Foliage Intensity is an Important Cue of Habitat Location for Empoasca onukii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects and Plants
2.2. Datamining, Gene Cloning, and Phylogenetic Analysis
2.3. Histology
2.4. Electroretinography Recordings
2.5. Reflectance Spectra of Host Foliage
2.6. Behavioral Experiments
2.7. Discrimination of Host Foliage Color
3. Results
3.1. Opsins and Compound Eye Structure
3.2. Spectral Sensitivity of the Compound Eyes
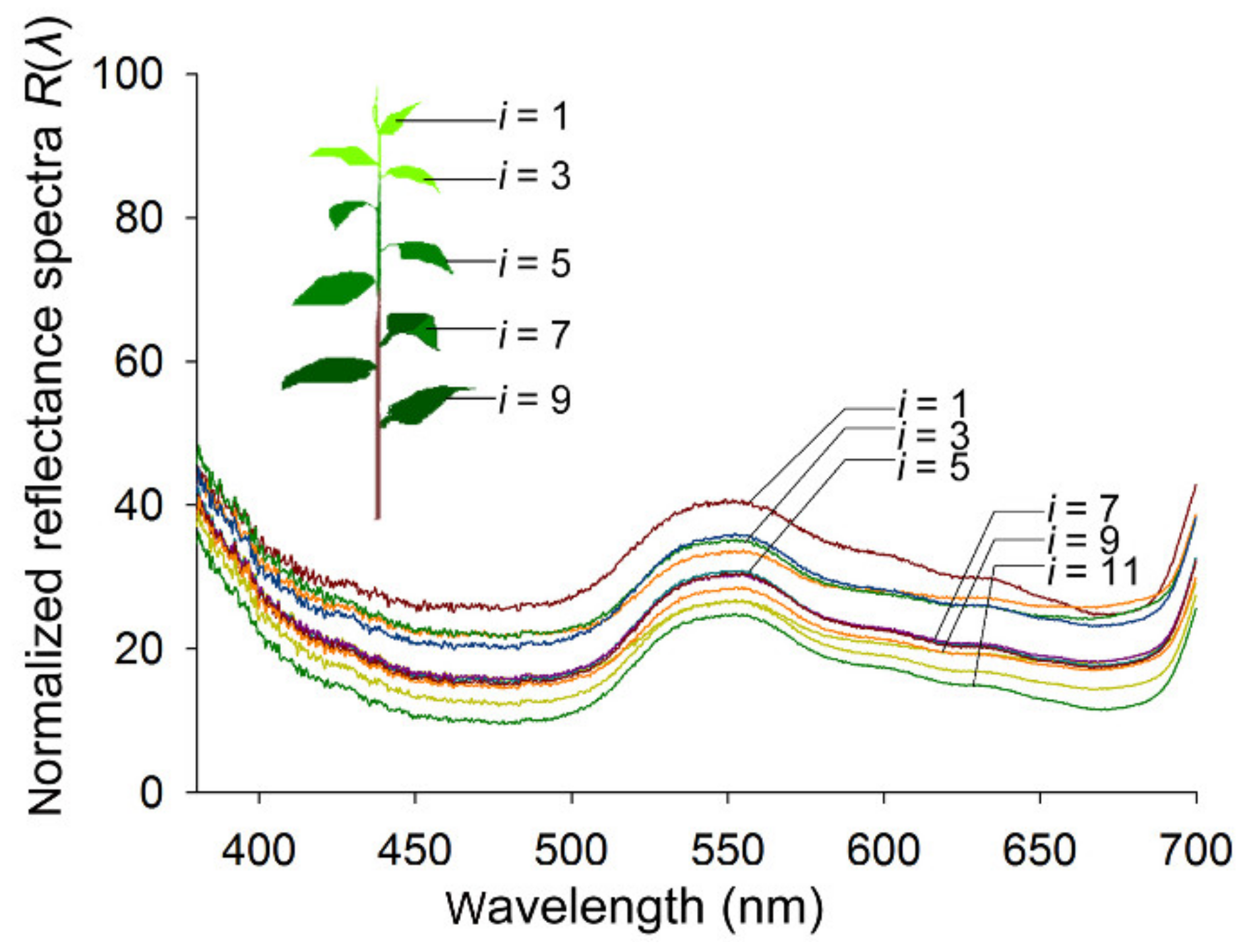
3.3. Reflectance Spectra of Host Foliage
3.4. Behavioral Experiments
3.5. Discrimination of Host Foliage Color
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bian, L.; Cai, X.M.; Luo, Z.X.; Li, Z.Q.; Xin, Z.J.; Chen, Z.M. Design of an attractant for Empoasca onukii (Hemiptera: Cicadellidae) based on the volatile components of fresh tea leaves. J. Econ. Entomol. 2018, 111, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Xie, Z.L.; Pang, X.F. Studies on the ecological niche of Emposca vitis (Göthe) and spiders in tea gardens. J. Tea Sci. 2008, 6, 401–406. [Google Scholar]
- Wang, Q.S.; Huang, J.; Gao, X.F. Studies on the spatial distribution of Empoasca vitis (Göthe) in organic tea garden. Chin. Agric. Sci. Bull. 2010, 26, 234–237. [Google Scholar]
- Bruce, T.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant. Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef]
- Knolhoff, L.M.; Heckel, D.G. Behavioral assays for studies of host plant choice and adaptation in herbivorous insects. Annu. Rev. Entomol. 2014, 59, 263–278. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Owens, E.D. Visual detection of plants by herbivorous insects. Annu. Rev. Entomol. 1983, 28, 337–364. [Google Scholar] [CrossRef]
- Saxena, K.N.; Saxena, R.C. Patterns of relationships between certain leafhoppers and plants, part III. Range and interaction of sensory stimuli. Entomol. Exp. Appl. 1975, 18, 194–206. [Google Scholar] [CrossRef]
- Zhang, X.; Pengsakul, T.; Tukayo, M.; Yu, L.; Fang, W.; Luo, D. Host-location behavior of the tea green leafhopper Empoasca vitis Gothe (Hemiptera: Cicadellidae): Olfactory and visual effects on their orientation. Bull. Entomol. Res. 2018, 108, 423–433. [Google Scholar] [CrossRef]
- Bian, L.; Sun, X.L.; Luo, Z.X.; Zhang, Z.Q.; Chen, Z.M. Design and selection of trap color for capture of the tea leafhopper, Empoasca vitis, by orthogonal optimization. Entomol. Exp. Appl. 2014, 151, 247–258. [Google Scholar] [CrossRef]
- Rodriguez–Saona, C.R.; Byers, J.A.; Schiffhauer, D. Effect of trap color and height on captures of blunt–nosed and sharp–nosed leafhoppers (Hemiptera: Cicadellidae) and non–target arthropods in cranberry bogs. Crop. Prot. 2012, 40, 132–144. [Google Scholar] [CrossRef]
