The Importance of Males to Bumble Bee (Bombus Species) Nest Development and Colony Viability
Abstract
:Simple Summary
Abstract
1. Introduction
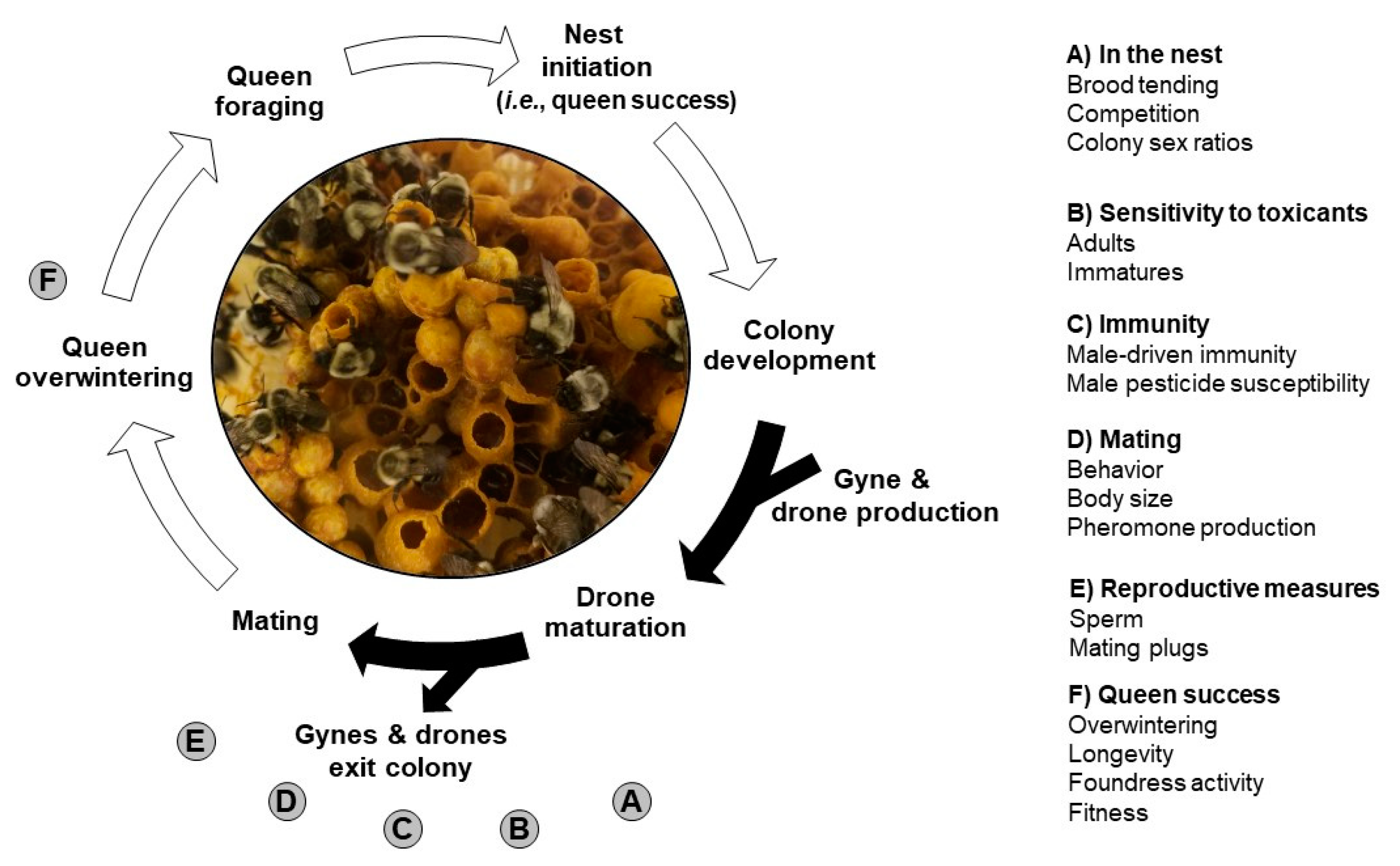
2. Methods
3. Drone Biology
4. Role within the Nest
5. Sensitivity to Toxicants
5.1. Sensitivity of Mature Drones to Pesticides
5.2. Sensitivity of Immature Drones to Pesticides
6. Immunity
7. Mating Behavior
8. Reproductive Measures
9. Queen Success as a Result of Mating
10. Discussion and Knowledge Gaps
10.1. Species Focus
10.2. Effects of Pesticides
10.3. Immune System
10.4. Nutritional Requirements
10.5. Risk Assessment Considerations
10.6. Future Research
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Martin, C.D.; Fountain, M.T.; Brown, M.J.F. Varietal and seasonal differences in the effects of commercial bumblebees on fruit quality in strawberry crops. Agric. Ecosyst. Environ. 2019, 281, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Baur, A.; Strange, J.P.; Koch, J.B. Foraging economics of the hunt bumble bee, a viable pollinator for commercial agriculture. Environ. Entomol. 2019, 48, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, J.; Williams, P.H.; Vaissière, B.E.; Zhou, Z.; Gai, Q.; Dong, J.; An, J. Managed bumblebees outperform honeybees in increasing peach fruit set in China: Different limiting processes with different pollinators. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desjardins, E.C.; De Oliveira, D. Commercial bumble bee Bombus impatiens (Hymenoptera: Apidae) as a pollinator in lowbush blueberry (Ericale: Ericaceae) fields. J. Econ. Entomol. 2006, 99, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Artz, D.R.; Nault, B.A. Performance of Apis mellifera, Bombus impatiens, and Peponapis pruinosa (Hymenoptera: Apidae) as pollinators of pumpkin. J. Econ. Entomol. 2011, 104, 1153–1161. [Google Scholar] [CrossRef]
- Shipp, J.L.; Whitfield, G.H.; Papadopoulos, A.P. Effectiveness of the bumble bee, Bombus impatiens Cr. (Hymenoptera: Apidae), as a pollinator of greenhouse sweet pepper. Sci. Hortic. 1994, 57, 29–39. [Google Scholar] [CrossRef]
- Isaacs, R.; Kirk, A.K. Pollination services provided to small and large highbush blueberry fields by wild and managed bees. J. Appl. Ecol. 2010, 47, 841–849. [Google Scholar] [CrossRef]
- Button, L.; Elle, E. Wild bumble bees reduce pollination deficits in a crop mostly visited by managed honey bees. Agric. Ecosyst. Environ. 2014, 197, 255–263. [Google Scholar] [CrossRef]
- Goulson, D. Bumblebees Behaviour and Ecology; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Hatten, T.D.; Looney, C.; Strange, J.P.; Bosque-Perez, N.A. Bumble bee fauna of Palouse prairie: Survey of native bee pollinators in a fragmented ecosystem. J. Insect Sci. 2013, 13, 1–19. [Google Scholar] [CrossRef]
- US Fish and Wildlife Service. U.S. Fish & Wildlife Service Endangered Species List: Insects. In Environmental Conservation Online System. Available online: https://ecos.fws.gov/ecp0/pub/SpeciesReport.do?groups=I&listingType=L&mapstatus=1 (accessed on 10 March 2020).
