Current Distribution and Diagnostic Features of Two Potentially Invasive Asian Buprestid Species: Agrilus mali Matsumura and A. fleischeri Obenberger (Coleoptera: Buprestidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Taxonomy, Distribution, and Host Plants of Agrilus mali and A. fleischeri
3.1.1. Agrilus mali Matsumura, 1924, Apple Buprestid
3.1.2. Agrilus fleischeri Obenberger, 1925
4. Discussion
5. Conclusions
- Distributional maps for two potentially invasive buprestid species, Agrilus mali and A. fleischeri, are compiled based on the documented records for 51 and 53 localities, correspondingly.
- A dangerous quarantine pest of wild and cultivated apple trees, A. mali, is distributed over the continental East Asia from the Russian Far East, Northeast China, and the Korean Peninsula to Mongolia and Eastern Siberia; however, its occurrence in Japan is not confirmed.
- Massive outbreak sites of A. mali on Malus sieversii in Xinjiang likely represent a newly forming invasion area, which indicates the expansion of its range; their proximity to the wild apple stands in the Kazakh part of the Tien Shan is a direct threat to Kazakhstan and other Central Asian countries.
- Agrilus fleischeri is more widely distributed in the Russian Far East and Siberia, Kazakhstan, Mongolia, China, and Japan than was assumed earlier; its westernmost limits come close but do not overlap with the range of the closely related European poplar pest A. ater.
- Outbreak sites of A. fleischeri in Liaoning (China) are situated within its range; the local switch from indigenous to introduced poplars is the most likely reason for the outbreak. Thus, there is no reason to claim an expansion of its native range. The situation is similar to the early stages of emerald ash borer invasion and A. fleischeri should be monitored as a potential invasion threat.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orlova–Bienkowskaja, M.J.; Volkovitsh, M.G. Are native ranges of the most destructive invasive pests well known? A case study of the native range of the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). Biol. Invasions 2018, 20, 1275–1286. [Google Scholar] [CrossRef]
- Orlova–Bienkowskaja, M.J.; Bieńkowski, A.O. The life cycle of the emerald ash borer Agrilus planipennis in European Russia and comparisons with its life cycles in Asia and North America. Agric. For. Entomol. 2016, 18, 182–188. [Google Scholar] [CrossRef]
- Orlova–Bienkowskaja, M.J.; Bieńkowski, A.O. Modeling long–distance dispersal of emerald ash borer in European Russia and prognosis of spread of this pest to neighboring countries within next 5 years. Ecol. Evol. 2018, 8, 9295–9304. [Google Scholar] [CrossRef] [PubMed]
- Haack, R.A.; Baranchikov, Y.N.; Bauer, L.S.; Poland, T.M. Emerald ash borer biology and invasion history. In Biology and Control of Emerald Ash Borer; Van Driesche, R.G., Reardon, R.C., Eds.; U.S. Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team: Morgantown, WV, USA, 2015; pp. 1–13. [Google Scholar]
- Herms, D.A.; McCullough, D.G. Emerald ash borer invasion of North America: History, biology, ecology, impacts, and management. Annu. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [Green Version]
- Chamorro, M.L.; Jendek, E.; Haack, R.A.; Petrice, T.R.; Woodley, N.E.; Konstantinov, A.S.; Volkovitsh, M.G.; Yang, X.K.; Grebennikov, V.V. Illustrated Guide to the Emerald Ash Borer, Agrilus Planipennis Fairmaire and Related Species (Coleoptera, Buprestidae); Pensoft Publisher: Sofia-Moscow, Bulgaria, 2015; pp. 1–198. [Google Scholar]
- Volkovitsh, M.G.; Mozolevskaya, E.G. The tenth «anniversary» of the invasion of emerald ash borer Agrilus planipennis Fairm. (Coleoptera: Buprestidae) in Russia: Results and prospects. Izvestia St.-Peterburg. Lesoteh. Akad. 2014, 207, 8–19. [Google Scholar]
- Orlova–Bienkowskaja, M.J. Ashes in Europe are in danger: The invasive range of Agrilus planipennis in European Russia is expanding. Biol. Invasions 2014, 16, 1345–1349. [Google Scholar] [CrossRef]
- Orlova–Bienkowskaja, M.J.; Drogvalenko, A.N.; Zabaluev, I.A.; Sazhnev, A.S.; Peregudova, E.Y.; Mazurov, S.G.; Komarov, E.V.; Struchaev, V.V.; Martynov, V.V.; Nikulina, T.V.; et al. Current range of Agrilus planipennis Fairmaire, an alien pest of ash trees, in European Russia and Ukraine. Ann. For. Sci. 2020, 77, 1–14. [Google Scholar] [CrossRef]
- Kelnarova, I.; Jendek, E.; Grebennikov, V.V.; Bocak, L. First molecular phylogeny of Agrilus (Coleoptera: Buprestidae), the largest genus on Earth, with DNA barcode database for forestry pest diagnostics. Bull. Entomol. Res. 2019, 109, 200–211. [Google Scholar] [CrossRef]
- Agrilus Anxius. EPPO Global Database. Available online: https://gd.eppo.int/taxon/AGRLAX (accessed on 3 May 2020).
