Feeding Preference of Crapemyrtle Bark Scale (Acanthococcus lagerstroemiae) on Different Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Source and Plant Material
2.2. Pomegranate Feeding Trials
2.3. Host Range Confirmation on Seven Genera
2.4. Data Analysis and Statistics
3. Results
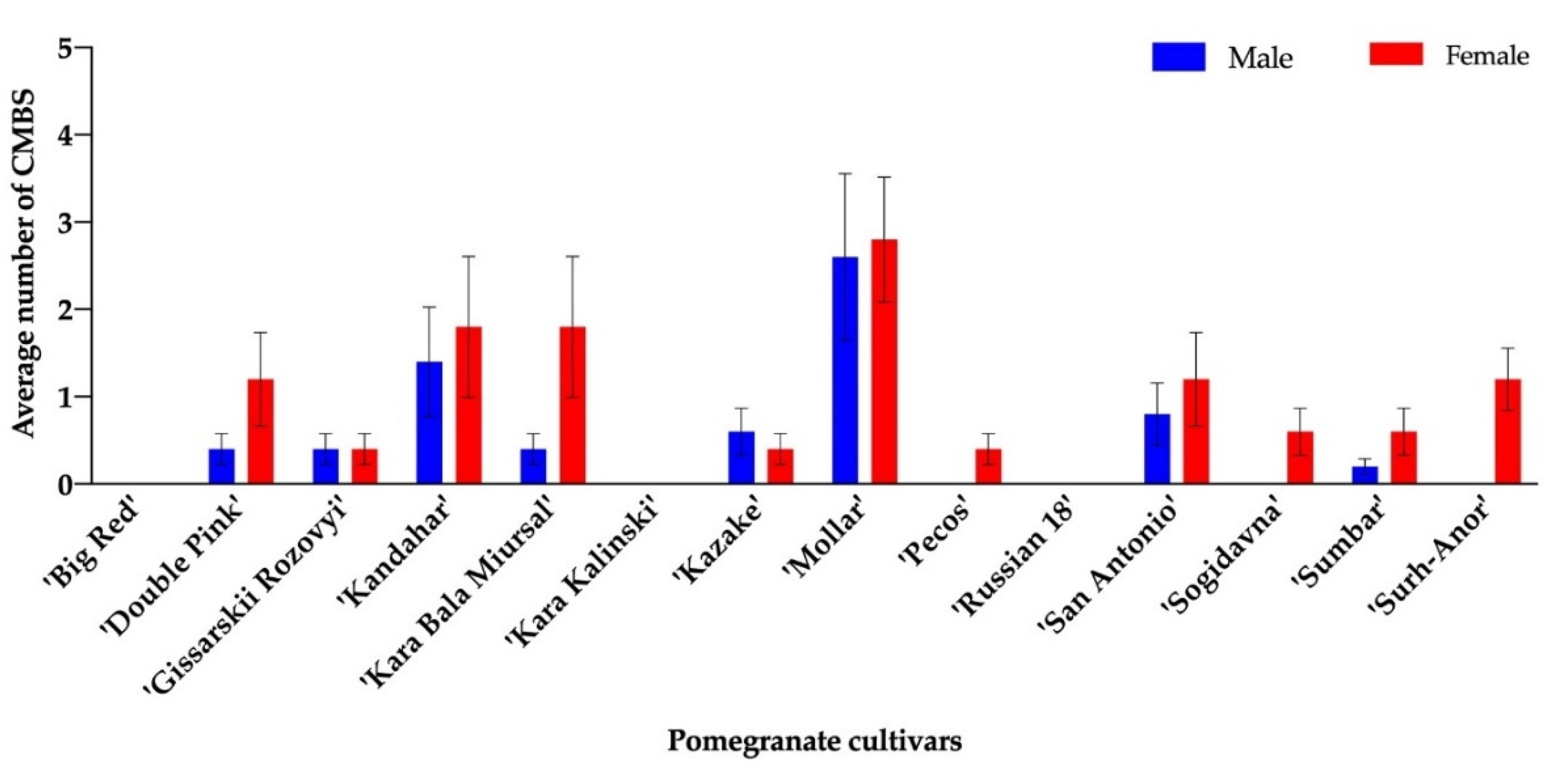
3.1. Pomegranate Feeding Trials
3.2. Host Range Confirmation on Seven Genera
3.3. The Effects of Plant Hosts on Insect Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Egolf, D.R.; Andrick, A.O. The Lagerstroemia Handbook-Checklist: A Guide to Crapemyrtle Cultivars; American Association of Botanical Gardens and Arboreta, Inc.: Chester County, PA, USA, 1978; Volume 2, p. 7. [Google Scholar]
- Gu, M.; Merchant, M.; Robbins, J.; Hopkins, J. Crape Myrtle Bark Scale: A New Exotic Pest. Texas A M AgriLife Ext. 2014. Available online: https://agrilifecdn.tamu.edu/citybugstest/files/2010/05/EHT-049-Crape-myrtle-bark-scale.pdf (accessed on 18 October 2018).