- Shimoda, M.; Honda, K. Insect reactions to light and its applications to pest management. Appl. Entomol. Zool. 2013, 48, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Wakakuwa, M.; Stewart, F.; Matsumoto, Y.; Matsunaga, S.; Arikawa, K. Physiological basis of phototaxis to near–infrared light in Nephotettix cincticeps. J. Comp. Physiol, A. 2014, 200, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, A.D.; Chittka, L. The evolution of color vision in insects. Annu. Rev. Entomol. 2001, 46, 471–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaccardi, G.; Kelber, A.; Sison–Mangus, M.P.; Briscoe, A.D. Color discrimination in the red range with only one long–wavelength sensitive opsin. J. Exp. Biol. 2006, 209, 1944–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsson, J.; Henze, M.J.; Svensson, G.P.; Lind, O.; Anderbrant, O. Visual cues of oviposition sites and spectral sensitivity of Cydia strobilella L. J. Insect Physiol. 2017, 101, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Finkbeiner, S.D.; Fishman, D.A.; Osorio, D.; Briscoe, A.D. Ultraviolet and yellow reflectance but not fluorescence is important for visual discrimination of conspecifics by Heliconius erato. J. Exp. Biol. 2017, 220, 1267–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, M.V. Honey bees as a model for vision, perception, and cognition. Annu. Rev. Entomol. 2010, 55, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Paulk, A.; Millard, S.S.; Van Swinderen, B. Vision in Drosophila: Seeing the world through a model’s eyes. Annu. Rev. Entomol. 2013, 58, 313–332. [Google Scholar] [CrossRef]
- Gates, D.M. Application to plants. In Biophysical Ecology; Springer: New York, NY, USA, 1980; pp. 25–56. [Google Scholar]
- Bian, L.; Li, Z.Q.; Ma, L.; Cai, X.M.; Luo, Z.X.; Chen, Z.M. Identification of the genes in tea leafhopper, Empoasca onukii (Hemiptera: Cicadellidae), that encode odorant–binding proteins and chemosensory proteins using transcriptome analyses of insect heads. Appl. Entomol. Zool. 2018, 53, 93–105. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Mortazavi, A.; Williams, B.A.; Mccue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA–Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Schirmer, A.E.; Prete, F.R.; Mantes, E.S.; Urdiales, A.F.; Bogue, W. Circadian rhythms affect electroretinogram, compound eye color, striking behavior and locomotion of the praying mantis Hierodula patellifera. J. Exp. Biol. 2014, 217, 3853–3861. [Google Scholar] [CrossRef] [Green Version]