- Bozorov, T.A.; Luo, Z.; Li, X.; Zhang, D. Agrilus mali Matsumara (Coleoptera: Buprestidae), a new invasive pest of wild apple in western China: DNA barcoding and life cycle. Ecol. Evol. 2018, 9, 1160–1172. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.-J.; Zhang, Y.-L.; Zhang, X.; Luo, Z.-H.; Zhang, P.; Golec, J.; Poland, T.M.; Zalucki, M.P.; Han, P.; Lu, Z.-Z. Life history and mortality factors of Agrilus mali Matsumura (Coleoptera: Buprestidae) in wild apples in Northwestern China. Agric. For. Ent. 2019, 21, 309–317. [Google Scholar] [CrossRef]
- Zang, K.; Wang, X.-Y.; Yang, Z.-Q.; Wei, K.; Duan, J.J. Biology and natural enemies of Agrilus fleischeri (Coleoptera: Buprestidae), a newly emerging destructive buprestid pest in Northeast China. J. Asia-Pac. Ent. 2017, 20, 47–52. [Google Scholar] [CrossRef] [Green Version]
- An Unified List of Quarantine Pests of the Eurasian Economic Union. (As Amended by: 8 August 2019.) (Decision of the Council of the Eurasian Economic Commission of 8 August 2019 No. 74). Available online: https://vniikr.ru/edinyij-perechen-karantinnyix-obektov-evrazijskogo-ekonomicheskogo-soyuza (accessed on 24 April 2020).
- Agrilus Mali. EPPO Global Database. Available online: https://gd.eppo.int/taxon/AGRLMA (accessed on 20 March 2020).
- EPPO A2 List of Pests Recommended for Regulation as Quarantine Pests (Version 2019-09). Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list (accessed on 24 April 2020).
- Matsumura, S. [Life history of Agrilus mali, Mats. (Buprestidae)]. In Bionomics on Apple Trees in CHINA and Corea; Muramatsu, S., Ed.; Korea Agricultural Experiment Station, 1924; Report No. 11; pp. 1–21. (In Japanese) [Google Scholar]
- Jendek, E.; Grebennikov, V. Agrilus (Coleoptera, Buprestidae) of East Asia; Jan Farkač: Prague, Czech Republic, 2011; pp. 1–362. [Google Scholar]
- Ji, Y.; Ji, R.; Huang, R.-X. Invasive Species—Agrilus mali Matsmura and damage in Xinjiang. Xinjiang Agric. Sci. 2004, 41, 31–33. [Google Scholar]
- Cui, X.N.; Liu, D.G.; Li, A.H. Research progress in integrated management of Agrilus mali. Plant Prot. 2015, 41, 16–23. [Google Scholar]
- Zhang, P.; Cui, Z.; Xu, H.; Ali, A.; Zhang, X.; Liu, X.; Zhang, Y.; Zhou, X.; Lu, Z. Thirst or Malnutrition: The impacts of invasive insect Agrilus mali on the physiological status of wild apple trees. Insects 2020, 11, 440. [Google Scholar] [CrossRef] [Green Version]
- Alexeev, A.V. New, previously unknown from the territory of the USSR, and little-known species of jewel-beetles (Coleoptera: Buprestidae) of Eastern Siberia and the Far East. In Beetles of the Far East and Eastern Siberia (New Data on the Fauna and Taxonomy); Krivolutskaya, G.O., Ed.; Far East Scientific Center of Academy of Sciences of the USSR: Vladivostok, Russia, 1979; pp. 123–139. (In Russian) [Google Scholar]
- Alexeev, A.V. 39. Fam. Buprestidae—Jewel-beetles. In Keys to the Identification of Insects of the Russian Far East. Vol. 1. Coleoptera or beetles. Part 1; Ler, P.A., Ed.; Nauka: Leningrad, Russia, 1989; Volume 1, pp. 463–489. (In Russian) [Google Scholar]
- Sergeeva, E.V.; Stolbov, V.A. The fauna of Jewel beetles (Coleoptera, Buprestidae) of Tyumen region. Acta Biol. Sib. 2019, 5, 159–166. [Google Scholar]
- Hijmans, R.J.; Guarino, L.; Cruz, M.; Rojas, E. Computer tools for spatial analysis of plant genetic resources data: 1. DIVA-GIS. Plant Genet. Res. Newsl. 2001, 127, 15–19. [Google Scholar]
- Kalashian, M.Y.; (Scientific Center of Zoology and Hydroecology, Yerevan, Armenia). Personal communication, 2020.
