Asymmetric Interaction between Aphis spiraecola and Toxoptera citricida on Sweet Orange Induced by Pre-Infestation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Plants and Aphid Culture
2.2. Aphid Pre-Infestation Procedure
2.3. Aphid Performance Bioassay
2.4. Aphid Feeding Behavior
2.5. Total Amino Acid and Soluble Sugar Concentration
2.6. Gene Relative Expression Detection
2.7. Statistical Analysis
3. Results
3.1. Effects on Life-History Parameters
3.2. Effects on Feeding Behavior via EPG Measurements
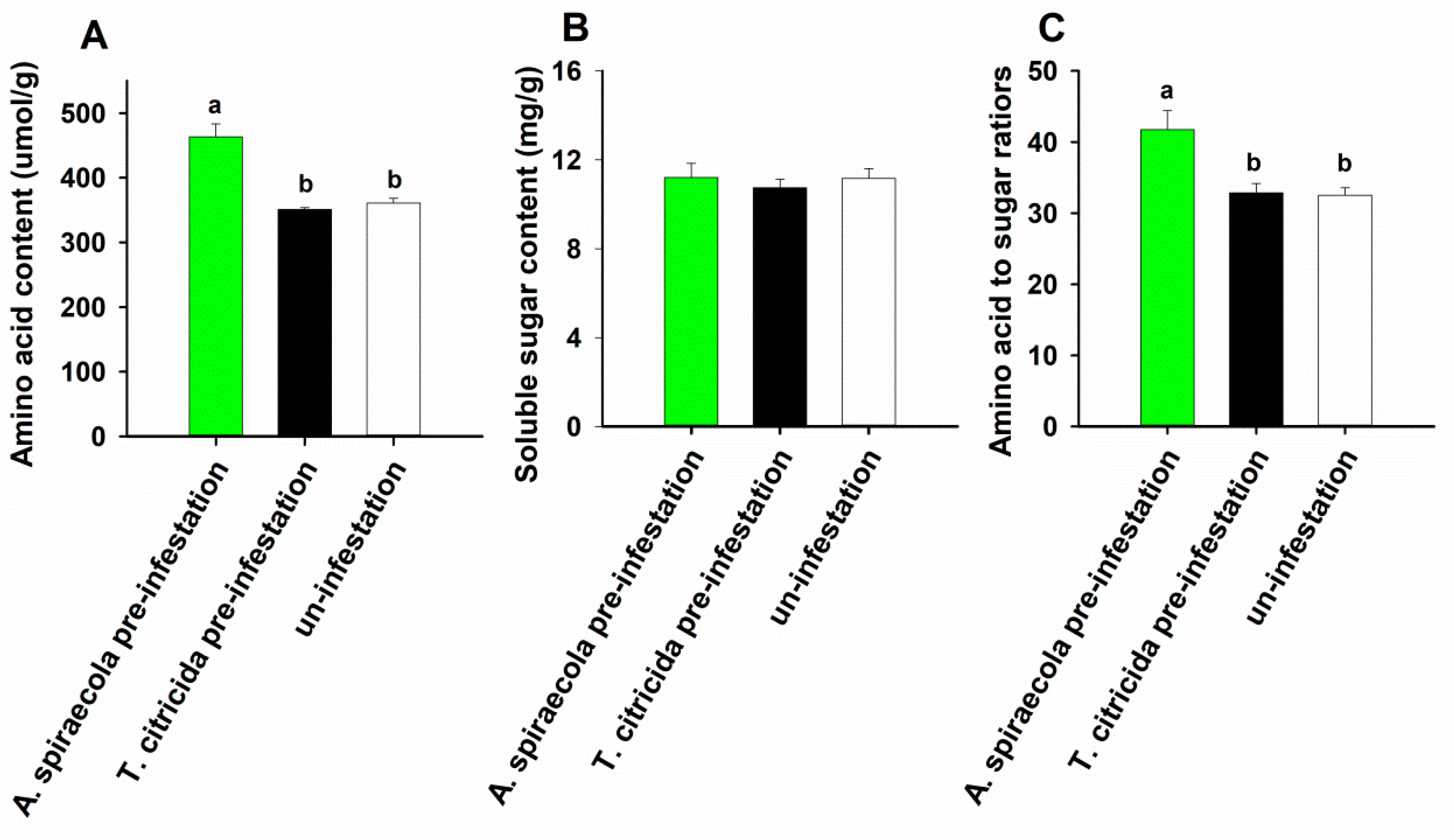
3.3. Amino Acid and Soluble Sugar Concentration
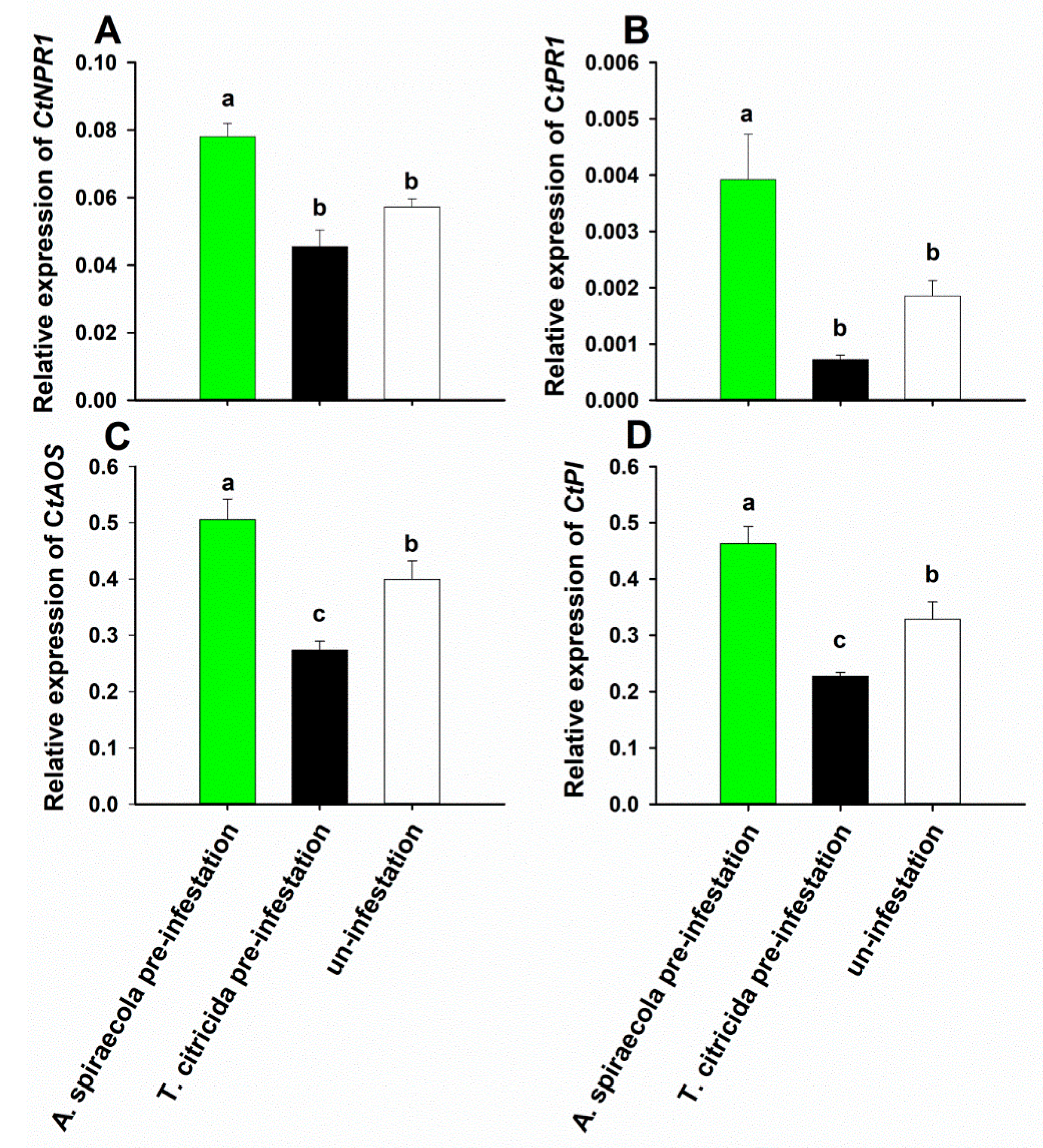
3.4. SA and JA Marker Gene Expression in Sweet Orange
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.; Xue, M.; Zhao, H.P. Species-specific effects on salicylic acid content and subsequent Myzus persicae (Sulzer) performance by three phloem-sucking insects infesting Nicotiana tabacum L. Anthr. Plant Interact. 2015, 9, 383–391. [Google Scholar] [CrossRef]
- Tan, X.L.; Wang, S.; Ridsdill-Smith, J.; Liu, T.X. Direct and Indirect Impacts of Infestation of Tomato Plant by Myzus persicae (Hemiptera: Aphididae) on Bemisia tabaci (Hemiptera: Aleyrodidae). PLoS ONE 2014, 9, e94310. [Google Scholar] [CrossRef]
- Ohgushi, T. Indirect interaction webs: Herbivore-induced effects through trait change in plants. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 81–105. [Google Scholar] [CrossRef] [Green Version]
- Saad, K.A.; Roff, M.M.; Hallett, R.H.; Idris, A. Aphid-induced defences in chilli affect preferences of the whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Sci Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.P.; Zhang, X.Y.; Xue, M.; Zhang, X. Feeding of whitefly on tobacco decreases aphid performance via increased salicylate signaling. PLoS ONE 2015, 10, e0138584. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Suzuki, Y. Direct and feeding-induced interactions between two rice planthoppers, Sogatella furcifera and Nilaparvata lugens: Effects on dispersal capability and performance. Ecol. Entomol. 2003, 28, 174–182. [Google Scholar] [CrossRef]
