic, D.; Benítez, H.A.; Püschel, T.A.; Gašparić, H.V.; Šatvar, M.; Bažok, R. Ecological morphology of the sugar beet weevil Croatian populations: Evaluating the role of environmental conditions on body shape. Zool. Anz. A J. Comp. Zool. 2016, 260, 25–32. [Google Scholar] [CrossRef]
- Sukhodolskaya, R.A.; Saveliev, A.A. Impact of environmental factors on the body shape variation and sexual shape dimorphism in Carabus granulatus L. (Coleoptera: Carabidae). Zool. Syst. 2017, 42, 71–89. [Google Scholar]
- Benítez, H.A.; Sanzana, M.-J.; Jerez, V.; Parra, L.E.; Hernandez, C.E.; Canales-Aguirre, C.B. Sexual Shape and Size Dimorphism in Carabid Beetles of the Genus Ceroglossus: Is Geometric Body Size Similar Between Sexes Due to Sex Ratio? Zool. Sci. 2013, 30, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Lemic, D.; Benítez, H.A.; Bažok, R. Intercontinental effect on sexual shape dimorphism and allometric relationships in the beetle pest Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae). Zool. Anz. A J. Comp. Zool. 2014, 253, 203–206. [Google Scholar] [CrossRef]
- Benítez, H.A.; Parra, L.E.; Sepulveda, E.; Sanzana, M.J. Geometric Perspectives of Sexual Dimorphism in the Wing Shape of Lepidoptera: The Case of Synneuria sp. (Lepidoptera: Geometridae). J. Entomol. Res. Soc. 2011, 13, 53–60. [Google Scholar]
- Sanaei, E.; Seiedy, M.; Momtazi, F. Evolutionary view on sexual dimorphism and shape variation in Iranian populations of Hypera postica (Coleoptera: Curculionidae). Zoomorphology 2015, 134, 541–552. [Google Scholar] [CrossRef]
- Prokopenko, E.; Zhukov, A. The population structure of spiders (Aranei) of the bayrak katena. Bull. Donetsk Univ. Ser. A Nat. Sci. 2011, 2, 145–150. [Google Scholar]
- Zhukov, A.; Kunah, O.; Prokopenko, E.; Konovalova, T. The pedoturbation activity of the mole rats (Spalax microphthalmus) as a factor of the spatial organization of the spider (Aranei). News Dnipropetr. State Agrar. Econ. Univ. 2011, 6, 28–35. [Google Scholar]
- Rohlf, F.J. TPSdig Version 2.17; State University at Stony Brook: New York, NY, USA, 2013. [Google Scholar]
- Rohlf, F.J.; Slice, D. Extensions of the Procustes methods for the optimal superimposition of landmarks. Syst. Zool. 1990, 39, 40–59. [Google Scholar] [CrossRef] [Green Version]
- Dryden, I.L.; Mardia, K.V. Statistical Shape Analysis; Wiley: Chichester, UK, 1998; Volume 4. [Google Scholar]
- Klingenberg, C.P.; McIntyre, G.S. Geometric morphometrics of developmental instability: Analyzing patterns of fluctuating asymmetry with procrustes methods. Evolution 1998, 52, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Fruciano, C. Measurment error in geometric morphometrics. Dev. Genes Evol. 2016, 226, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Monteiro, L.R. Multivariate regression models and geometric morphometrics: The search for causal factors in the analysis of shape. Syst. Biol. 1999, 48, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Polak, M. Developmental Instability: Causes and Consequences; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Cox, R.M.; Calsbeek, R. Sexually Antagonistic Selection, Sexual Dimorphism, and the Resolution of Intralocus Sexual Conflict. Am. Nat. 2009, 173, 176–187. [Google Scholar] [CrossRef] [Green Version]
- Cox, R.M.; Calsbeek, R. Sex-specific selection and intraspecific variation in sexual size dimorphism. Evol. Int. J. Org. Evol. 2010, 64, 798–809. [Google Scholar] [CrossRef]
- Holloway, B.A. Elytral surface structures as indicators of relationships in stag beetles, with special reference to the New Zealand species (Coleoptera: Lucanidae). N. Z. J. Zool. 1997, 24, 47–64. [Google Scholar] [CrossRef]