- Makarov, K.V.; (Moscow State Pedagogical University, Moscow, Russia). Personal communication, 2020.
- Shabalin, S.A.; (Federal Scientific Centre of Biodiversity of Terrestrial Biota of East Asia, Far East Department of Russian Academy of Sciences, Vladivostok, Russia). Personal communication, 2020.
- Smirnov, M.E.; (Ivanovo, Russia). Personal communication, 2020.
- Tleppaeva, A.M.; (Institute of Zoology, Ministry of Education and Science of the Republic of Kazakhstan, Almaty, Kazakhstan). Personal communication, 2020.
- Hattori, T.; (Yokohama City, Japan). Personal communication, 2020.
- Song, H.; (Fuzhou, China). Personal communication, 2020.
- Akiyama, K.; Ohmomo, S. The Buprestid Beetles of the World. In Mushi-Sha’s Iconographic Series of Insects 4; Mushi-Sha: Tokyo, Japan, 2000; pp. 1–341. (In Japanese) [Google Scholar]
- Cao, L.M.; Zhang, Y.L.; van Achterberg, C.; Wang, Z.Y.; Wang, X.Y.; Zhao, W.X.; Yang, Z.Q. Notes on braconid wasps (Hymenoptera, Braconidae) parasitising on Agrilus mali Matsumura (Coleoptera, Buprestidae) in China. ZooKeys 2019, 867, 97–121. [Google Scholar] [CrossRef] [PubMed]
- Fukutomi, K.; Hori, S. The buprestid beetles of Hokkaido in color (Coleoptera, Buprestidae). Jezoensis Sapporo 2004, 30, 1–25. [Google Scholar]
- GBIF.org GBIF Occurrence Download. Available online: https://www.gbif.org/ru/occurrence/search?taxon_key=9379101 (accessed on 24 April 2020).
- Kadyrbekov, R.K.; Tleppaeva, A.M.; Childebaev, M.K. To the fauna of cerambycid (Cerambycidae) and buprestid (Buprestidae) beetles of the National Nature Park “Burabai”. News Nat. Acad. Sci. Rep. Kazakhstan Biol. Med. Ser. 2003, 6, 34–42. (In Russian) [Google Scholar]
- Kurosawa, Y. A comment to the Japanese Buprestidae (11). Coleopts News Tokyo 1974, 21/22, 1–2. (In Japanese) [Google Scholar]
- Ohmomo, S.; Fukutomi, H. The Buprestid Beetles of Japan. In Mushi-Sha’s Iconographic Series of Insects 7; Mushi-Sha: Tokyo, Japan, 2013; pp. 1–206. (In Japanese) [Google Scholar]
- Beetles (Coleoptera) and Coleopterists. Available online: https://www.zin.ru/Animalia/Coleoptera/rus/agrflems.htm (accessed on 28 April 2020).