- EDDMapS. Early Detection and Distribution Mapping System. The University of Georgia-Center for Invasive Species and Ecosystem Health. Available online: http://www.eddmaps.org/cmbs/distribution.cfm?map=distribution (accessed on 8 August 2019).
- Wang, Z.; Chen, Y.; Gu, M.; Vafaie, E.; Merchant, M.; Diaz, R. Crapemyrtle Bark Scale: A New Threat for Crapemyrtles, a Popular Landscape Plant in the U.S. Insects 2016, 7, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USDA. Census of Horticultural Specialties (2014); USDA: Washington, DC, USA, 2014.
- Knox, G.W. Crape Myrtle in Florida; University of Florida Cooperative Extension Service: Gainesville, FL, USA, 2000. [Google Scholar]
- Jiang, N.; Xu, H. Observertion on Eriococcus lagerostroemiae Kuwana. J. Anhui Agric. Coll. 1998, 25, 142–144. [Google Scholar]
- He, D.; Cheng, J.; Zhao, H.; Chen, S. Biological characteristic and control efficacy of Eriococcus lagerstroemiae. Chin. Bull. Entomol. 2008, 45, 812–814. [Google Scholar]
- Wang, Z.; Chen, Y.; Knox, G.; Ring, D.; Diaz, R. Crape Myrtle Bark Scale. Available online: http://www.lsuagcenter.com/~/media/system/7/8/d/1/78d165df43ac0d4767607d88dadfb841/pub3440bugbizcrapemyrtlebarkscale_final.pdf (accessed on 16 May 2016).
- Luo, Q.; Xie, X.; Zhou, L.; Wang, S.; Xu, Z. A study on the dynamics and biological characteristics of Eriococcus lagerstroemiae Kuwanae population in Guiyang. Acta Entomol. Sin. 2000, 43, 35–42. [Google Scholar]
- Wang, Z.; Chen, Y.; Diaz, R.; Laine, R.A. Physiology of crapemyrtle bark scale, Acanthococcus lagerstroemiae (Kuwana), associated with seasonally altered cold tolerance. J. Insect Physiol. 2019, 112, 1–8. [Google Scholar] [CrossRef]
- Robbins, J.A.; Hopkins, J.; Merchant, M.; Gu, M. Crapemyrtle Bark Scale: A New Insect Pest; Cooperative Extension Service, University of Arkansas: Little Rock, AR, USA, 2014; Available online: https://www.uaex.edu/publications/PDF/fsa-7086.pdf (accessed on 19 September 2015).
