Modified Atmosphere Does Not Reduce the Efficacy of Phytosanitary Irradiation Doses Recommended for Tephritid Fruit Flies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tephritids
2.2. Fruit Infestation, Incubation, and Maintenance
2.3. Low Oxygen Treatments
2.4. Radiation Treatments
2.5. Post-Treatment Evaluations
2.6. Statistics
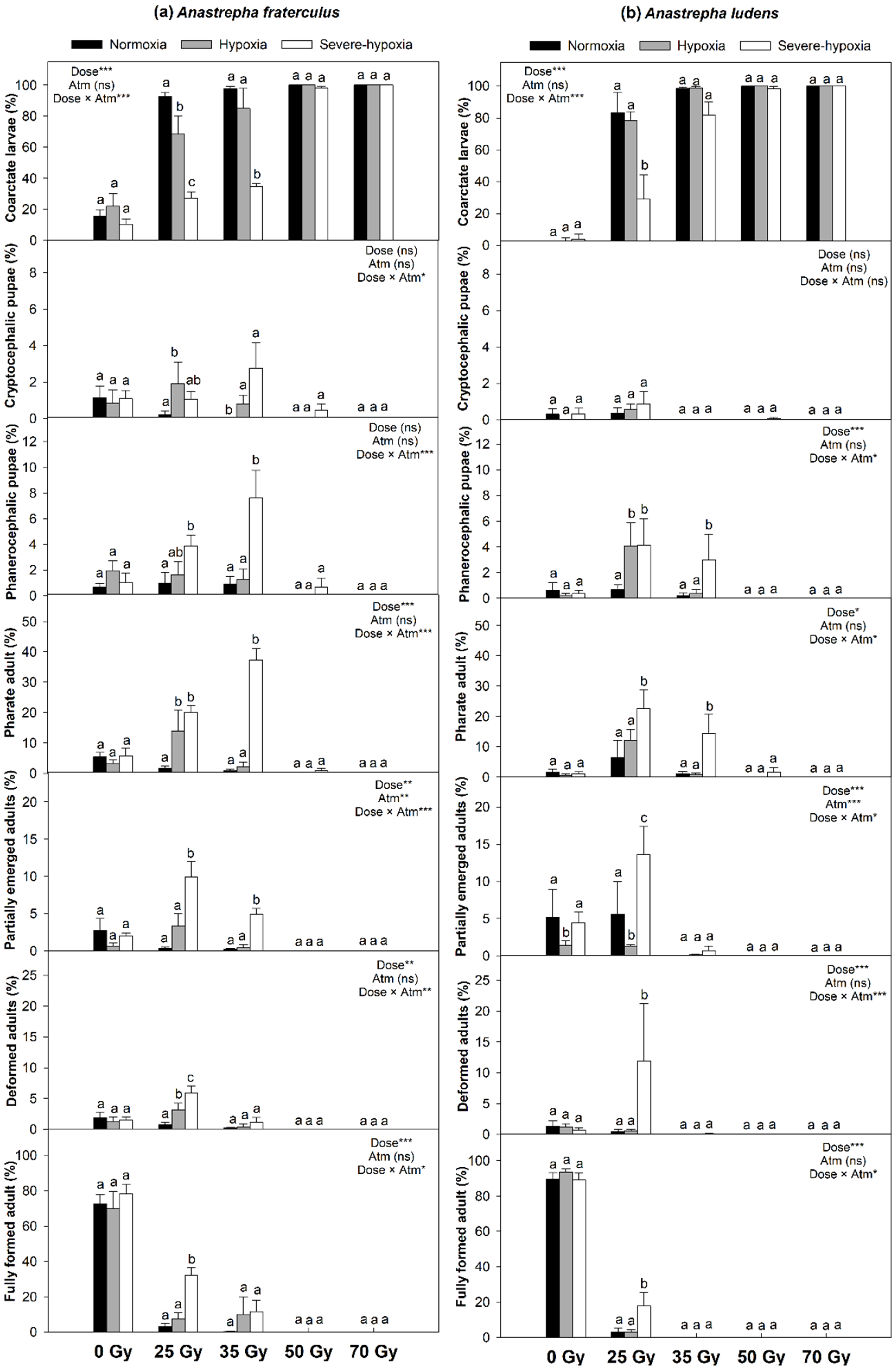
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hallman, G.J.; Hénon, Y.M.; Parker, A.G.; Blackburn, C.M. Phytosanitary irradiation: An overview. Fla. Entomol. 2016, 99, 1–13. [Google Scholar]
- Hallman, G.J.; Levang-Brilz, M.; Zettler, J.L.; Winborne, I.C. Factors affecting ionizing radiation phytosanitary treatments, and implications for research and generic treatments. J. Econ. Entomol. 2010, 103, 1950–1963. [Google Scholar] [CrossRef]
- Follett, P. Phytosanitary irradiation for fresh horticultural commodities: Generic treatments, current issues, and next steps. Stewart Postharvest Rev. 2014, 10, 1–7. [Google Scholar]
- Yahia, E.M. Introduction. In Modified and Controlled Atmospheres for the Storage, Transportation, and Packaging of Horticultural Commodities; Yahia, E.M., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009; pp. 1–16. [Google Scholar]
- Kader, A.A. Postharvest technology of horticultural crops—An overview from farm to fork. Ethiop. J. Appl. Sci. Technol. 2013, 1, 1–8. [Google Scholar]
- IPPC ISPM 18. Guidelines for the Use of Irradiation as a Phytosanitary Measure; FAO: Rome, Italy, 2003. [Google Scholar]
- USDA Treatment Manual. Available online: https://www.aphis.usda.gov/import_export/plants/manuals/ports/downloads/treatment.pdf (accessed on 1 May 2020).
- IPPC ISPM 28. Annex 1 PT 1: Irradiation Treatment for Anastrepha Ludens; FAO: Rome, Italy, 2009. [Google Scholar]
- IPPC ISPM 28. Annex 2 PT 2: Irradiation Treatment for Anastrepha Obliqua; FAO: Rome, Italy, 2009. [Google Scholar]
- IPPC ISPM 28. Annex 3 PT 3: Irradiation Treatment for Anastrepha Serpentina; FAO: Rome, Italy, 2009. [Google Scholar]
- IPPC ISPM 28. Annex 4 PT 4: Irradiation Treatment for Bactrocera Jarvisi; FAO: Rome, Italy, 2009. [Google Scholar]
- IPPC ISPM 28. Annex 5 PT 5: Irradiation Treatment for Bactrocera Tryoni; FAO: Rome, Italy, 2009. [Google Scholar]
- IPPC ISPM 28. Annex 14 PT 14: Irradiation Treatment for Ceratitis Capitata; FAO: Rome, Italy, 2011. [Google Scholar]
- IPPC ISPM 28. Annex 8 PT 8: Irradiation Treatment for Rhagoletis Pomonella; FAO: Rome, Italy, 2009. [Google Scholar]
- IPPC ISPM 28. Annex 11 PT 11: Irradiation Treatment for Grapholita Molesta under Hypoxia; FAO: Rome, Italy, 2010. [Google Scholar]