- Tleppaeva, A.M.; Kadyrbekov, R.K.; Kolov, S.V.; Zlatanov, B.V. Xylophagous Insects of Trees and Shrubs of Mountains of Almaty Province; TOO 378: Almaty, Kazakhstan, 2017; pp. 1–196. (In Russian) [Google Scholar]
- Tleppaeva, A.M.; Gabdullina, A.U. To the fauna of buprestid beetles (Coleoptera, Buprestidae) of the Katon-Karagay State National Nature Park (Southwest Altai). Bull. Nat. Acad. Sci. Rep. Kazakhstan Biol. Ser. 2010, 2, 68–70. (In Russian) [Google Scholar]
- Volkovitsh, M.G. Buprestidae. In Insects of Lazovsky Nature Reserve; Storozhenko, S.Y., Ed.; Dalnauka: Vladivostok, Russia, 2009; pp. 132–137. (In Russian) [Google Scholar]
- Volkovitsh, M.G.; Orlova-Bienkowskaja, M.J.; Kovalev, A.V.; Bieńkowski, A.O. An illustrated guide to distinguish emerald ash borer (Agrilus planipennis) from its congeners in Europe. Forestry 2020, 93, 316–325. [Google Scholar] [CrossRef]
- Kubáň, V.; Jendek, E.; Kalashian, M.Y.; Volkovitsh, M.G. Superfamily BUPRESTOIDEA Leach, 1815. In Catalogue of Palaearctic Coleoptera). Scarabaeoidea, Scirtoidea, Dascilloidea, Buprestoidea and Byrrhoidea; Revised and Updated Ed., Löbl, I., Löbl, D., Eds.; BRILL: Leiden, The Netherlands, 2016; Volume 3, pp. 19–32, 432–574. [Google Scholar]
- Jendek, E.; Poláková, J. Host Plants of World Agrilus (Coleoptera, Buprestidae). A Critical Review; Springer: New York, NY, USA, 2014; pp. 1–706. [Google Scholar]
- Li, M.L.; Zhang, Z.Q. Discussion on biology and life history associated with Agrilus mali Matsumura. J. Northwest For. Univ. 2017, 32, 139–146. [Google Scholar]
- Liu, Z.Q.; Chen, W.M.; Xu, Z.; Liang, Q.L. Malus sieversii forest distribution and Agrilus mali Mastsumura status of damage in the west part of Tianshan Mountains. North. Hortic. 2014, 17, 121–124. [Google Scholar]