- Chappell, M.R.; Braman, S.K.; Williams-Woodward, J.; Knox, G.J. Optimizing plant health and pest management of Lagerstroemia spp. in commercial production and landscape situations in the southeastern United States: A review. HortScience. 2012, 30, 161–172. [Google Scholar]
- Wang, Z.; Chen, Y.; Diaz, R. Thermal Tolerance and Prediction of Northern Distribution of the Crapemyrtle Bark Scale (Hemiptera: Eriococcidae). Environ. Entomol. 2019, 48, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.-J. Notes on some parasitoids (Hymenoptera: Chalcidoidea) associated with Acanthococcus lagerstroemiae (Kuwana)(Hemiptera: Eriococcidae) in the Republic of Korea. Insecta Mundi 2019, 0690, 1–5. [Google Scholar]
- Liu, S.S.; Meng, X.D. Modelling development time of Myzus persicae (Hemiptera: Aphididae) at constant and natural temperatures. Bull. Entomol. Res. 1999, 89, 53–63. [Google Scholar] [CrossRef]
- Pandey, A.K.; Tripathi, C.J.B. Effect of temperature on the development, fecundity, progeny sex ratio and life-table of Campoletis chlorideae, an endolarval parasitoid of the pod borer, Helicoverpa armigera. BioControl 2008, 53, 461. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Diaz, R. Temperature-dependent development and host range of crapemyrtle bark scale, Acanthococcus lagerstroemiae (Kuwana)(Hemiptera: Eriococcidae). Fla. Entomol. 2019, 102, 181–186. [Google Scholar]
- Brodbeck, B.V.; Andersen, P.C.; Oden, S.; Mizell, R.F.; McKamey, S.H.; Zapata, M. The distribution of Cicadellinae leafhoppers and other Auchenorrhyncha on coffee and citrus in Puerto Rico. Environ. Entomol. 2017, 46, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Yu, X.; Wu, G.; Tao, L.; Chen, J.; Zheng, X. The virulence change and damage characteristics of various geographic populations of brown planthopper. Insect Sci. 1999, 6, 146–154. [Google Scholar] [CrossRef]
- Truzi, C.C.; Vieira, N.F.; de Laurentis, V.L.; Vacari, A.M.; De Bortoli, S.A. Development and feeding behavior of Helicoverpa armigera (Hübner)(Lepidoptera: Noctuidae) on different sunflower genotypes under laboratory conditions. Antrhopod Plant Inetract. 2017, 11, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.K.; Roy, S. Role of host switching in the development of pesticide tolerance in Helopeltis theivora (Hemiptera: Miridae), the major pest of tea in India. In Annales de la Société Entomologique de France (NS); Taylor & Francis: Milton Park, UK, 2017; Volume 53, pp. 428–433. [Google Scholar]
- Morrison, C.R.; Aubert, C.; Windsor, D.M. Variation in Host Plant Usage and Diet Breadth Predict Sibling Preference and Performance in the Neotropical Tortoise Beetle Chelymorpha alternans (Coleoptera: Chrysomelidae: Cassidinae). Environ. Èntomol. 2019, 48, 382–394. [Google Scholar] [CrossRef]
- Castiglione, E.; Manti, F.; Bonsignore, C.P. First record from Calabria (southern Italy) of the “bronze bug” Thaumastocoris peregrinus Carpintero & Dellapé, 2006, alien Eucalyptus pest native to Australia (Hemiptera: Heteroptera: Thaumastocoridae). J. Entomol. Acarol. Res. 2020, 52. [Google Scholar] [CrossRef]
- Nibouche, S.; Mississipi, S.; Fartek, B.; Delatte, H.; Reynaud, B.; Costet, L. Host plant specialization in the sugarcane aphid Melanaphis sacchari. PLoS ONE 2015, 10, e0143704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troncoso, A.J.; Vargas, R.; Tapia, D.; Olivares-Donoso, R.; Niemeyer, H.M. Host selection by the generalist aphid Myzus persicae (Hemiptera: Aphididae) and its subspecies specialized on tobacco, after being reared on the same host. Bull. Entomol. Res. 2005, 95, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaud, J.P. Assessment of cotton as an alternative host plant for the brown citrus aphid, Toxoptera citricida (Homoptera: Aphididae). Fla. Entomol. 2004, 87, 105–112. [Google Scholar] [CrossRef]