- Cresswell, J.E. A demographic approach to evaluating the impact of stressors on bumble bee colonies. Ecol. Entomol. 2017, 42, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Kent, C.F.; Dey, A.; Patel, H.; Tsvetkov, N.; Tiwari, T.; MacPhail, V.J.; Gobell, Y.; Harpur, B.A.; Gurtowski, J.; Schatz, M.C.; et al. Conservation genomics of the declining North American bumblebee Bombus terricola reveals inbreeding and selection on immune genes. Front. Genet. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dance, C.; Botias, C.; Goulson, D. The combined effects of a monotonous diet and exposure to thiamethoxam on the performance of bumblebee micro-colonies. Ecotoxicol. Environ. Saf. 2017, 139, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Gradish, A.E.; van der Steen, J.; Scott-Dupree, C.D.; Cabrera, A.R.; Cutler, G.C.; Goulson, D.; Klein, O.; Lehmann, D.M.; Luckmann, J.; O’Neill, B.; et al. Comparison of pesticide exposure in honey bees (Hymenoptera: Apidae) and bumble bees (Hymenoptera: Apidae): Implications for risk assessments. Environ. Entomol. 2019, 48, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D. Bumblebees: Behavior, Ecology and Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Williams, P.H.; Thorp, R.W.; Richardson, L.L.; Colla, S.R. Bumble Bees of North America: An Identification Guide; Princeton University Press: Princeton, NJ, USA, 2014. [Google Scholar]
- Schmid-Hempel, P. Parasites in Social Insects; Princeton University Press: Princeton, NJ, USA, 1998. [Google Scholar]
- Alford, D.V. Bumblebees; Davis-Poynter: London, UK, 1975. [Google Scholar]
- Flottum, K. The Backyard Beekeeper: An Absolute Beginner’s Guide to Keeping Bees in Your Yard and Garden, 4th ed.; Quarry Books: Beverly, MA, USA, 2018. [Google Scholar]
- Eeraerts, M.; Ceuppens, B.; Asselman, J.; De Schamphelaere, K.; Smagghe, G. 4.12 Effects of imidacloprid in combination with λ-cyhalothrin on the model pollinator Bombus terrestris at different levels of complexity. In Proceedings of the Hazards of Pesticides to Bees—12th International Symposium of the ICP-PR Bee Protection Group, Ghent, Belgium, 15–17 September 2014; pp. 219–224. [Google Scholar]
- Whitehorn, P.R.; O’Connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laycock, I.; Lenthall, K.M.; Barratt, A.T.; Cresswell, J.E. Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology 2012, 21, 1937–1945. [Google Scholar] [CrossRef]
- Laycock, I.; Cotterell, K.C.; O’Shea-Wheller, T.A. Effects of the neonicotinoid pesticide thiamethoxam at field-realistic levels on microcolonies of Bombus terrestris worker bumble bees. Ecotoxicol. Environ. Saf. 2014, 100, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Babendreier, D.; Reichhart, B.; Romeis, J.; Bigler, F. Impact of insecticidal proteins expressed in transgenic plants on bumblebee microcolonies. Entomol. Exp. Appl. 2008, 126, 148–157. [Google Scholar] [CrossRef]
- Smagghe, G.; Deknopper, K.; Meeus, I.; Mommaerts, V. Dietary chlorantraniliprole suppresses reproduction in worker bumblebees. Pest Manag. Sci. 2013, 69, 787–791. [Google Scholar] [CrossRef]
- Besard, L.; Mommaerts, V.; Abdu-Alla, G.; Smagghe, G. Lethal and sublethal side-effect assessment supports a more benign profile of spinetoram compared with spinosad in the bumblebee Bombus terrestris. Pest Manag. Sci. 2011, 67, 541–547. [Google Scholar] [CrossRef]
- Barbosa, W.F.; De Meyer, L.; Guedes, R.N.C.; Smagghe, G. Lethal and sublethal effects of azadirachtin on the bumblebee Bombus terrestris (Hymenoptera: Apidae). Ecotoxicology 2015, 24, 130–142. [Google Scholar] [CrossRef] [Green Version]