- Yao, Y.-X.; Duan, J.J.; Mottern, J.L.; Wang, X.-Y.; Yang, Z.-Q.; Bauer, L.S.; Gates, M.W. Two new species of Oobius (Hymenoptera: Encyrtidae) and their phylogenetic relationship with other congeners from northeastern Asia. Can. Entomol. 2018, 150, 303–316. [Google Scholar] [CrossRef]
- Zhao, T.H.; Zhao, W.X.; Gao, R.T.; Zhang, Q.W.; Li, G.H.; Liu, X.X. Induced outbreaks of indigenous insect species by exotic tree species. Acta Entomol. Sin. 2007, 50, 826–833. [Google Scholar]
- Orlova-Bienkowskaja, M.J.; Bieńkowski, A.O. Minimum winter temperature as a limiting factor of the potential spread of Agrilus planipennis, an alien pest of ash trees, in Europe. Insects 2020, 11, 258. [Google Scholar] [CrossRef]
- Sobek-Swant, S.; Kluza, D.A.; Cuddington, K.; Lyons, D.B. Potential distribution of emerald ash borer: What can we learn from ecological niche models using Maxent and GARP? For. Ecol. Manag. 2012, 281, 23–31. [Google Scholar] [CrossRef]
- Sobek-Swant, S.; Crosthwaite, J.C.; Lyons, D.B.; Sinclair, B.J. Could phenotypic plasticity limit an invasive species? Incomplete reversibility of mid-winter deacclimation in emerald ash borer. Biol. Invasions 2012, 14, 115–125. [Google Scholar] [CrossRef]
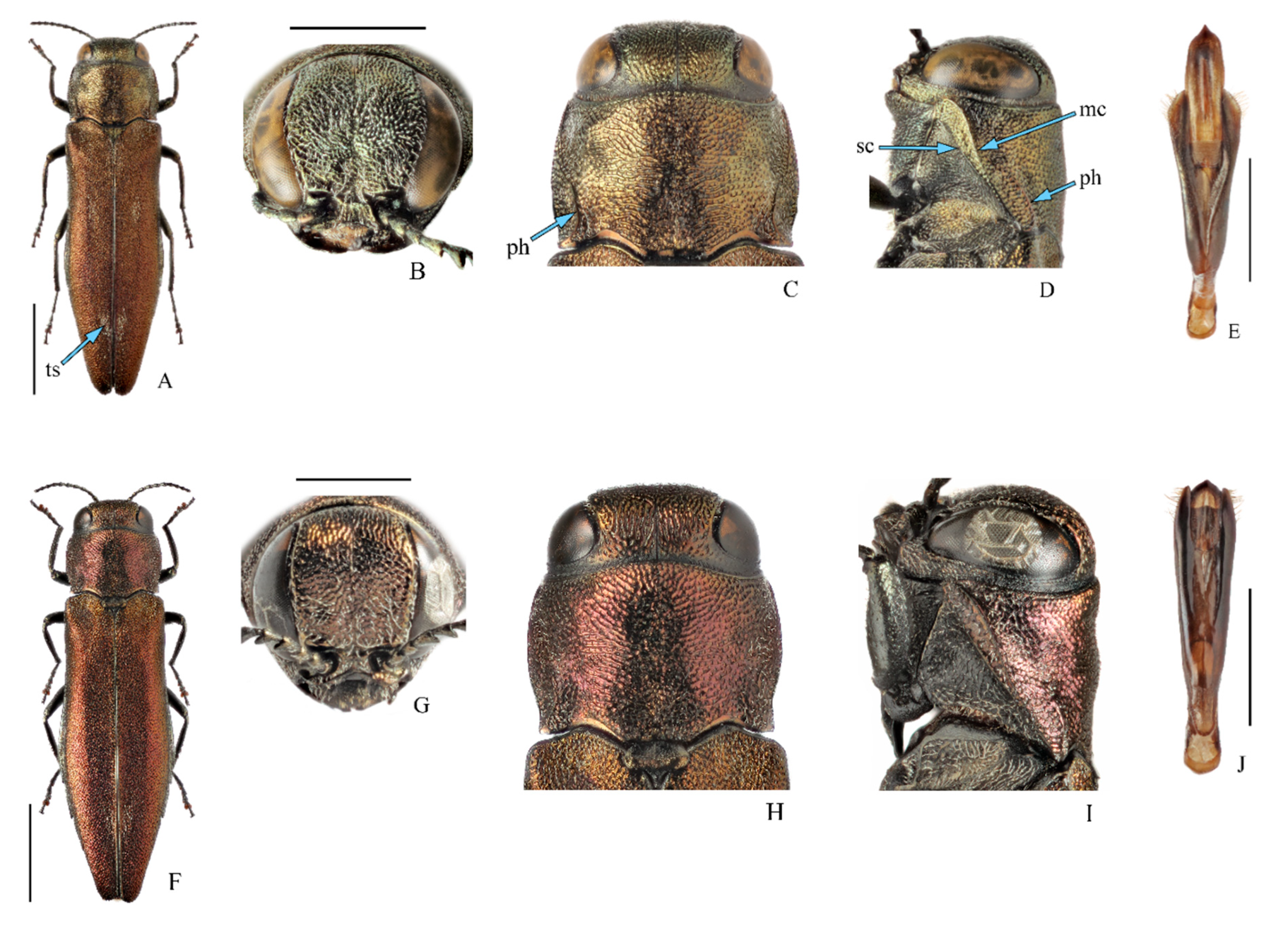

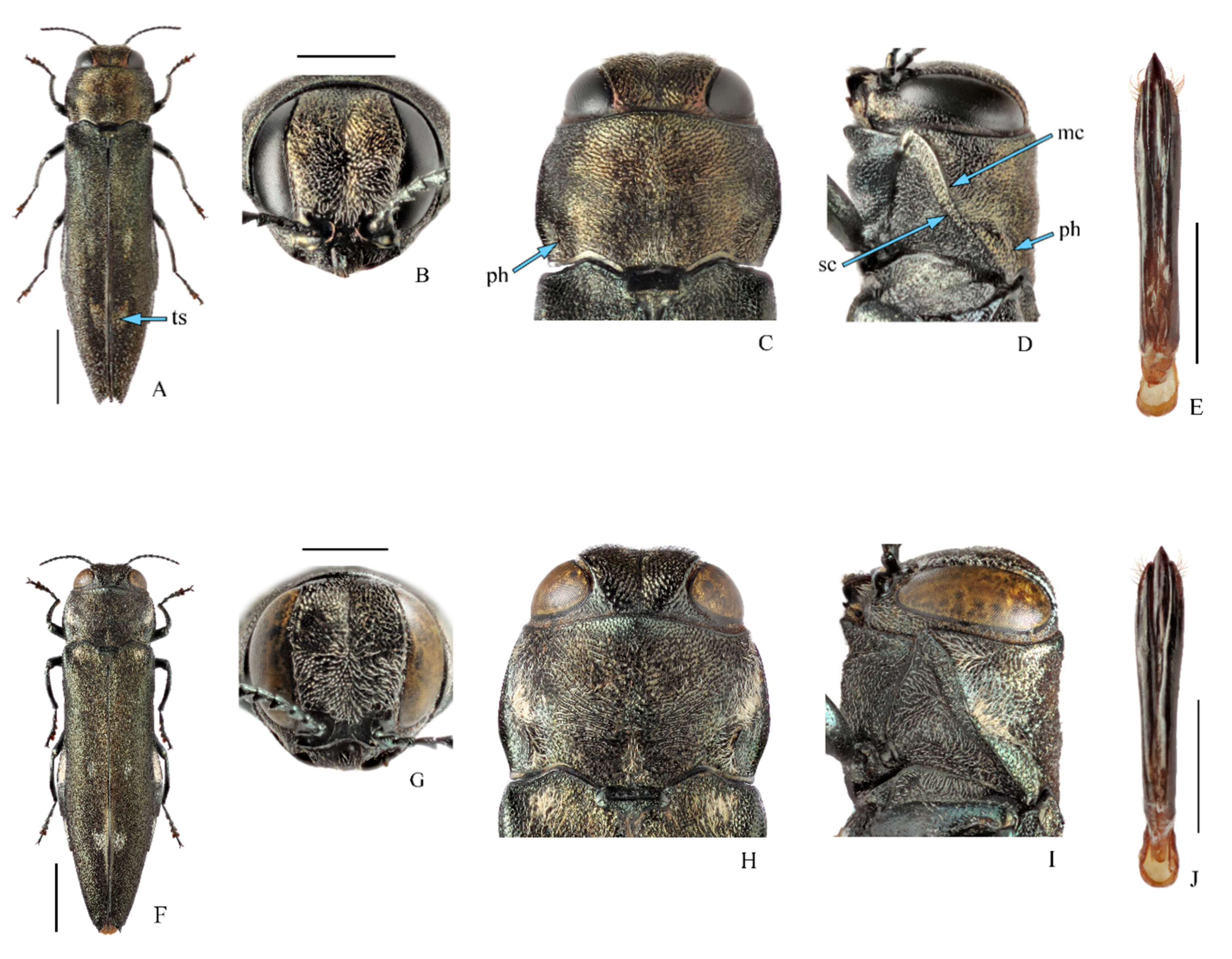

| Region | Number of Mapped Localities | Years of Collection | Host Plants | Sources of Information |
|---|---|---|---|---|
| RU: Primorskii Krai | 10 | 1926–1977 | Malus sp. (part.) | ZIN; [28,44] |
| RU: Khabarovskii Krai | 0 | - | - | [19] |
| RU: Amurskaya Oblast | 3 | 1927, 1958 | - | ZIN; [19] |
| RU: Zabaikalskii Krai | 1 | 1898 | - | ZIN |
| RU: Chitinskaya Oblast | 2 | 1923, 1924 | Malus sp. | ZIN; [27] |
| MG: Dornod | 3 | 1976 | Wild Malus sp. | ZIN |
| MG: Tuv | 2 | 1976 | Wild Malus sp. | ZIN |