- Tapia, D.H.; Silva, A.X.; Ballesteros, G.I.; Figueroa, C.C.; Niemeyer, H.M.; Ramírez, C.C. Differences in learning and memory of host plant features between specialist and generalist phytophagous insects. Anim. Behav. 2015, 106, 1–10. [Google Scholar] [CrossRef]
- Wilkinson, T.L.; Douglas, A.E. Phloem amino acids and the host plant range of the polyphagous aphid, Aphis fabae. Entomol. Exp. Appl. 2003, 106, 103–113. [Google Scholar] [CrossRef]
- Adachi, S.; Matsumoto, Y.; Yoshitomi, H.; Sasaki, D.; Tokuda, M.J. New Distribution Records of Aphis nasturtii (Hemiptera: Aphididae) in Japan. Jpn. J. Syst. Entomol. 2017, 23, 191–193. [Google Scholar]
- Guidolin, A.S. Multipartite Interactions of Aphis (Toxoptera) and Their Associated Symbionts. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2016. [Google Scholar] [CrossRef]
- Miller, G.L.; Favret, C.; Carmichael, A.; Voegtlin, D.J. Is there a cryptic species within Aulacorthum solani (Hemiptera: Aphididae)? J. Econ. Entomol. 2009, 102, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Madjdzadeh, S.M.; Mehrparvar, M.; Abolhasanzadeh, F.J.R. Morphometric discrimination of host-adapted populations of Brachycaudus helichrysi (Kaltenbach)(Hemiptera Aphididae). Redia 2009, 92, 143–145. [Google Scholar]
- Raboudi, F.; Chavigny, P.; Marrakchi, M.; Makni, H.; Makni, M.; Vanlerberghe-Masutti, F. Characterization of polymorphic microsatellite loci in the aphid species Macrosiphum euphorbiae (Hemiptera: Aphididae). Mol. Ecol. Notes 2005, 5, 490–492. [Google Scholar] [CrossRef]
- Sylvester, E.S.; Richardson, J. Consecutive serial passage of strawberry crinkle virus in Myzus ornatus by injection and its occasional transmission to Fragaria vesca. Phytopathology 1986, 76, 1161–1164. [Google Scholar] [CrossRef]
- Francis, F.; Haubruge, E.; Gaspar, C. Influence of host plants on specialist/generalist aphids and on the development of Adalia bipunctata (Coleoptera: Coccinellidae). Eur. J. Entomol. 2000, 97, 481–486. [Google Scholar] [CrossRef] [Green Version]
- Moran, N.A. A 48-million-year-old aphid—Host plant association and complex life cycle: Biogeographic evidence. Science 1989, 245, 173–175. [Google Scholar] [CrossRef]
- Ocimati, W.; Were, E.; Groot, J.C.; Tittonell, P.; Nakato, G.V.; Blomme, G. Risks posed by intercrops and weeds as alternative hosts to Xanthomonas campestris pv. musacearum in banana fields. Front. Plant Sci. 2018, 9, 1471. [Google Scholar] [CrossRef]
- Bawin, T.; Dujeu, D.; De Backer, L.; Francis, F.; Verheggen, F.J. Ability of Tuta absoluta (Lepidoptera: Gelechiidae) to develop on alternative host plant species. Can. Entomol. 2016, 148, 434–442. [Google Scholar] [CrossRef]
- Unruh, T.R.; Luck, R.F. Deme formation in scale insects: A test with the pinyon needle scale and a review of other evidence. Ecol. Entomol. 1987, 12, 439–449. [Google Scholar] [CrossRef]
- Spitzer, B. Local maladaptation in the soft scale insect Saissetia coffeae (Hemiptera: Coccidae). Evolution 2006, 60, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Schultz, P.B.; Szalanski, A.L. Hypericum kalmianum (St. Johnswort) Confirmed as a New Host of the Crapemyrtle Bark Scale in Virginia, USA. J. Agric. Urban Entomol. 2019, 35, 12–15. [Google Scholar] [CrossRef]
- Gregory, E.; USDA Systematic Entomology Laboratory, Beltsville, MD, USA; Gu, M.; Department of Horticultural Sciences, Texas A&M AgriLife Extension Service, College Station, TX, USA. Personal Communication, 2020.