- Hutchinson, F. Molecular basis for action of ionizing radiations. Science 1961, 134, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.A. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. 1994, 65, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cheng, W.; Meng, J.; Speakmon, M.; Qiu, J.; Pillai, S.; Zhu-Salzman, K. Hypoxic environment protects cowpea bruchid (Callosobruchus maculatus) fromelectron beam irradiation damage. Pest Manag. Sci. 2019, 75, 726–735. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, G.; Hahn, D.A. Short-term anoxic conditioning hormesis boosts antioxidant defenses, lowers oxidative damage following irradiation and enhances male sexual performance in the Caribbean fruit fly, Anastrepha suspensa. J. Exp. Biol. 2012, 215, 2150–2161. [Google Scholar] [CrossRef] [Green Version]
- Hermes-Lima, M.; Moreira, D.C.; Rivera-Ingraham, G.A.; Giraud-Billoud, M.; Genaro-Mattos, T.C.; Campos, É.G. Preparation for oxidative stress under hypoxia and metabolic depression: Revisiting the proposal two decades later. Free Radic. Biol. Med. 2015, 89, 1122–1143. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Geihs, M.A.; França, T.F.A.; Moreira, D.C.; Hermes-Lima, M. Is “Preparation for Oxidative Stress” a Case of Physiological Conditioning Hormesis? Front. Physiol. 2018, 9, 945. [Google Scholar] [CrossRef] [Green Version]
- Hallman, G.J. Irradiation disinfestation of apple maggot (Diptera: Tephritidae) in hypoxic and low-temperature storage. J. Econ. Entomol. 2004, 97, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Hallman, G.J. Ionizing irradiation quarantine treatment against oriental fruit moth (Lepidoptera: Tortricidae) in ambient and hypoxic atmospheres. J. Econ. Entomol. 2004, 97, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Hallman, G.J. Hypoxia reduces reproductive susceptibility of plum curculio (Coleoptera: Curculionidae) to ionizing radiation. Fla. Entomol. 2005, 88, 208–210. [Google Scholar] [CrossRef]
- Follett, P.A.; Wall, M.; Bailey, W. Influence of modified atmosphere packaging on radiation tolerance in the phytosanitary pest melon fly (Diptera: Tephritidae). J. Econ. Entomol. 2013, 106, 2020–2026. [Google Scholar] [CrossRef] [Green Version]
- Follett, P.A.; Swedman, A.; Mackey, B. Effect of low-oxygen conditions created by modified atmosphere packaging on radiation tolerance in Drosophila suzukii (Diptera: Drosophilidae) in sweet cherries. J. Econ. Entomol. 2018, 111, 141–145. [Google Scholar] [CrossRef]
- López-Martínez, G.; Meagher, R.L.; Jeffers, L.A.; Bailey, W.D.; Hahn, D.A. Low oxygen atmosphere enhances post-irradiation survival of Trichoplusia ni (Lepidoptera: Noctuidae). Fla. Entomol. 2016, 99, 24–33. [Google Scholar]
- Condon, C.H.; White, S.; Meagher, R.L.; Jeffers, L.A.; Bailey, W.D.; Hahn, D.A. Effects of low-oxygen environments on the radiation tolerance of the cabbage looper moth (Lepidoptera: Noctuidae). J. Econ. Entomol. 2017, 110, 80–86. [Google Scholar] [CrossRef]