- Mcculloch, K.J.; Osorio, D.; Briscoe, A.D. Determination of photoreceptor cell spectral sensitivity in an insect model from in vivo intracellular recordings. J. Vis. Exp. 2016, 26, 53829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnsen, S. How to measure color using spectrometers and calibrated photographs. J. Exp. Biol. 2016, 219, 772–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhoutte, K.; Stavenga, D.G. Visual pigment spectra of the comma butterfly, Polygonia c–album, derived from in vivo epi–illumination nation microspectrophotometry. J. Comp. Physiol. A. 2005, 191, 461–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelber, A.; Vorobyev, M.; Osorio, D. Animal colour vision–behavioural tests and physiological concepts. Biol. Rev. Camb. Philos. Soc. 2003, 78, 81–118. [Google Scholar] [CrossRef]
- Vorobyev, M.; Osorio, D. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. B 1998, 265, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Vorobyev, M.; Brandt, R.; Peitsch, D.; Laughlin, S.B.; Menzel, R. Colour thresholds and receptor noise: Behaviour and physiology compared. V. Res. 2001, 41, 639–653. [Google Scholar] [CrossRef] [Green Version]
- Kukkonen, H.; Rovamo, J.; Tiippana, K.; Nasanen, R. Michelson contrast, RMS contrast and energy of various spatial stimuli at threshold. V. Res. 1993, 33, 1431–1436. [Google Scholar] [CrossRef]
- Jia, L.; Liang, A. Fine Structure of the Compound Eyes of Callitettix versicolor (Insecta: Hemiptera). Ann. Entomol. Soc. Am. 2015, 108, 316–324. [Google Scholar] [CrossRef]
- Stavenga, D.G. On visual pigment templates and the spectral shape of invertebrate rhodopsins and metarhodopsins. J. Comp. Physiol. A 2010, 196, 869–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govardovskii, V.I.; Fyhrquist, N.; Reuter, T.; Kuzmin, D.G.; Donner, K. In search of the visual pigment template. V. Neurosci. 2000, 17, 509–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakakuwa, M.; Stavenga, D.G.; Arikawa, K. Spectral organization of ommatidia in flower–visiting insects. Photochem. Photobiol. 2007, 83, 27–34. [Google Scholar] [CrossRef]
- Mcculloch, K.J.; Osorio, D.; Briscoe, A.D. Sexual dimorphism in the compound eye of Heliconius erato: A nymphalid butterfly with at least five spectral classes of photoreceptor. J. Exp. Biol. 2016, 219, 2377–2387. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, A.; Vogt, A.; Friedmann, K.; Paulsen, R.; Huber, A. Rhodopsin patterning in central photoreceptor cells of the blowfly Calliphora vicina: Cloning and characterization of Calliphora rhodopsins Rh3, Rh5 and Rh6. J. Exp. Biol. 2005, 208, 1247–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnaitmann, C.; Garbers, C.; Wachtler, T.; Tanimoto, H. Color discrimination with broadband photoreceptors. Curr. Biol. 2013, 23, 2375–2382. [Google Scholar] [CrossRef] [Green Version]