- Ma, J.-H. Occurrence and biological characteristics of Eriococcus lagerostroemiae Kuwana in Panxi district. Biology 2011, 5, 3. [Google Scholar]
- Guo, D.L.; Luo, Z.R. Genetic relationships of the Japanese persimmon Diospyros kaki (Ebenaceae) and related species revealed by SSR analysis. Genet. Mol. Res. 2011, 10, 1060–1068. [Google Scholar] [CrossRef]
- Rauf, A.; Uddin, G.; Patel, S.; Khan, A.; Halim, S.A.; Bawazeer, S.; Ahmad, K.; Muhammad, N.; Mubarak, M.S. Diospyros, an under-utilized, multi-purpose plant genus: A review. Biomed. Pharm. 2017, 91, 714–730. [Google Scholar] [CrossRef]
- Celik, A.; Ercisli, S. Persimmon cv. Hachiya (Diospyros kaki Thunb.) fruit: Some physical, chemical and nutritional properties. Int. J. Food Sci. Nutr. 2008, 59, 599–606. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Noncitrus Fruits and Nuts 2018 Summary; US Department of Agriculture, National Agricultural Statistics Service: Washington, DC, USA, 2019.
- Brown, S. Apple. In Fruit Breeding; Springer: Berlin/Heidelberg, Germany, 2012; pp. 329–367. [Google Scholar]
- United States Department of Agriculture. Census of Agriculture; US Department of Agriculture, National Agricultural Statistics Service: Washington, DC, USA, 2017; Volume 1.
- Holland, D.; Hatib, K.; Bar-Ya’akov, I. Pomegranate: Botany, Horticulture, Breeding. Hortic. Rev. 2009, 35, 127–191. [Google Scholar]
- Iskenderova, Z.D. Double forms of pomegranate and their uses in ornamental horticulture. Byulleten’Glavnogo Bot. Sada 1980, 115, 58–62. [Google Scholar]
- Jalikop, S.H. Pomegranate breeding. Fruit Veg. Cereal Sci. Biothec 2010, 4, 26–34. [Google Scholar]
- USDA. USA National Plant Germplasm System. Available online: https://npgsweb.ars-grin.gov/gringlobal/site.aspx?id=4 (accessed on 23 April 2020).
- Bonsignore, C.P. Apate monachus (Fabricius, 1775), a bostrichid pest of pomegranate and carob trees in nurseries-Short Communication. Plant Prot. Sci. 2012, 48, 94–97. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Dan, J.; Tang, L.; Cao, S. Fruit scientific research in New China in the past 70 years: Pomegranate. J. Fruit Sci. 2019, 36, 1389–1398. [Google Scholar]
- Deng, Q. Control Eriococcus Lagerstroemiae Kuwana for Pomegranate Bonsai. China Flower Penjing 2001, 3, 33. [Google Scholar]
- Stover, E.; Mercure, E.W. The pomegranate: A new look at the fruit of paradise. HortScience 2007, 42, 1088–1092. [Google Scholar] [CrossRef] [Green Version]
- Park, J.D.; Kim, Y.H.; Kim, S.S.; Park, I.S.; Kim, K.C. Seasonal occurrence, host preference and hatching behavior of Eriococcus lagerstroemiae. Korean J. Appl. Entomol. 1993, 32, 83–89. [Google Scholar]
- Hua, L.-Z. List of Chinese Insects; Zhongshan University Press: Guangzhou, China, 2000; Volume I, p. 448. [Google Scholar]
- Kozár, F. Catalogue of Palaearctic Coccoidea; Plant Protection Institute: Budapest, Hungary, 1998. [Google Scholar]
- Castle, B. Cultivar Selection Guide for Florida-Grown Pomegranates: Horticultural Traits. Available online: https://crec.ifas.ufl.edu/extension/pomegranates/pom_guide.html (accessed on 21 May 2020).