- Ceuppens, B.; Eeraerts, M.; Vleugels, T.; Cnops, G.; Roldan-Ruiz, I.; Smagghe, G. Effects of dietary lambda-cyhalothrin exposure on bumblebee survival, reproduction, and foraging behavior in laboratory and greenhouse. J. Pest Sci. 2015, 88, 777–783. [Google Scholar] [CrossRef]
- Sutcliffe, G.H.; Plowright, R.C. The effects of food supply on adult size in the bumble bee Bombus terricola Kirby (Hymenoptera: Apidae). Can. Entomol. 1988, 120, 1051–1058. [Google Scholar] [CrossRef]
- Sutcliffe, G.H.; Plowright, R.C. The effects of pollen availability on development time in the bumble bee Bombus terricola K. (Hymenoptera: Apidae). Can. J. Zool. 1990, 68, 1120–1123. [Google Scholar] [CrossRef]
- OECD. Test No. 247: Bumblebee, Acute Oral Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2, No. 247.; OECD Publishing: Paris, France, 2017. [Google Scholar] [CrossRef]
- OECD. Test No. 246: Bumblebee, Acute Contact Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2, No. 246.; OECD Publishing: Paris, France, 2017. [Google Scholar] [CrossRef]
- Klinger, E.G.; Camp, A.A.; Strange, J.P.; Cox-Foster, D.; Lehmann, D.M. Bombus (Hymenoptera: Apidae) microcolonies as a tool for biological understanding and pesticide risk assessment. Environ. Entomol. 2019, 48, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Baer-Imhoof, B.; Millar, A.H.; Baer, B. Consequences of Nosema apis infection for male honey bees and their fertility. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Roberts, K.E.; Evison, S.E.F.; Baer, B.; Hughes, W.O.H. The cost of promiscuity: Sexual transmission of Nosema microsporidian parasites in polyandrous honey bees. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Rangel, J.; Fisher, A.I. Factors affecting the reproductive health of honey bee (Apis mellifera) drones—A review. Apidologie 2019, 50, 759–778. [Google Scholar] [CrossRef] [Green Version]
- Duchateau, M.J.; Velthuis, H.H.W. Development and reproductive strategies in Bombus terrestris colonies. Behaviour 1988, 107, 186–207. [Google Scholar] [CrossRef]
- Padilla, S.C.; Cure, J.R.; Riano, D.A.; Gutierrez, A.P.; Rodriguez, D.; Romero, E. Gyne and drone production in Bombus atratus (Hymenoptera: Apidae). J. Apic. Sci. 2017, 61, 55–72. [Google Scholar] [CrossRef] [Green Version]
- Röseler, P.F. Vergleichende Untersuchungen zur Oogenese bei weiselrichtigen und weisellosen Arbeiterinnen der Hummelart Bombus terrestris. Insectes Sociaux 1974, 21, 249–274. [Google Scholar] [CrossRef]
- Pomeroy, N.; Plowright, R.C. The relation between worker numbers and the production of males and queens in the bumble bee Bombus perplexus. Can. J. Zool. 1982, 60, 954–957. [Google Scholar] [CrossRef]
- Duchateau, M.J.; Marien, J. Sexual biology of haploid and diploid males in the bumble bee Bombus terrestris. Ins. Soc. 1995, 42, 255–266. [Google Scholar] [CrossRef]
- Tasei, J.N.; Moinard, C.; Moreau, L.; Himpens, B.; Guyonnaud, S. Relationship between aging, mating and sperm production in captive Bombus terrestris. J. Apic. Res. 1998, 37, 107–113. [Google Scholar] [CrossRef]
- Yoon, H.J.; Lee, K.Y.; Ko, H.-J. Sexual maturity time of reproductive organ development and mating in the Korean native bumblebee. Korean J. Appl. Entomol. 2018, 57, 329–337. [Google Scholar] [CrossRef]