- Nombela, G.; Garzo, E.; Duque, M.; Muñiz, M. Preinfestations of tomato plants by whiteflies (Bemisia tabaci) or aphids (Macrosiphum euphorbiae) induce variable resistance or susceptibility responses. Bull. Entomol. Res. 2009, 99, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Davidson, L.E.; Szendrei, Z.; Ali, J.G. Asymmetric effects of a leaf-chewing herbivore on aphid population growth. Ecol. Entomol. 2019, 44, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.H.; Liu, H.R.; Zhang, Z.F.; Liu, T.X. The green peach aphid Myzus persicae perform better on pre-infested Chinese cabbage Brassica pekinensis by enhancing host plant nutritional quality. Sci Rep. 2016, 6, 21954. [Google Scholar] [CrossRef] [Green Version]
- McNutt, D.W.; Underwood, N. Variation in plant-mediated intra- and interspecific interactions among insect herbivores: Effects of host genotype. Ecosphere 2016, 7, e01520. [Google Scholar] [CrossRef] [Green Version]
- Inbar, M.; Eshel, A.; Wool, D. Interspecific competition among phloem-feeding insects mediated by induced host-plant sinks. Ecology 1995, 76, 1506–1515. [Google Scholar] [CrossRef]
- Triyogo, A.; Yasuda, H. Effect of host-plant manipulation by a gall-inducing insect on abundance of herbivores on chestnut trees. Appl. Entomol. Zoolog. 2013, 48, 345–353. [Google Scholar] [CrossRef]
- Erb, M. Plant Defenses against Herbivory: Closing the Fitness Gap. Trends Plant Sci. 2018, 23, 187–194. [Google Scholar] [CrossRef]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef]
- Stewart, S.A.; Hodge, S.; Bennett, M.; Mansfield, J.W.; Powell, G. Aphid induction of phytohormones in Medicago truncatula is dependent upon time post-infestation, aphid density and the genotypes of both plant and insect. Anthr. Plant Interact. 2016, 10, 41–53. [Google Scholar] [CrossRef]
- Cui, H.Y.; Sun, Y.C.; Su, J.W.; Li, C.Y.; Ge, F. Reduction in the Fitness of Bemisia tabaci Fed on Three Previously Infested Tomato Genotypes Differing in the Jasmonic Acid Pathway. Environ. Entomol. 2012, 41, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, H.; Uefune, M.; Ozawa, R.; Arimura, G.-I.; Takabayashi, J. Previous infestation of pea aphids Acyrthosiphon pisum on broad bean plants resulted in the increased performance of conspecific nymphs on the plants. J. Plant Interact. 2013, 8, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Gao, J.; Guo, H.J.; Sun, Y.C.; Ge, F. Differential accumulation of leucine and methionine in red and green pea aphids leads to different fecundity in response to nitrogen fertilization. Pest. Manag. Sci. 2018, 74, 1779–1789. [Google Scholar] [CrossRef]