- Hanks, L.M.; Millar, J.G.; Paine, T.D. Biological constraints on host-range expansion by the wood-boring beetle Phoracantha semipunctata (Coleoptera: Cerambycidae). Ann. Entomol. Soc. Am. 1995, 88, 183–188. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Zhou, D.-S.; Gao, S.-X.; Zhao, X.-C.; Tang, Q.-B.; Wang, C.-Z.; van Loon, J.J.A. Higher plasticity in feeding preference of a generalist than a specialist: Experiments with two closely related Helicoverpa species. Sci. Rep. 2017, 7, 17876. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Tanga, C.M.; Khamis, F.M.; Rasowo, B.A.; Mohamed, S.A.; Badii, B.K.; Salifu, D.; Sétamou, M.; Ekesi, S.; Borgemeister, C. Host suitability and feeding preference of the African citrus triozid Trioza erytreae Del Guercio (Hemiptera: Triozidae), natural vector of “Candidatus Liberibacter africanus”. J. Appl. Entomol. 2019, 143, 262–270. [Google Scholar] [CrossRef]
- Karowe, D.N. Facultative monophagy as a consequence of prior feeding experience: Behavioral and physiological specialization in Colias philodice larvae. Oecologia 1989, 78, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-J.; Zhang, S.-S.; Niu, B.-L.; Ji, D.-F.; Liu, X.-J.; Li, M.-W.; Bai, H.; Palli, S.R.; Wang, C.-Z.; Tan, A. A determining factor for insect feeding preference in the silkworm, Bombyx mori. PLoS Biol. 2019, 17, e3000162. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, S.; Blanchet, E.; Egas, M.; Olivieri, I.J.B.E.B. Are adaptation costs necessary to build up a local adaptation pattern? BMC Evol. Biol. 2009, 9, 182. [Google Scholar]
- Kellerhals, M. Introduction to apple (Malus × domestica). In Genetics and Genomics of Rosaceae; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2009; pp. 73–84. [Google Scholar]
- Hokanson, S.; Lamboy, W.; Szewc-McFadden, A.; McFerson, J. Microsatellite (SSR) variation in a collection of Malus (apple) species and hybrids. Euphytica 2001, 118, 281–294. [Google Scholar] [CrossRef]
- Wu, B.; Xie, R.; Knox, G.W.; Qin, H.; Gu, M. Crapemyrtle bark scale (Hemiptera: Eriococcidae) showed different acceptance among crapemyrtle species significantly. 2020; unpublished; manuscript in preparation. [Google Scholar]
- Denno, R.; Roderick, G. Influence of patch size, vegetation texture, and host plant architecture on the diversity, abundance, and life history styles of sapfeeding herbivores. In Habitat Structure; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1991; pp. 169–196. [Google Scholar]
- Thompson, J.N. Selection pressures on phytophagous insects feeding on small host plants. Oikos 1983, 438–444. [Google Scholar] [CrossRef]
- Pecot, H.C. Influence of Planting Depth and Mulch on the Growth of Nine Species of Ornamental Plants in Landscape and Container Settings. Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2004. [Google Scholar]