| CH: Heilongjiang | 1 | 1931 | - | ZIN |
| CH: Liaoning | 1 | - | - | [18] |
| CH: Xinjiang | 15 | 2011–2017 | M. sieversii, M. domestica | [12,13,35,37] |
| CH: Gansu | 2 | 1992, 1996 | - | [19] |
| CH: Qinghai | 1 | 2008 | - | [35] |
| CH: Hubei | 1 | 2002 | - | [19] |
| CH: Sichuan | 3 | 1990–2001 | - | [19] |
| CH: Shaanxi | 1 | 2006 | - | [35] |
| CH: Guangxi | 0 | - | - | [19] |
| CH: Hebei | 0 | - | - | [19] |
| CH: Henan | 0 | - | - | [19] |
| CH: Nei Mongol | 0 | - | - | [19] |
| CH: Shandong | 0 | - | - | [19] |
| CH: Xizang | 0 | - | - | [19] |
| NC: Pyongyang | 1 | - | M. domestica | [18] |
| SK: Gyeonggi-do | 1 | - | M. domestica | [18] |
| SK: Taegu | 1 | - | M. domestica | [18] |
| SK: Inchon | 1 | - | M. domestica | [18] |
| SK: Seoul | 1 | 1940 | - | [32] |
| 51 | 1898–2017 |
| Region | Number of Mapped Localities | Years of Collection | Host Plants | Sources of Information |
|---|---|---|---|---|
| RU: Sakhalin | 1 | 1930 | - | [19] |
| RU: Primorskii Krai | 18 | 1899–2015 | - | ZIN; [19,27,28,30] |
| RU: Khabarovskii Krai | 2 | 1976 | - | ZIN |
| RU: Evreiskaya AO | 1 | - | - | ZIN |
| RU: Amurskaya Oblast | 0 | - | - | [19] |
| RU: Byrjatia Republic | 0 | - | - | [19] |
| RU: Tuva Republic | 2 | 1949, 1979 | Populus sp. | [19,27] |
| RU: Altai Republic | 1 | 1909 | - | ZIN |
| RU: Altaiskii Krai | 1 | 1910 | - | ZIN |
| RU: Tyumenskaya Oblast | 1 | 2002 | - | [25] |
| RU: Krasnoyarskii Krai | 1 | 1989 | - | [27] |
| KZ: Almaty Oblast | 1 | 2016 | Populus tremula | ZIN; [42] |
| KZ: Vostochno-Kazakhstanskaya Oblast | 2 | 2001, 2009 | Populus sp. | [19,43] |
| KZ: Akmolinskaya Oblast 1 | 1 | 2002 | - | [38] |
| MG: Tuv | 2 | 2003 | - | [19] |
| CH: Heilongjiang | 5 | 1931, 1939, 1940 | - | ZIN; [19] |