- Talon, M.; Gmitter, F.G. Citrus genomics. Int. J. Plant. Gen. 2008, 2008, 528361. [Google Scholar] [CrossRef]
- Brlansky, R.H.; Damsteegt, V.D.; Howd, D.S.; Roy, A. Molecular analyses of Citrus tristeza virus subisolates separated by aphid transmission. Plant Dis. 2003, 87, 397–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, D.G.; Albrigo, L.G. Estimating the relative abundance of flush shoots in citrus with implications on monitoring insects associated with flush. HortScience 2007, 42, 364–368. [Google Scholar] [CrossRef] [Green Version]
- Powell, C.A.; Burton, M.S.; Pelosi, R.R.; Rundell, P.A.; Ritenour, M.A.; Bullock, R.C. Six-year evaluation of brown citrus and spirea aphid populations in a citrus grove and the effects of insecticides on these populations. HortScience 2006, 41, 688–690. [Google Scholar] [CrossRef]
- Brlansky, R.H.; Roy, A.; Damsteegt, V.D. Stem-pitting Citrus tristeza virus predominantly transmitted by the brown citrus aphid from mixed infections containing non-stem-pitting and stem-pitting isolates. Plant Dis. 2011, 95, 913–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostefaoui, H.; Allal-Benfekih, L.; Djazouli, Z.E.; Petit, D.; Saladin, G. Why the aphid Aphis spiraecola is more abundant on clementine tree than Aphis gossypii? C. R. Biol. 2014, 337, 123–133. [Google Scholar] [CrossRef]
- Elhaddad, A.; ElAmrani, A.; Fereres, A.; Moreno, A. Spatial and temporal spread of Citrus tristeza virus and its aphid vectors in the North western area of Morocco. Insect Sci. 2016, 23, 903–912. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wang, Y.L.; Wang, Q.J.; Cao, M.; Zhou, C.Y.; Zhou, Y. Identification of Aphis spiraecola as a vector of Citrus yellow vein clearing virus. Eur. J. Plant Pathol. 2018, 152, 841–844. [Google Scholar] [CrossRef]
- Hu, X.; Liu, X.; Zhao, H. Development and application of electrical penetration graph (EPG) technique. Plant. Prot. 2006, 32, 1–4. [Google Scholar]