- Srimartpirom, M.; Burikam, I.; Limohpasmanee, W.; Kongratarporn, T.; Thannarin, T.; Bunsiri, A.; Follett, P.A. Low-dose irradiation with modified atmosphere packaging for mango against the oriental fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 2018, 111, 135–140. [Google Scholar] [CrossRef]
- Chen, C.; Condon, C.H.; Boardman, L.; Meagher, R.L.; Jeffers, L.A.; Beam, A.; Bailey, W.D.; Hahn, D.A. Critical PO2 as a diagnostic biomarker for the effects of low-oxygen modified and controlled atmospheres on phytosanitary irradiation treatments in the cabbage looper Trichoplusia ni (Hübner). Pest Manag. Sci. 2020, 76, 5768. [Google Scholar] [CrossRef]
- Tanaka, N.; Okamoto, R.; Chambers, D.L. Methods of Mass Rearing the Mediterranean Fruit Fly Currently Used by the U.S. Department of Agriculture; IAEA: Vienna, Austria, 1970; pp. 19–23. [Google Scholar]
- Rempoulakis, P.; Afshar, N.; Osorio, B.; Barajas-Aceves, M.; Szular, J.; Ahmad, S.; Dammalage, T.; Tomas, U.S.; Nemny-Lavy, E.; Salomon, M.; et al. Conserved metallomics in two insect families evolving separately for a hundred million years. BioMetals 2014, 27, 1323–1335. [Google Scholar] [CrossRef] [Green Version]
- Sobrinho, R.B.; Caceres, C.; Islam, A.; Wornoayporn, V.; Enkerlin, W.R. Diets based on soybean protein for Mediterranean fruit fly. Pesqui. Agropecuária Bras. 2006, 41, 705–708. [Google Scholar] [CrossRef]
- Rennie, T.J.; Sunjka, P.S. Modified atmosphere for storage, transportation, and packaging. In Novel Postharvest Treatments of Fresh Produce; Pareek, S., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 433–480. [Google Scholar]
- Fraenkel, G.; Bhaskaran, G. Pupariation and pupation in cyclorrhaphous flies (Diptera): Terminology and interpretation. Ann. Entomol. Soc. Am. 1973, 66, 418–422. [Google Scholar] [CrossRef]
- Thomas, D.B.; Hallman, G.J. Developmental arrest in Mexican fruit fly (Diptera: Tephritidae) irradiated in Grapefruit. Ann. Entomol. Soc. Am. 2011, 104, 1367–1372. [Google Scholar] [CrossRef]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 65–70. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer Science & Business Media: New York, NY, USA, 2002. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kosmidis, I.; Pagui, E.C.K.; Sartori, N. Mean and median bias reduction in generalized linear models. Stat. Comput. 2020, 30, 43–59. [Google Scholar] [CrossRef] [Green Version]
- Bolker, B.; R Development Core Team. bbmle: Tools for General Maximum Likelihood Estimation. R Package Version 1.0.23.1. Available online: https://cran.r-project.org/web/packages/bbmle/bbmle.pdf (accessed on 15 May 2020).