| CH: Liaoning | 2 | 2013–2015 | Populus davidiana, P. nigra var. italica | [14,50] |
| CH: Beijiing | 2 | 2000 (1) | Populus spp. in plantations; Salix sp. | [14,19] |
| CH: Hebei | 1 | - | Populus spp. in plantations; Salix sp. | [14] |
| CH: Shaanxi | 1 | 1998 | - | [19] |
| CH: Sichuan | 1 | 2001 | - | [19] |
| NC | 0 | - | - | [19] |
| SC | 0 | - | - | [19] |
| JA: Honsu | 5 | 1996 (1) | - | [19,34,39,40] |
| JA: Hokkaido | 2 | 2015 (1) | - | [19,36] |
| 53 | 1899–2016 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkovitsh, M.G.; Kovalev, A.V.; Orlova-Bienkowskaja, M.J. Current Distribution and Diagnostic Features of Two Potentially Invasive Asian Buprestid Species: Agrilus mali Matsumura and A. fleischeri Obenberger (Coleoptera: Buprestidae). Insects 2020, 11, 493. https://doi.org/10.3390/insects11080493
Volkovitsh MG, Kovalev AV, Orlova-Bienkowskaja MJ. Current Distribution and Diagnostic Features of Two Potentially Invasive Asian Buprestid Species: Agrilus mali Matsumura and A. fleischeri Obenberger (Coleoptera: Buprestidae). Insects. 2020; 11(8):493. https://doi.org/10.3390/insects11080493
Chicago/Turabian StyleVolkovitsh, Mark G., Alexey V. Kovalev, and Marina J. Orlova-Bienkowskaja. 2020. "Current Distribution and Diagnostic Features of Two Potentially Invasive Asian Buprestid Species: Agrilus mali Matsumura and A. fleischeri Obenberger (Coleoptera: Buprestidae)" Insects 11, no. 8: 493. https://doi.org/10.3390/insects11080493
APA StyleVolkovitsh, M. G., Kovalev, A. V., & Orlova-Bienkowskaja, M. J. (2020). Current Distribution and Diagnostic Features of Two Potentially Invasive Asian Buprestid Species: Agrilus mali Matsumura and A. fleischeri Obenberger (Coleoptera: Buprestidae). Insects, 11(8), 493. https://doi.org/10.3390/insects11080493