- Gao, J.; Guo, H.J.; Sun, Y.C.; Ge, F. Juvenile hormone mediates the positive effects of nitrogen fertilization on weight and reproduction in pea aphid. Pest. Manag. Sci. 2018, 74, 2511–2519. [Google Scholar] [CrossRef]
- Alvarez, A.E.; Tjallingii, W.F.; Garzo, E.; Vleeshouwers, V.; Dicke, M.; Vosman, B. Location of resistance factors in the leaves of potato and wild tuber-bearing Solanum species to the aphid Myzus persicae. Entomol. Exp. Appl. 2006, 121, 145–157. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Vamvatsikos, P.; Ward, D.; Gravanis, F. Changes in the levels of plant total phenols and free amino acids induced by two cereal aphids and effects on aphid fecundity. J. Appl. Entomol. 2006, 130, 15–19. [Google Scholar] [CrossRef]
- Weibull, J.; Brishammar, S.; Pettersson, J. Amino acid analysis of phloem sap from oats and barley: A combination of aphid stylet excision and high performance liquid chromatography. Entomol. Exp. Appl. 1986, 42, 27–30. [Google Scholar] [CrossRef]
- Nehela, Y.; Hijaz, F.; Elzaawely, A.A.; El-Zahaby, H.M.; Killiny, N. Citrus phytohormonal response to Candidatus Liberibacter asiaticus and its vector Diaphorina citri. Physiol. Mol. Plant Pathol. 2018, 102, 24–35. [Google Scholar] [CrossRef]
- Ibanez, F.; Suh, J.H.; Wang, Y.; Stelinski, L.L. Long-term, sustained feeding by Asian citrus psyllid disrupts salicylic acid homeostasis in sweet orange. BMC Plant Biol. 2019, 19, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Wang, C.X.; Bi, M.J.; Li, Q.L.; Liu, T.X. Induced Defense by Bemisia tabaci Biotype B (Hemiptera: Aleyrodidae) in Tobacco Against Myzus persicae (Hemiptera: Aphididae). Environ. Entomol. 2010, 39, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Inbar, M.; Doostdar, H.; Leibee, G.L.; Mayer, R.T. The role of plant rapidly induced responses in asymmetric interspecific interactions among insect herbivores. J. Chem. Ecol. 1999, 25, 1961–1979. [Google Scholar] [CrossRef]
- Zhang, L.P.; Zhang, G.Y.; Zhang, Y.J.; Zhang, W.J.; Liu, Z. Interspecific interactions between Bemisia tabaci (Hem., Aleyrodidae) and Liriomyza sativae (Dipt., Agromyzidae). J. Appl. Entomol. 2005, 129, 443–446. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Huang, H.; Shan, H.W.; Zhang, F.; Wan, F.H.; Liu, T.X. Defense against Pieris rapae in cabbage plants induced by Bemisia tabaci biotype B. Entomol. Exp. Appl. 2013, 147, 293–300. [Google Scholar] [CrossRef]