- Bian, L.; Cai, X.M.; Luo, Z.X.; Li, Z.Q.; Chen, Z.M. Decreased capture of natural enemies of pests in light traps with light–emitting diode technology. Ann. Appl. Biol. 2018, 173, 251–260. [Google Scholar] [CrossRef]
- Leboulle, G.; Niggebrugge, C.; Roessler, R.; Briscoe, A.D.; Menzel, R.; Hempel, D.I.N. Characterisation of the RNA interference response against the long–wavelength receptor of the honeybee. Insect Biochem. Mol. Biol. 2013, 43, 959–969. [Google Scholar] [CrossRef]
- Yan, S.; Zhu, J.L.; Zhu, W.L.; Zhang, X.F.; Li, Z.; Liu, X.X.; Zhang, Q.W. The expression of three opsin genes from the compound eye of Helicoverpa armigera (Lepidoptera: Noctuidae) is regulated by a circadian clock, light conditions and nutritional status. PLoS ONE 2014, 9, e111683. [Google Scholar] [CrossRef] [Green Version]
- Jane Telles, F.; Lind, O.; Henze, M.J.; Angel Rodriguez–girones, M.; Goyret, J.; Kelber, A. Out of the blue: The spectral sensitivity of hummingbird hawkmoths. J. Comp. Physiol. A 2014, 200, 537–546. [Google Scholar] [CrossRef]
- Shi, L.; Zeng, Z.; Huang, H.; Zhou, Y.; Vasseur, L.; You, M. Identification of Empoasca Onukii (Hemiptera: Cicadellidae) and monitoring of its populations in the tea plantations of south China. J. Econ. Entomol. 2015, 108, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Nieri, R.; Mazzoni, V. The reproductive strategy and the vibrational duet of the leafhopper Empoasca vitis. Insect Sci. 2018, 25, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Kelber, A. Receptor based models for spontaneous colour choices in flies and butterflies. Entomol. Exp. Appl. 2001, 99, 231–244. [Google Scholar] [CrossRef]
- Mooney, H.A.; Gulmon, S.L. Constraints on leaf structure and function in reference to herbivory. Bioscience 1982, 32, 198–206. [Google Scholar] [CrossRef]
- Kumashiro, S.; Matsukura, K.; Adachi, S.; Matsumura, M. Oviposition site preference and developmental performance of a gall-inducing leafhopper on galled and non-galled host plants. Entomol. Exp. Appl. 2016, 160, 18–27. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, R. Oviposition preference of cotton leafhopper in relation to leaf-vein morphology. J. Appl. Entomol. 2002, 126, 538–544. [Google Scholar] [CrossRef]
- Heil, M. Direct defense or ecological costs: Responses of herbivorous beetles to volatiles released by wild lima bean (Phaseolus Lunatus). J. Chem. Ecol. 2004, 30, 1289–1295. [Google Scholar] [CrossRef]
- Xin, Z.; Ge, L.; Chen, S.; Sun, X. Enhanced transcriptome responses in herbivore-infested tea plants by the green leaf volatile (Z)-3-hexenol. J. Plant Res. 2019, 132, 285–293. [Google Scholar] [CrossRef]
- Xin, Z.; Li, X.; Bian, L.; Sun, X. Tea green leafhopper, Empoasca vitis, chooses suitable host plants by detecting the emission level of (3Z)-hexenyl acetate. Bull. Entomol. Res. 2017, 107, 77–84. [Google Scholar] [CrossRef]