- Lenth, R. emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.4.6. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 15 May 2020).
- Follett, P.A.; Neven, L.G. Phytosanitary irradiation: Does modified atmosphere packaging or controlled atmosphere storage creating a low oxygen environment threaten treatment efficacy? Radiat. Phys. Chem. 2020, 173, 108874. [Google Scholar] [CrossRef]
- Wang, L.; Cui, S.; Ma, L.; Kong, L.; Geng, X. Current advances in the novel functions of hypoxia-inducible factor and prolyl hydroxylase in invertebrates: Hypoxia-inducible factor and prolyl hydroxylase. Insect Mol. Boil. 2015, 24, 634–648. [Google Scholar] [CrossRef]
- Harrison, J.F.; Greenlee, K.J.; Verberk, W.C.E.P. Functional Hypoxia in Insects: Definition, Assessment, and Consequences for Physiology, Ecology, and Evolution. Annu. Rev. Entomol. 2018, 63, 303–325. [Google Scholar] [CrossRef]
- López-Barneo, J.; Pardal, R.; Ortega-Sáenz, P. Cellular mechanism of oxygen sensing. Annu. Rev. Physiol. 2001, 63, 259–287. [Google Scholar] [CrossRef]
- Graham, A.M.; Barreto, F.S. Loss of the HIF pathway in a widely distributed intertidal crustacean, the copepod Tigriopus californicus. Proc. Natl. Acad. Sci. USA 2019, 116, 12913–12918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, N.A.; Turnbull, D.W.; Johnson, E.A. Induction of the heat shock pathway during hypoxia requires regulation of heat shock factor by hypoxia-inducible factor-1. J. Biol. Chem. 2006, 281, 38675–38681. [Google Scholar] [CrossRef] [Green Version]
- Centanin, L.; Gorr, T.A.; Wappner, P. Tracheal remodelling in response to hypoxia. J. Insect Physiol. 2010, 56, 447–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, D.B. Behavioral responses to hypoxia and hyperoxia in Drosophila larvae: Molecular and neuronal sensors. Fly 2011, 5, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, J.F.; Haddad, G.G. Effects of oxygen on growth and size: Synthesis of molecular, organismal, and evolutionary studies with Drosophila melanogaster. Annu. Rev. Physiol. 2011, 73, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Hoback, W.W. Ecological and experimental exposure of insects to anoxia reveals surprising tolerance. In Anoxia: Evidence for Eukaryote Survival and Paleontological Strategies; Altenbach, A.V., Bernhard, J.M., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 167–188. [Google Scholar]