- Hansen, H.A. Lagerstroemia Plant Named ‘Spiced Plum’. U.S. Patent 15/530,535, 16 July 2018. [Google Scholar]
- Thorsteinson, A.J. Host selection in phytophagous insects. Annu. Rev. Entomol. 1960, 5, 193–218. [Google Scholar] [CrossRef]
- Kumar, H.; Bhattacharya, S. Biology of Spodoptera litura (Fabricius) on different crop plants. J. Entomol. Res. 2019, 43, 165–168. [Google Scholar] [CrossRef]
- Mani, C.; Lawrence, L.; Ranjith, M.J.E. Biology and morphometry of Paracoccus marginatus Williams and Granara de Willink (Hemiptera: Pseudococcidae). Entomon 2013, 38, 97–110. [Google Scholar]
- Raja, S.; Gillani, W.; Copland, M.J.B.S.-P. Effect of Different Temperatures and Host Plants on the Biology of the Long-Tailed Mealy Bug Pseudococcus longispinus (Targioni and Tozzetti)(Homoptera: Pseudococcidae). Pak. J. Sci. Ind. Res. Ser. B Biol. Sci. 2011, 54, 142–151. [Google Scholar]
- Chaudhari, V.; Desai, H.; Patel, N. Determination of Economic Threshold Level of Cotton Leafhopper (Amarasca biguttula biguttula Ishida) on Cotton under South Gujarat Condition. Trends Biosci. 2018, 11, 52–57. [Google Scholar]
- Soliman, A.; Hendawy, A.; El-hefny, A.S.; Sherif, M. Determination of Economic Threshold Level for Chilo agamemnon Bles. Infestation In Rice Plants, Based on Simulated Dead Hearts. Egypt. J. Agric. Res. 2016, 94. [Google Scholar]
- McCarville, M.T.; Kanobe, C.; Macintosh, G.C.; O’Neal, M. What is the economic threshold of soybean aphids (Hemiptera: Aphididae) in enemy-free space? J. Econ. Entomol. 2011, 104, 845–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alatawi, F.; Margolies, D.; Nechols, J. Aesthetic damage thresholds for twospotted spider mites (Acari: Tetranychidae) on impatiens: Effect of plant age and level of infestation. J. Econ. Entomol. 2007, 100, 1904–1909. [Google Scholar] [CrossRef] [PubMed]
- Short, B.D.; Janisiewicz, W.; Takeda, F.; Leskey, T.C. UV-C irradiation as a management tool for Tetranychus urticae on strawberries. Pest. Manag. Sci. 2018, 74, 2419–2423. [Google Scholar] [CrossRef] [PubMed]


| 2016 | 2019 | |
|---|---|---|
| ‘Al-Sirin-Nar’ | ‘Kazake’ | ‘Big Red’ |
| ‘Angel Red’ | ‘Larkin’ | ‘Double Pink’ |
| ‘Apseronski Krasnyi’ | ‘Molla Nepes’ | ‘Gissarskii Rozovyi’ |
| ‘Austin’ | ‘Mollar’ | ‘Kandahar’ |
| ‘Azadi’ | ‘Mridula’ | ‘Kara Bala Miursal’ |
| ‘Bala Miursal’ | ‘Russian 18’ | ‘Kara Kalinski’ |
| ‘Christina’ | ‘Salavatski’ | ‘Kazake’ |
| ‘Desertnyi’ | ‘Sirenevyi’ | ‘Mollar’ |
| ‘Elf’ | ‘Sogidavna’ | ‘Pecos’ |
| ‘Entek Habi Saveh’ | ‘Spanish Sweet’ | ‘Russian 18’ |
| ‘Girkanets’ | ‘Sumbar’ | ‘San Antonio’ |
| ‘Gissarskii Rozovyi’ | ‘Surh-Anor’ | ‘Sogidavna’ |
| ‘JD’ | ‘Sweet’ | ‘Sumbar’ |
| ‘Kandahar’ | ‘Vkusanyi’ | ‘Surh-Anor’ |