- Cameron, S.A. Brood care by male bumble bees. Proc. Natl. Acad. Sci. USA 1985, 82, 6371–6373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartar, R.V.; Dill, L.M. Costs of energy shortfall for bumble bee colonies: Predation, social parasitism, and brood development. Can. Entomol. 1991, 123, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Owen, R.E.; Plowright, R.S. Worker-queen conflict and male parentage in bumble bees. Behav. Ecol. Sociobiol. 1982, 11, 91–99. [Google Scholar] [CrossRef]
- Paxton, R.J.; Thoren, P.A.; Estoup, A.; Tengo, J. Queen-worker conflict over male production and the sex ratio in a facultatively polyandrous bumblebee, Bombus hypnorum: The consequences of nest usurpation. Mol. Ecol. 2001, 10, 2489–2498. [Google Scholar] [CrossRef]
- Bourke, A.F.G.; Ratnieks, F.L.W. Kin-selected conflict in the bumble-bee Bombus terrestris (Hymenoptera: Apidae). Proc. R. Soc. Lond. B 2001, 268, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Alaux, C.; Savarit, F.; Jaisson, P.; Hefetz, A. Does the queen win it all? Queen–worker conflict over male production in the bumblebee, Bombus terrestris. Naturwissenschaften 2004, 91, 400–403. [Google Scholar] [CrossRef]
- Owen, R.E.; Rodd, F.H.; Plowright, R.C. Sex ratios in bumble bee colonies: Complications due to orphaning? Behav. Ecol. Sociobiol. 1980, 7, 287–291. [Google Scholar] [CrossRef]
- Bourke, A.F.G. Sex ratios in bumble bees. Phil. Trans. R. Soc. Lond. B 1997, 352, 1921–1933. [Google Scholar] [CrossRef]
- Bulmer, M.G. The significance of protandry in social Hymenoptera. Am. Nat. 1983, 121, 540–551. [Google Scholar] [CrossRef]
- Bulmer, M.G. Models for the evolution of protandry in insects. Theor. Popul. Biol. 1983, 23, 314–322. [Google Scholar] [CrossRef]
- Beekman, M.; van Stratum, P. Bumblebee sex ratios: Why do bumblebees produce so many males? Proc. R. Soc. Lond. B 1998, 265, 1535–1543. [Google Scholar] [CrossRef]
- Ogilvie, J.E.; Thomson, J.D. Male bumble bees are important pollinators of a late-blooming plant. Arthropod-Plant Interact. 2015, 9, 205–213. [Google Scholar] [CrossRef]
- Bertsch, A. Foraging in male bumblebees (Bombus lucorum L.): Maximizing energy or minimizing water load? Oecologia 1984, 62, 325–336. [Google Scholar] [CrossRef]
- Ostevik, K.L.; Manson, J.S.; Thomson, J.D. Pollination potential of male bumble bees (Bombus impatiens): Movement patterns and pollen-transfer efficiency. J. Pollinat. Ecol. 2010, 2, 21–26. [Google Scholar] [CrossRef]
- Colgan, T.J.; Fletcher, I.K.; Arce, A.N.; Gill, R.J.; Rodrigues, A.R.; Stolle, E.; Chittka, L.; Wurm, Y. Caste- and pesticide-specific effects of neonicotinoid pesticide exposure on gene expression in bumblebees. Mol. Ecol. 2019, 28, 1964–1974. [Google Scholar] [CrossRef] [Green Version]
- Kenna, D.; Cooley, H.; Pretelli, I.; Rodrigues, A.R.; Gill, S.D.; Gill, R.J. Pesticide exposure affects flight dynamics and reduces flight endurance in bumblebees. Ecol. Evol. 2019, 9, 5637–5650. [Google Scholar] [CrossRef] [Green Version]
- Mobley, M.W.; Gegear, R.J. One size does not fit all: Caste and sex differences in the response of bumblebees (Bombus impatiens) to chronic oral neonicotinoid exposure. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholer, J.; Krischik, V. Chronic exposure of imidacloprid and clothianidin reduce queen survival, foraging, and nectar storing in colonies of Bombus impatiens. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Fauser-Misslin, A.; Sadd, B.M.; Neumann, P.; Sandrock, C. Influence of combined pesticide and parasite exposure on bumblebee colony traits in the laboratory. J. Appl. Ecol. 2014, 51, 450–459. [Google Scholar] [CrossRef]