- Cao, Y.; Xu, K.; Zhu, X.; Bai, Y.; Yang, W.; Li, C. Role of modified atmosphere in pest control and mechanism of its effect on insects. Front. Physiol. 2019, 10, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Hu, F.; Ren, L.; Gao, X.; Wang, Y. Effects of anoxia on survival and gene expression in Bactrocera dorsalis. J. Insect Physiol. 2018, 107, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.B.; Lopez-Martinez, G. Role of conventional and unconventional stress proteins during the response of insects to traumatic environmental conditions. In Hemolymph Proteins and Functional Peptides: Recent Advances in Insects and Other Arthropods; Tufail, M., Takeda, M., Eds.; Bentham Science: Oak Park, IL, USA, 2012; pp. 128–160. [Google Scholar]
- Gao, X.-M.; Jia, F.-X.; Shen, G.-M.; Jiang, H.-Q.; Dou, W.; Wang, J.-J. Involvement of superoxide dismutase in oxidative stress in the oriental fruit fly, Bactrocera dorsalis: Molecular cloning and expression profiles. Pest Manag. Sci. 2013, 69, 1315–1325. [Google Scholar] [CrossRef]
- López-Martínez, G.; Carpenter, J.E.; Hight, S.D.; Hahn, D.A. Low-oxygen atmospheric treatment improves the performance of irradiation-sterilized male cactus moths used in SIT. J. Econ. Entomol. 2014, 107, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Balock, J.W.; Burditt, A.K.; Christenson, L.D. Effects of gamma radiation on various stages of three fruit fly species. J. Econ. Entomol. 1963, 56, 42–46. [Google Scholar] [CrossRef]
- Hallman, G.J.; Nisperos-Carriedo, M.O.; Baldwin, E.A.; Campbell, C.A. Mortality of Caribbean fruit fly (Diptera: Tephritidae) immatures in coated fruits. J. Econ. Entomol. 1994, 87, 752–757. [Google Scholar] [CrossRef]
- Neven, L.G.; Yahia, E.M.; Hallman, G.J. Effects on insects. In Modified and Controlled Atmospheres for the Storage, Transportation, and Packaging of Horticultural Commodities; Yahia, E.M., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009; pp. 233–316. [Google Scholar]

| Dose (Gy) | Atmospheric Conditions 1 | n | No. Larvae Per Fruit | Total No. Larvae Treated | Total No. Insects Dead | Adult Emergence (%) 2 |
|---|---|---|---|---|---|---|
| 0 | Normoxia | 36 | 180 ± 33 | 8976 | 2051 | 75.8 ± 2.4 AB |
| Hypoxia | 33 | 222 ± 36 | 7318 | 1078 | 80.5 ± 2.3 A | |
| Severe hypoxia | 40 | 162 ± 26 | 6475 | 1243 | 70.6 ± 3.7 B | |
| 25 | Normoxia | 30 | 210 ± 29 | 6287 | 6141 | 2.2 ± 0.5 A |
| Hypoxia | 32 | 268 ± 37 | 8578 | 8187 | 6.0 ± 1.3 A | |
| Severe hypoxia | 27 | 265 ± 50 | 7145 | 4771 | 38.7 ± 4.3 B | |
| 35 | Normoxia | 12 | 207 ± 47 | 2483 | 2476 | 0.2 ± 0.3 A |
| Hypoxia | 10 | 327 ± 94 | 3275 | 3274 | 0.3 ± 0.0 A | |
| Severe hypoxia | 12 | 225 ± 78 | 2701 | 2625 | 5.5 ± 3.0 A | |
| 50 | Normoxia | 17 | 131 ± 24 | 2224 | 2224 | 0.0 ± 0.0 A |
| Hypoxia | 11 | 224 ± 66 | 2462 | 2462 | 0.0 ± 0.0 A | |
| Severe hypoxia | 17 | 136 ± 27 | 2318 | 2318 | 0.0 ± 0.0 A | |
| 70 | Normoxia | 28 | 196 ± 32 | 5501 | 5501 | 0.0 ± 0.0 A |