- Ramírez, C.C.; Niemeyer, H.M. The influence of previous experience and starvation on aphid feeding behavior. J. Insect Behav. 2000, 13, 699–709. [Google Scholar] [CrossRef]
- Dardeau, F.; Pointeau, S.; Ameline, A.; Laurans, F.; Cherqui, A.; Lieutier, F.; Sallé, A. Host manipulation by a herbivore optimizes its feeding behaviour. Anim. Behav. 2014, 95, 49–56. [Google Scholar] [CrossRef]
- Brunissen, L.; Cherqui, A.; Pelletier, Y.; Vincent, C.; Giordanengo, P. Host-plant mediated interactions between two aphid species. Entomol. Exp. Appl. 2009, 132, 30–38. [Google Scholar] [CrossRef]
- Gonzales, W.L.; Ramirez, C.C.; Olea, N.; Niemeyer, H.M. Host plant changes produced by the aphid Sipha flava: Consequences for aphid feeding behaviour and growth. Entomol. Exp. Appl. 2002, 103, 107–113. [Google Scholar] [CrossRef]
- Braendle, C.; Davis, G.K.; Brisson, J.A.; Stern, D.L. Wing dimorphism in aphids. Heredity 2006, 97, 192–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purandare, S.R.; Tenhumberg, B.; Brisson, J.A. Comparison of the wing polyphenic response of pea aphids (Acyrthosiphon pisum) to crowding and predator cues. Ecol. Entomol. 2014, 39, 263–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoysted, G.A.; Lilley, C.J.; Field, K.J.; Dickinson, M.; Hartley, S.E.; Urwin, P.E. A Plant-Feeding Nematode Indirectly Increases the Fitness of an Aphid. Front. Plant Sci. 2017, 8, 1897. [Google Scholar] [CrossRef] [Green Version]
- Van Dam, N.M.; Wondafrash, V.; Mathur, V.; Tytgat, T.O.G. Differences in Hormonal Signaling Triggered by Two Root-Feeding Nematode Species Result in Contrasting Effects on Aphid Population Growth. Front. Ecol. Evol. 2018, 6, 88. [Google Scholar] [CrossRef] [Green Version]
- Denno, R.F.; Peterson, M.A.; Gratton, C.; Cheng, J.A.; Langellotto, G.A.; Huberty, A.F.; Finke, D.L. Feeding-induced changes in plant quality mediate interspecific competition between sap-feeding herbivores. Ecology 2000, 81, 1814–1827. [Google Scholar] [CrossRef]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf whitefly induces salicylic acid Defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef] [Green Version]