- Faccoli, M. Morphological separation of Tomicus piniperda and T. destruens (Coleoptera: Curculionidae: Scolytinae): New and old characters. Eur. J. Entomol. 2006, 103, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Kirejtshuk, A.G.; Poschmann, M.; Prokop, J.; Garrouste, R.; Nel, A. Evolution of the elytral venation and structural adaptations in the oldest Palaeozoic beetles (Insecta: Coleoptera: Tshekardocoleidae). J. Syst. Palaeontol. 2014, 12, 575–600. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Multivariate allometry. In Advances in Morphometrics; Marcus, L.F., Marco, C., Loy, A., Naylor, G.J.P., Slice, D.E., Eds.; Plenum Press: New York, NY, USA, 1996; pp. 23–49. [Google Scholar]
- Klingenberg, C.P.; Marugan-Lobon, J. Evolutionary Covariation in Geometric Morphometric Data: Analyzing Integration, Modularity, and Allometry in a Phylogenetic Context. Syst. Biol. 2013, 62, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Abouheif, E.; Fairbairn, D.J. A comparative analysis of allometry for sexual size dimorphism: Assessing Rensch’s rule. Am. Nat. 1997, 149, 540–562. [Google Scholar] [CrossRef]
- Tseng, M.; Rowe, L. Sexual dimorphism and allometry in the giant water strider Gigantometra gigas. Can. J. Zool. 1999, 77, 923–929. [Google Scholar] [CrossRef]
- Guillermo-Ferreira, R.; Novaes, M.; Lecci, L.; Bispo, P.C. Allometry for sexual size dimorphism in stoneflies defies the Rensch’s rule. Neotrop. Entomol. 2014, 43, 172–175. [Google Scholar] [CrossRef]
- Kawano, K. Horn and wing allometry and male dimorphism in giant rhinoceros beetles (Coleoptera: Scarabaeidae) of tropical Asia and America. Ann. Entomol. Soc. Am. 1995, 88, 92–99. [Google Scholar] [CrossRef]
- Kelly, C.D. Allometry and sexual selection of male weaponry in Wellington tree weta, Hemideina crassidens. Behav. Ecol. 2005, 16, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Sukhodolskaya, R. Variation in body size and body shape in ground beetle Pterostichus melanarius Ill. (Coleoptera, Carabidae). J. Agric. Food Appl. Sci. 2014, 2, 196–205. [Google Scholar]
- Sukhodolskaya, R. Intra-specific body size variation of ground beetles (Coleoptera: Carabidae) in latitudinal gradient. Period. Biol. 2016, 118, 273–280. [Google Scholar] [CrossRef]
- Vesović, N.; Ivanović, A.; Ćurčić, S. Sexual size and shape dimorphism in two ground beetle taxa, Carabus (Procrustes) coriaceus cerisyi and C. (Morphocarabus) kollari praecellens (Coleoptera: Carabidae)-A geometric morphometric approach. Arthropod Struct. Dev. 2019, 49, 1–9. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Hsu, Y.; Lin, C.-P. Allometry and fighting behaviour of a dimorphic stag beetle Cyclommatus mniszechi (Coleoptera: Lucanidae). Insects 2020, 11, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinoza-Donoso, S.; Angulo-Bedoya, M.; Lemic, D.; Benítez, H.A. Assessing the influence of allometry on sexual and non-sexual traits: An example in Cicindelidia trifasciata (Coleoptera: Cicindelinae) using geometric morphometrics. Zool. Anz. 2020. [Google Scholar] [CrossRef]
- Vilaseca, C.; Méndez, M.A.; Pinto, C.F.; Benítez, H.A. Assessment of Shape Variation Patterns in Triatoma infestans (Klug 1834)(Hemiptera: Reduviidae: Triatominae): A First Report in Populations from Bolivia. Insects 2020, 11, 274. [Google Scholar] [CrossRef] [PubMed]



| Centroid size | Elytra | ||||
| Effect | SS | MS | df | F | p(param) |
| Individual | 28.834975 | 1.517630 | 19 | 553 | <0.0001 |
| Error 1 | 0.054883 | 0.002744 | 20 | ||
| Shape | |||||
| Effect | SS | MS | df | F | p(param.) |
| Individual | 0.01459804 | 0.0000225976 | 646 | 35.84 | <0.0001 |
| Error 1 | 0.00042873 | 0.0000006305 | 680 | ||
| Centroid size | Ventral View | ||||
| Effect | SS | MS | df | F | p(param) |
| Individual | 47.130744 | 2.772397 | 17 | 20.45 | <0.0001 |