| Hypoxia | 31 | 191 ± 30 | 5911 | 5911 | 0.0 ± 0.0 A | |
| Severe hypoxia | 32 | 216 ± 39 | 6896 | 6896 | 0.0 ± 0.0 A |
| Dose (Gy) | Atmospheric Conditions 1 | n | No. Larvae Per Fruit | Total No. Larvae Treated | Total No. Insects Dead | Adult Emergence (%) 2 |
|---|---|---|---|---|---|---|
| 0 | Normoxia | 27 | 109 ± 15 | 5985 | 1723 | 68.3 ± 3.1 A |
| Hypoxia | 18 | 209 ± 44 | 3757 | 648 | 81.3 ± 2.6 B | |
| Severe hypoxia | 24 | 161 ± 30 | 3863 | 1247 | 64.7 ± 4.5 A | |
| 25 | Normoxia | 38 | 232 ± 30 | 8797 | 8602 | 2.1 ± 0.5 A |
| Hypoxia | 25 | 222 ± 38 | 5539 | 5261 | 9.3 ± 4.3 A | |
| Severe hypoxia | 26 | 102 ± 23 | 2639 | 1891 | 35.6 ± 5.4 B | |
| 35 | Normoxia | 25 | 164 ± 27 | 4088 | 4082 | 0.1 ± 0.1 A |
| Hypoxia | 12 | 239 ± 65 | 2864 | 2863 | 0.1 ± 0.1 A | |
| Severe hypoxia | 16 | 145 ± 35 | 2315 | 2293 | 1.5 ± 0.9 A | |
| 50 | Normoxia | 23 | 119 ± 30 | 2731 | 2731 | 0.0 ± 0.0 A |
| Hypoxia | 12 | 250 ± 62 | 2996 | 2996 | 0.0 ± 0.0 A | |
| Severe hypoxia | 19 | 170 ± 36 | 3237 | 3233 | 0.1 ± 0.1 A | |
| 70 | Normoxia | 20 | 100 ± 26 | 1990 | 1990 | 0.0 ± 0.0 A |
| Hypoxia | 14 | 176 ± 49 | 2468 | 2468 | 0.0 ± 0.0 A | |
| Severe hypoxia | 19 | 128 ± 38 | 2435 | 2435 | 0.0 ± 0.0 A |
| Dose (Gy) | Atmospheric Conditions 1 | n | No. Larvae Per Fruit | Total No. Larvae Treated | Total No. Insects Dead | Adult Emergence (%) 2 |
|---|---|---|---|---|---|---|
| 0 | Normoxia | 36 | 123 ± 10 | 18,397 | 3057 | 84.7 ± 1.5 A |
| Hypoxia | 24 | 86 ± 11 | 4050 | 844 | 82.4 ± 2.7 A | |
| Severe hypoxia | 47 | 108 ± 10 | 11,168 | 2269 | 81.0 ± 2.2 A | |
| 30 | Normoxia | 8 | 78 ± 17 | 1172 | 1105 | 5.5 ± 1.8 A |
| Hypoxia | 16 | 141 ± 25 | 4523 | 3872 | 14.4 ± 3.6 A | |
| Severe hypoxia | 7 | 101 ± 21 | 1508 | 816 | 45.9 ± 5.6 B | |
| 40 | Normoxia | 41 | 119 ± 17 | 4899 | 4787 | 3.8 ± 1.1 A |
| Hypoxia | 34 | 133 ± 21 | 4523 | 4264 | 12.0 ± 3.2 A | |
| Severe hypoxia | 43 | 89 ± 14 | 3820 | 3143 | 21.8 ± 3.8 B | |
| 80 | Normoxia | 16 | 143 ± 33 | 2289 | 2288 | 0.01 ± 0.01 A |
| Hypoxia | 31 | 116 ± 22 | 3699 | 3699 | 0.0 ± 0.0 A | |
| Severe hypoxia | 14 | 76 ± 23 | 1069 | 1069 | 0.0 ± 0.0 A | |
| 116 | Normoxia | 48 | 70 ± 8 | 6405 | 6405 | 0.0 ± 0.0 A |
| Hypoxia | 32 | 80 ± 12 | 4967 | 4967 | 0.0 ± 0.0 A | |
| Severe hypoxia | 36 | 63 ± 9 | 4511 | 4511 | 0.0 ± 0.0 A | |
| 150 | Normoxia | 33 | 187 ± 31 | 6175 | 6175 | 0.0 ± 0.0 A |
| Hypoxia | 28 | 66 ± 10 | 1852 | 1852 | 0.0 ± 0.0 A | |
| Severe hypoxia | 28 | 141 ± 20 | 3938 | 3938 | 0.0 ± 0.0 A |
| Dose (Gy) | Atmospheric Conditions 1 | n | No. Larvae Per Fruit | Total No. Larvae Treated | Total No. Insects Dead | Adult Emergence (%) 2 |