- Ellis, C.; Park, K.J.; Whitehorn, P.; David, A.; Goulson, D. The neonicotinoid insecticide thiacloprid impacts upon bumblebee colony development under field conditions. Environ. Sci. Technol. 2017, 51, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef] [Green Version]
- Tasei, J.N.; Lerin, J.; Ripault, G. Sub-lethal effects of imidacloprid on bumblebees, Bombus terrestris (Hymenoptera: Apidae), during a laboratory feeding test. Pest Manag. Sci. 2000, 56, 784–788. [Google Scholar] [CrossRef]
- de Wael, L.; de Greef, M.; van Laere, O. Toxicity of pyriproxifen and fenoxycarb to bumble bee brood using a new method for testing insect growth regulators. J. Apic. Res. 1995, 34, 3–8. [Google Scholar] [CrossRef]
- Mommaerts, V.; Sterk, G.; Smagghe, G. Hazards and uptake of chitin synthesis inhibitors in bumblebees Bombus terrestris. Pest Manag. Sci. 2006, 62, 752–758. [Google Scholar] [CrossRef]
- Camp, A.A.; Batres, M.A.; Williams, W.C.; Lehmann, D.M. Impact of diflubenzuron on Bombus impatiens (Hymenoptera: Apidae) microcolony development. Environ. Entomol. 2020, 49, 203–210. [Google Scholar] [CrossRef]
- Besard, L.; Mommaerts, V.; Vandeven, J.; Cuvelier, X.; Sterk, G.; Smagghe, G. Compatibility of traditional and novel acaricides with bumblebees (Bombus terrestris): A first laboratory assessment of toxicity and sublethal effects. Pest Manag. Sci. 2010, 66, 786–793. [Google Scholar] [CrossRef]
- Ramanaidu, K.; Cutler, G.C. Different toxic and hormetic responses of Bombus impatiens to Beauveria bassiana, Bacillus subtilis and spirotetramat. Pest Manag. Sci. 2012, 69, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Riddell, C.E.; Sumner, S.; Adams, S.; Mallon, E.B. Pathways to immunity: Temporal dynamics of the bumblebee (Bombus terrestris) immune response against a trypanosomal gut parasite. Insect Mol. Biol. 2011, 20, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, P. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 2005, 50, 529–551. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G.; Hultmark, D. Cell-free immunity in insects. Ann. Rev. Microbiol. 1987, 41, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Barribeau, S.M.; Sadd, B.M.; du Plessis, L.; Brown, M.J.; Buechel, S.D.; Cappelle, K.; Carolan, J.C.; Christiaens, O.; Colgan, T.J.; Erler, S.; et al. A depauperate immune repertoire precedes evolution of sociality in bees. Genome Biol. 2015, 16, 83. [Google Scholar] [CrossRef] [Green Version]
- Baer, B.; Schmid-Hempel, P. Phenotypic variation in male and worker encapsulation response in the bumblebee Bombus terrestris. Ecol. Entomol. 2006, 31, 591–596. [Google Scholar] [CrossRef]
- Moret, Y.; Schmid-Hempel, P. Immune defence in bumble-bee offspring. Nature 2001, 414, 506. [Google Scholar] [CrossRef]
- Wilfert, L.; Gadau, J.; Schmid-Hempel, P. The genetic architecture of immune defense and reproduction in male Bombus terrestris bumblebees. Evolution 2007, 61, 804–815. [Google Scholar] [CrossRef]
- Svensson, B.G.; Bergstrom, G. Marking pheromones of Alpinobombus males. J. Chem. Ecol. 1979, 5, 603–615. [Google Scholar] [CrossRef]
- Benton, T. Bumble Bees: The Natural History and Identification of the Species Found in Britain; Collins: London, UK, 2006. [Google Scholar]