| Error 1 | 2.440455 | 0.135581 | 18 | ||
| Shape | SS | MS | df | F | p(param.) |
| Individual | 0.03155336 | 0.0000580025 | 544 | 34.30 | <0.0001 |
| Error 1 | 0.00097397 | 0.0000016909 | 576 | ||
| Elytral View Mahalanobis Distance | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex/Species | F/Ce | F/Cg | F/Pm | F/Pn | F/Po | M/Ce | M/Cg | M/Pm | M/Pn |
| F/Cg | 5.2613 | ||||||||
| p-value | <0.0001 | ||||||||
| F/Pm | 18.8655 | 16.2569 | |||||||
| p-value | <0.0001 | <0.0001 | |||||||
| F/Pn | 17.953 | 15.4154 | 5.7224 | ||||||
| p-value | <0.0001 | <0.0001 | 00.0001 | ||||||
| F/Po | 18.7788 | 17.0917 | 11.8677 | 12.4862 | |||||
| p-value | <0.0001 | <0.0001 | <0.0001 | 00.0001 | |||||
| M/Ce | 7.6355 | 4.5361 | 14.2137 | 12.9436 | 16.0726 | ||||
| p-value | <0.0001 | 00.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| M/Cg | 9.1398 | 5.348 | 15.1158 | 14.1775 | 17.6648 | 4.9439 | |||
| p-value | <0.0001 | 00.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0002 | |||
| M/Pm | 18.9741 | 16.2627 | 4.0372 | 6.3808 | 12.1244 | 13.9327 | 14.6692 | ||
| p-value | <0.0001 | <0.0001 | 00.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| M/Pn | 18.474 | 15.8697 | 4.3609 | 4.1095 | 10.5852 | 13.5252 | 14.6722 | 4.5779 | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| M/Po | 20.3573 | 18.5016 | 11.0318 | 11.8492 | 4.1636 | 17.0685 | 18.5699 | 11.0096 | 9.5144 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Ventral View Mahalanobis’ Distance | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex/Species | F/Ce | F/Cg | F/Pm | F/Pn | F/Po | M/Ce | M/Cg | M/Pm | M/Pn |
| F/Cg | 12.632 | ||||||||
| p-value | 0.0001 | ||||||||
| F/Pm | 16.19 | 16.2206 | |||||||
| p-value | 0.0004 | <0.0001 | |||||||
| F/Pn | 17.0903 | 16.4136 | 7.5436 | ||||||
| p-value | 0.0006 | <0.0001 | <0.0001 | ||||||
| F/Po | 15.2336 | 13.855 | 10.1876 | 14.1468 | |||||
| p-value | 0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| M/Ce | 6.1634 | 14.2034 | 14.9344 | 15.6364 | 15.2469 | ||||
| p-value | 0.0009 | 0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| M/Cg | 12.5781 | 8.0316 | 12.5788 | 11.8953 | 11.6517 | 11.9983 | |||
| p-value | 0.0007 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0001 | |||
| M/Pm | 17.3343 | 17.167 | 3.7356 | 6.6073 | 10.6839 | 15.9838 | 12.9631 | ||
| p-value | 0.0002 | <0.0001 | 0.0004 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| M/Pn | 17.7948 | 15.3642 | 7.5476 | 6.4857 | 11.7805 | 16.3224 | 11.0813 | 5.9446 | |
| p-value | 0.0002 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| M/Po | 15.6674 | 15.7041 | 10.0926 | 13.7685 | 5.2142 | 14.7927 | 11.6578 | 10.3142 | 11.4985 |
| p-value | 0.0003 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0001 | <0.0001 | <0.0001 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez, H.A.; Sukhodolskaya, R.A.; Órdenes-Clavería, R.; Avtaeva, T.A.; Kushalieva, S.A.; Saveliev, A.A. Measuring the Inter and Intraspecific Sexual Shape Dimorphism and Body Shape Variation in Generalist Ground Beetles in Russia. Insects 2020, 11, 361. https://doi.org/10.3390/insects11060361
Benítez HA, Sukhodolskaya RA, Órdenes-Clavería R, Avtaeva TA, Kushalieva SA, Saveliev AA. Measuring the Inter and Intraspecific Sexual Shape Dimorphism and Body Shape Variation in Generalist Ground Beetles in Russia. Insects. 2020; 11(6):361. https://doi.org/10.3390/insects11060361
Chicago/Turabian StyleBenítez, Hugo A., Raisa A. Sukhodolskaya, Rodrigo Órdenes-Clavería, Tamara A. Avtaeva, Shapaat A. Kushalieva, and Anatoly A. Saveliev. 2020. "Measuring the Inter and Intraspecific Sexual Shape Dimorphism and Body Shape Variation in Generalist Ground Beetles in Russia" Insects 11, no. 6: 361. https://doi.org/10.3390/insects11060361