- Koyama, Y.; Yao, I.; Akimoto, S.I. Aphid galls accumulate high concentrations of amino acids: A support for the nutrition hypothesis for gall formation. Entomol. Exp. Appl. 2004, 113, 35–44. [Google Scholar] [CrossRef]
- Castañeda, L.E.; Figueroa, C.C.; Fuentes-Contreras, E.; Niemeyer, H.M.; Nespolo, R.F. Energetic costs of detoxification systems in herbivores feeding on chemically defended host plants: A correlational study in the grain aphid. Sitobion avenae J. Exp. Biol. 2009, 212, 1185–1190. [Google Scholar] [CrossRef] [Green Version]
- Ali, J.G.; Agrawal, A.A. Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci. 2012, 17, 293–302. [Google Scholar] [CrossRef] [PubMed]



| Gene Function | Gene | Primer Sequence (5’–3’) |
|---|---|---|
| Reference gene | CtGAPC1 | F: ACTCCAGAGGGATGATGTGG |
| R: ATGGGATCTCCTCTGGGTTC | ||
| Salicylic acid | CtNPR1 | F: TGATAAGACCTTGCCACAACAC |
| signaling | R: ACCGCAGGATTCAGATCTATGT | |
| CtPR1 | F: ACTGCAATCTTGTGCATTCG | |
| R: TTCACCCACAGTTTCACAGC | ||
| Jasmonic acid | CtAOS | F: GTTTCAGCTCGCTCCGTTAC |
| signaling | R: GAGGTTGTGACACGCTTCCT | |
| CtPI | F: AATCTTCTCATCGCTTTATC | |
| R: TGCTTCGCACTTACAACT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Arthurs, S.; Mao, R. Asymmetric Interaction between Aphis spiraecola and Toxoptera citricida on Sweet Orange Induced by Pre-Infestation. Insects 2020, 11, 414. https://doi.org/10.3390/insects11070414
Gao J, Arthurs S, Mao R. Asymmetric Interaction between Aphis spiraecola and Toxoptera citricida on Sweet Orange Induced by Pre-Infestation. Insects. 2020; 11(7):414. https://doi.org/10.3390/insects11070414
Chicago/Turabian StyleGao, Jing, Steve Arthurs, and Runqian Mao. 2020. "Asymmetric Interaction between Aphis spiraecola and Toxoptera citricida on Sweet Orange Induced by Pre-Infestation" Insects 11, no. 7: 414. https://doi.org/10.3390/insects11070414
APA StyleGao, J., Arthurs, S., & Mao, R. (2020). Asymmetric Interaction between Aphis spiraecola and Toxoptera citricida on Sweet Orange Induced by Pre-Infestation. Insects, 11(7), 414. https://doi.org/10.3390/insects11070414