- Darvill, B.; Ellis, J.S.; Lye, G.C.; Goulson, D. Population structure and inbreeding in a rare and declining bumblebee, Bombus muscorum (Hymenoptera: Apidae). Mol. Ecol. 2006, 15, 601–611. [Google Scholar] [CrossRef]
- Amin, M.R.; Than, K.K.; Kwon, Y.J. Mating status of bumblebees, Bombus terrestris (Hymenoptera: Apidae) with notes on ambient temperature, age and virginity. Appl. Entomol. Zool. 2010, 45, 363–367. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.R.; Bussière, L.F.; Goulson, D. Effects of male age and size on mating success in the bumblebee Bombus terrestris. J. Insect Behav. 2012, 25, 362–374. [Google Scholar] [CrossRef]
- Paxton, R.J. Male mating behaviour and mating systems of bees: An overview. Apidologie 2005, 36, 145–156. [Google Scholar] [CrossRef]
- Gosterit, A.; Gurel, F. Male remating and its influences on queen colony foundation success in the bumblebee, Bombus terrestris. Apidologie 2016, 47, 828–834. [Google Scholar] [CrossRef] [Green Version]
- Darvill, B.; Lye, G.C.; Goulson, D. Aggregations of male Bombus muscorum (Hymenoptera: Apidae) at mature nests. Incestuous brothers or amorous suitors? Apidologie 2007, 38, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Foster, R.L. Nestmate recognition as an inbreeding avoidance mechanism in bumble bees (Hymenoptera: Apidae). J. Kans. Entomol. Soc. 1992, 65, 238–243. [Google Scholar]
- Zacek, P.; Kalinova, B.; Sobotnik, J.; Hovorka, O.; Ptacek, V.; Coppee, A.; Verheggen, F.; Valterova, I. Comparison of age-dependent quantitative changes in the male labial gland secretion of Bombus terrestris and Bombus lucorum. J. Chem. Ecol. 2009, 35, 698–705. [Google Scholar] [CrossRef] [Green Version]
- Zacek, P.; Prchalova-Hornakova, D.; Tykva, R.; Kindl, J.; Vogel, H.; Svatos, A.; Pichova, I.; Valterova, I. De novo biosynthesis of sexual pheromone in the labial gland of bumblebee males. Chembiochem 2013, 14, 361–371. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Vogt, R.G. The biosynthesis and detection of pheromones and plant volatiles. In Insect Pheromone Biochemistry and Molecular Biology; Blomquist, G.J., Vogt, R.G., Eds.; Elsevier Academic Press: London, UK, 2003; pp. 323–340. [Google Scholar]
- Korner, P.; Schmid-Hempel, P. Effects of sperm on female longevity in the bumble-bee Bombus terrestris L. Proc. R. Soc. Lond. Suppl. Biol. Lett. 2003, 270, S227–S229. [Google Scholar] [CrossRef] [Green Version]
- Baer, B.; Schmid-Hempel, P. Sperm influences female hibernation success, survival and fitness in the bumble-bee Bombus terrestris. Proc. R. Soc. B 2005, 272, 319–323. [Google Scholar] [CrossRef] [Green Version]
- Baer, B.; de Jong, G.; Schmid-Hempel, R.; Schmid-Hempel, P.; Høeg, J.T.; Boomsma, J.J. Heritability of sperm length in the bumblebee Bombus terrestris. Genetica 2006, 127, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Baer, B.; Schmid-Hempel, P.; Høeg, J.T.; Boomsma, J.J. Sperm length, sperm storage and mating system characteristics in bumblebees. Insectes Soc. 2003, 50, 101–108. [Google Scholar] [CrossRef]