|---|---|---|---|---|---|---|
| 0 | Normoxia | 21 | 69 ± 7 | 5901 | 1293 | 78.3 ± 2.5 A |
| Hypoxia | 9 | 63 ± 19 | 1004 | 267 | 74.8 ± 3.1 A | |
| Severe hypoxia | 29 | 46 ± 6 | 2462 | 424 | 77.6 ± 3.6 A | |
| 20 | Normoxia | 4 | 76 ± 21 | 529 | 514 | 10.5 ± 5.6 A |
| Hypoxia | 10 | 49 ± 14 | 977 | 796 | 29.1 ± 6.2 B | |
| Severe hypoxia | 10 | 66 ± 18 | 1320 | 591 | 67.3 ± 6.0 C | |
| 30 | Normoxia | 12 | 78 ± 15 | 2590 | 2470 | 3.6 ± 1.6 A |
| Hypoxia | 9 | 32 ± 9 | 543 | 489 | 12.2 ± 6.0 AB | |
| Severe hypoxia | 12 | 44 ± 9 | 1053 | 846 | 19.8 ± 5.2 B | |
| 50 | Normoxia | 9 | 73 ± 36 | 654 | 653 | 0.6 ± 0.6 A |
| Hypoxia | 20 | 62 ± 16 | 1248 | 1247 | 0.2 ± 0.2 A | |
| Severe hypoxia | 20 | 44 ± 11 | 880 | 880 | 0.0 ± 0.0 A | |
| 70 | Normoxia | 6 | 70 ± 18 | 1031 | 1031 | 0.0 ± 0.0 A |
| Hypoxia | 11 | 124 ± 27 | 2727 | 2727 | 0.0 ± 0.0 A | |
| Severe hypoxia | 10 | 86 ± 21 | 1334 | 1334 | 0.0 ± 0.0 A | |
| 100 | Normoxia | 39 | 39 ± 5 | 2969 | 2969 | 0.0 ± 0.0 A |
| Hypoxia | 19 | 59 ± 9 | 2138 | 2138 | 0.0 ± 0.0 A | |
| Severe hypoxia | 33 | 27 ± 4 | 1550 | 1550 | 0.0 ± 0.0 A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, V.S.; Hallman, G.J.; Martínez-Barrera, O.Y.; Hurtado, N.V.; Cardoso, A.A.S.; Parker, A.G.; Caravantes, L.A.; Rivera, C.; Araújo, A.S.; Maxwell, F.; et al. Modified Atmosphere Does Not Reduce the Efficacy of Phytosanitary Irradiation Doses Recommended for Tephritid Fruit Flies. Insects 2020, 11, 371. https://doi.org/10.3390/insects11060371
Dias VS, Hallman GJ, Martínez-Barrera OY, Hurtado NV, Cardoso AAS, Parker AG, Caravantes LA, Rivera C, Araújo AS, Maxwell F, et al. Modified Atmosphere Does Not Reduce the Efficacy of Phytosanitary Irradiation Doses Recommended for Tephritid Fruit Flies. Insects. 2020; 11(6):371. https://doi.org/10.3390/insects11060371
Chicago/Turabian StyleDias, Vanessa S., Guy J. Hallman, Olga Y. Martínez-Barrera, Nick V. Hurtado, Amanda A. S. Cardoso, Andrew G. Parker, Luis A. Caravantes, Camilo Rivera, Alexandre S. Araújo, Florence Maxwell, and et al. 2020. "Modified Atmosphere Does Not Reduce the Efficacy of Phytosanitary Irradiation Doses Recommended for Tephritid Fruit Flies" Insects 11, no. 6: 371. https://doi.org/10.3390/insects11060371
APA StyleDias, V. S., Hallman, G. J., Martínez-Barrera, O. Y., Hurtado, N. V., Cardoso, A. A. S., Parker, A. G., Caravantes, L. A., Rivera, C., Araújo, A. S., Maxwell, F., Cáceres-Barrios, C. E., Vreysen, M. J. B., & Myers, S. W. (2020). Modified Atmosphere Does Not Reduce the Efficacy of Phytosanitary Irradiation Doses Recommended for Tephritid Fruit Flies. Insects, 11(6), 371. https://doi.org/10.3390/insects11060371