- Mei, X.; Liu, X.; Zhou, Y.; Wang, X.; Zeng, L.; Fu, X.; Li, J.; Tang, J.; Dong, F.; Yang, Z. Formation and emission of linalool in tea (Camellia sinensis) leaves infested by tea green leafhopper (Empoasca (Matsumurasca) onukii Matsuda). Food Chem. 2017, 237, 356–363. [Google Scholar] [CrossRef]
- Mayer, C.J.; Vilcinskas, A.; Gross, J. Chemically mediated multitrophic interactions in a plant-insect vector-phytoplasma system compared with a partially nonvector species. Agricult. For. Entomol. 2011, 13, 25–35. [Google Scholar] [CrossRef]
- Mayer, C.J.; Vilcinskas, A.; Gross, J. Pathogen-induced release of plant allomone manipulates vector insect behavior. J. Chem. Ecol. 2008, 34, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.J.; Vilcinskas, A.; Gross, J. Phytopathogen lures its insect vector by altering host plant odor. J. Chem. Ecol. 2008, 34, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Reeves, J.L. Vision should not be overlooked as an important sensory modality for finding host plants. Environ. Entomol. 2011, 40, 855–863. [Google Scholar] [CrossRef]





| i | Equation Constants | ||
|---|---|---|---|
| a | b | R | |
| 2 | 1.125 | −7.31 | 0.983 |
| 3 | 1.273 | −18.315 | 0.978 |
| 4 | 1.279 | −11.879 | 0.986 |
| 5 | 1.35 | −19.891 | 0.991 |
| 6 | 1.232 | −11.363 | 0.993 |
| 7 | 1.283 | −17.695 | 0.99 |
| 8 | 1.307 | −18.925 | 0.991 |
| 9 | 1.312 | −19.929 | 0.987 |
| 10 | 1.357 | −23.509 | 0.988 |
| 11 | 1.374 | −26.361 | 0.99 |
| Equation Constants | ||||
|---|---|---|---|---|
| Male | a | b | R | Sig. |
| Ii | 0.001 | −12.975 | 0.701 | 0.016 |
| S | 0.411 | −11.12 | 0.878 | 0 |
| B | 0.922 | −28.408 | 0.817 | 0.002 |
| Female | a | b | R | Sig. |
| Ii | 0.001 | −11.306 | 0.722 | 0.012 |
| S | 0.375 | −10.286 | 0.943 | 0 |
| B | 0.832 | −25.705 | 0.866 | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, L.; Cai, X.M.; Luo, Z.X.; Li, Z.Q.; Chen, Z.M. Foliage Intensity is an Important Cue of Habitat Location for Empoasca onukii. Insects 2020, 11, 426. https://doi.org/10.3390/insects11070426
Bian L, Cai XM, Luo ZX, Li ZQ, Chen ZM. Foliage Intensity is an Important Cue of Habitat Location for Empoasca onukii. Insects. 2020; 11(7):426. https://doi.org/10.3390/insects11070426
Chicago/Turabian StyleBian, Lei, Xiao Ming Cai, Zong Xiu Luo, Zhao Qun Li, and Zong Mao Chen. 2020. "Foliage Intensity is an Important Cue of Habitat Location for Empoasca onukii" Insects 11, no. 7: 426. https://doi.org/10.3390/insects11070426
APA StyleBian, L., Cai, X. M., Luo, Z. X., Li, Z. Q., & Chen, Z. M. (2020). Foliage Intensity is an Important Cue of Habitat Location for Empoasca onukii. Insects, 11(7), 426. https://doi.org/10.3390/insects11070426