- Duvoisin, N.; Baer, B.; Schmid-Hempel, P. Sperm transfer and male competition in a bumblebee. Anim. Behav. 1999, 58, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Sauter, A.; Brown, M.J.F.; Baer, B.; Schmid-Hempel, P. Males of social insects can prevent queens from multiple mating. Proc. Roy. Soc. Lond. B 2001, 268, 1449–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baer, B.; Morgan, E.D.; Schmid-Hempel, P. A nonspecific fatty acid within the bumblebee mating plug prevents females from remating. Proc. Natl. Acad. Sci. USA 2001, 98, 3926–3928. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.J.F.; Baer, B.; Schmid-Hempel, R.; Schmid-Hempel, P. Dynamics of multiple-mating in the bumble bee Bombus hypnorum. Insectes Soc. 2002, 49, 315–319. [Google Scholar] [CrossRef]
- Baer, B.; Maile, R.; Schmid-Hempel, P.; Morgan, E.D.; Jones, G.R. Chemistry of a mating plug in bumblebees. J. Chem. Ecol. 2000, 26, 1869–1875. [Google Scholar] [CrossRef]
- Chen, P.S.; Stumm-Zollinger, E.; Aigaki, T.; Balmer, J.; Bienz, M.; Bohlen, P. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell 1988, 54, 291–298. [Google Scholar] [CrossRef]
- Liang, C.; Ding, G.; Huang, J.; Zhang, X.; Miao, C.; An, J. Characteristics of the two Asian bumblebee species Bombus friseanus and Bombus breviceps (Hymenoptera: Apidae). Insects 2020, 11, 163. [Google Scholar] [CrossRef] [Green Version]
- Crowther, L.P.; Wright, D.J.; Richardson, D.S.; Carvell, C.; Bourke, A.F.G. Spatial ecology of a range-expanding bumble bee pollinator. Ecol. Evol. 2019, 9, 986–997. [Google Scholar] [CrossRef] [Green Version]
- Owen, R.E.; Whidden, T.L. Monandry and polyandry in three species of North American bumble bees (Bombus) determined using microsatellite DNA markers. Can. J. Zool. 2013, 91, 523–528. [Google Scholar] [CrossRef]
- Estoup, A.; Scholl, A.; Pouvreau, A.; Solignac, M. Monoandry and polyandry in bumble bees (Hymenoptera: Bombinae) as evidenced by highly variable microsatellites. Mol. Ecol. 1995, 4, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Röseler, P.F. The number of spermatozoa in the spermatheca of bumble bee queens (Hym., Apoidea, Bombinae). Apidologie 1973, 4, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Sherman, P.W.; Seeley, T.D.; Reeve, H.K. Parasites, pathogens, and polyandry in social Hymenoptera. Am. Nat. 1988, 131, 602–610. [Google Scholar] [CrossRef]
- Shykoff, J.A.; Schmid-Hempel, P. Parasites and the advantage of genetic variability within social insect colonies. Proc. R. Soc. Lond. B 1991, 243, 55–58. [Google Scholar]
- Baer, B.; Schmid-Hempel, P. Experimental variation in polyandry affects parasite loads and fitness in a bumble-bee. Nature 1999, 397, 151–154. [Google Scholar] [CrossRef]
- Baer, B.; Schmid-Hempel, P. Unexpected consequences of polyandry for parasitism and fitness in the bumblebee, Bombus terrestris. Evolution 2001, 55, 1639–1643. [Google Scholar] [CrossRef]
- Bogo, G.; de Manincor, N.; Fisogni, A.; Galloni, M.; Bortolotti, L. Effects of queen mating status, pre-diapause weight and pupae’s sex on colony initiation in small-scale rearing of Bombus terrestris. Apidologie 2017, 48. [Google Scholar] [CrossRef] [Green Version]
- Greeff, M.; Schmid-Hempel, P. Sperm reduces female longevity and increases melanization of the spermatheca in the bumblebee Bombus terrestris L. Insectes Sociaux 2008, 55, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Colgan, T.J.; Finlay, S.; Brown, M.J.F.; Carolan, J. Mating precedes selective immune priming which is maintained throughout bumblebee queen diapause. BMC Genom. 2019, 20, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Goulson, D.; Hanley, M.E.; Darvill, B.; Ellis, J.S.; Knight, M.E. Causes of rarity in bumblebees. Biol. Conserv. 2005, 122, 1–8. [Google Scholar] [CrossRef]