| ‘Kara Kalinski’ | ‘Wonderful’ | |
| Scientific Name | Common Name | Family | USDA Cold Hardiness Zone |
|---|---|---|---|
| Buxus harlandii | Harland boxwood | Buxaceae | 7–9 |
| Buxus microphylla var. koreana × B. sempervirens ‘Green Gem’ | Boxwood | Buxaceae | 7–9 |
| Chaenomeles speciosa ‘Texas Scarlet’ | Common quince | Rosaceae | 4–8 |
| Diospyrosrhombifolia | Diamond-leaf Persimmon | Ebenaceae | 7–11 |
| Diospyros virginiana | Common persimmon | Ebenaceae | 4–9 |
| Heimia salicifolia | Sinicuichi | Lythraceae | 9–11 |
| Lagerstroemia ‘Spiced Plum’ | Crapemyrtle | Lythraceae | 6–10 |
| Malus angustifolia | Southern crabapple | Rosaceae | 3–8 |
| Malus domestica ‘Fuji’ | Apple | Rosaceae | 3–8 |
| Malus domestica ‘Red Delicious’ | Apple | Rosaceae | 3–8 |
| Rubus ‘Arapaho’ | Blackberry | Rosaceae | 4–9 |
| Rubus ‘Navaho’ | Raspberry | Rosaceae | 6–10 |
| Rubus fruticosus ‘Prime Ark Freedom’ | Blackberry | Rosaceae | 6–9 |
| Rubus idaeus ‘Dorman Red’ | Raspberry | Rosaceae | 5–9 |
| Plant Species | No. Male Pupae | No. Gravid Females | Sex Ratio |
|---|---|---|---|
| Lagerstroemia ‘Spiced Plum’ | 555.7a Z | 166.5a | 3.3:1 |
| Heimia salicifolia | 25.7b | 9.3b | 2.8:1 |
| Malus domestica ‘Red Delicious’ | 4.2b | 1.0b | 4.2:1 |
| M. domestica ‘Fuji’ | 3.7b | 3.7b | 1.0:1 |
| Chaenomeles speciosa | 3.2b | 2.8b | 1.1:1 |
| Plant Species | No. Male Pupae | No. Gravid Females | Sex Ratio |
|---|---|---|---|
| Lagerstroemia ‘Spiced Plum’ | 321.5a Z | 87.7a | 3.7:1 |
| Heimia salicifolia | 12.6b | 5.6b | 2.3:1 |
| Malus domestica ‘Red Delicious’ | 2.4b | 0.3b | 7.2:1 |
| M. domestica ‘Fuji’ | 1.9b | 1.9b | 1.0:1 |
| Chaenomeles speciosa | 1.1b | 1.4b | 0.8:1 |
| M. angustifolia | 0.2b | - | - |
| Diospyrosrhombifolia | 0.03b | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, R.; Wu, B.; Dou, H.; Liu, C.; Knox, G.W.; Qin, H.; Gu, M. Feeding Preference of Crapemyrtle Bark Scale (Acanthococcus lagerstroemiae) on Different Species. Insects 2020, 11, 399. https://doi.org/10.3390/insects11070399
Xie R, Wu B, Dou H, Liu C, Knox GW, Qin H, Gu M. Feeding Preference of Crapemyrtle Bark Scale (Acanthococcus lagerstroemiae) on Different Species. Insects. 2020; 11(7):399. https://doi.org/10.3390/insects11070399
Chicago/Turabian StyleXie, Runshi, Bin Wu, Haijie Dou, Cuiyu Liu, Gary W. Knox, Hongmin Qin, and Mengmeng Gu. 2020. "Feeding Preference of Crapemyrtle Bark Scale (Acanthococcus lagerstroemiae) on Different Species" Insects 11, no. 7: 399. https://doi.org/10.3390/insects11070399
APA StyleXie, R., Wu, B., Dou, H., Liu, C., Knox, G. W., Qin, H., & Gu, M. (2020). Feeding Preference of Crapemyrtle Bark Scale (Acanthococcus lagerstroemiae) on Different Species. Insects, 11(7), 399. https://doi.org/10.3390/insects11070399