- Mah, Y.I.; Lee, M.Y.; Bilinski, M. Some characteristics of Korean indigenous bumblebee species (Hymenoptera; Bombus spp.) under laboratory conditions. Acta Hortic. 2001, 561, 287–291. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, S.E.; Kim, Y.S. Temperature and humidity favorable for colony development of the indoor-reared bumblebee, Bombus ignitus. Appl. Entomol. Zool. 2002, 37, 419–423. [Google Scholar] [CrossRef] [Green Version]
- Hannan, M.D.; Maeta, Y.; Hoshikawa, K. Colony development of two species of Japanese bumble bees Bombus ignitus and Bombus hypocrita reared under artificial condition. Jpn. J. Entomol. 1997, 65, 343–354. [Google Scholar]
- Asada, S.; Ono, M. Difference in colony development of two Japanese bumblebees, Bombus hypocrita and B. ignitus (Hymenoptera: Apidae). Appl. Entomol. Zool. 2000, 35, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Prieto, D.L.R.; Cure, J.R. Desarrollo de colonias de Bombus atratus (Hymenoptera: Apidae) en cautiverio durante la etapa subsocial. Rev. Fac. Cienc. Basicas. 2012, 8, 28–33. [Google Scholar]
- Salvarrey, S.; Arbulo, N.; Santos, E.; Invernizzi, C. Cría artificial de abejorros nativos Bombus atratus y Bombus bellicosus (Hymenoptera, Apidae). Agrociencia Urug. 2013, 17, 75–82. [Google Scholar]
- Ono, M.; Mitsuhata, M.; Sasaki, M. Use of Introduced Bombus Terrestris Worker Helpers for Rapid Development of Japanese Native B. Hypocrita Colonies (Hymenoptera, Apidae). Appl. Entomol. Zool. 1994, 29, 413–419. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhou, Z.; Huang, J.; Yuan, X.; Ding, G.; An, J. Queen traits and colony size of four bumblebee species of China. Insectes Sociaux 2018, 65, 537–547. [Google Scholar] [CrossRef]
- Huang, J. Species diversity, pollination application and strategy for conservation of the bumblebees of China. Biodivers. Sci. 2018, 26, 486–497. [Google Scholar] [CrossRef]
- Strange, J.P. Bombus huntii, Bombus impatiens, and Bombus vosnesenskii (Hymenoptera: Apidae) Pollinate Greenhouse-Grown Tomatoes in Western North America. J. Econ. Entomol. 2015, 108, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Baer, B. Bumblebees as model organisms to study male sexual selection in social insects. Behav. Ecol. Sociobiol. 2003, 54, 521–533. [Google Scholar] [CrossRef]
| Endpoint Evaluated | Number of Publications |
|---|---|
| Drone biology | 10 |
| Role within the nest | 13 |
| Sensitivity to toxicants | 37 |
| Immunity | 8 |
| Mating behavior | 17 |
| Reproductive measures | 10 |
| Queen success as a result of mating | 16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belsky, J.E.; Camp, A.A.; Lehmann, D.M. The Importance of Males to Bumble Bee (Bombus Species) Nest Development and Colony Viability. Insects 2020, 11, 506. https://doi.org/10.3390/insects11080506
Belsky JE, Camp AA, Lehmann DM. The Importance of Males to Bumble Bee (Bombus Species) Nest Development and Colony Viability. Insects. 2020; 11(8):506. https://doi.org/10.3390/insects11080506
Chicago/Turabian StyleBelsky, Joseph E., Allison A. Camp, and David M. Lehmann. 2020. "The Importance of Males to Bumble Bee (Bombus Species) Nest Development and Colony Viability" Insects 11, no. 8: 506. https://doi.org/10.3390/insects11080506





