Genetic Variation in the Feeding Behavior of Isofemale Lines of Nesidiocoris tenuis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isofemale Lines and Plants
2.2. Phytophagy Experiment
2.3. Zoophagy Experiment
2.4. Statistical Analysis
2.5. Heritability
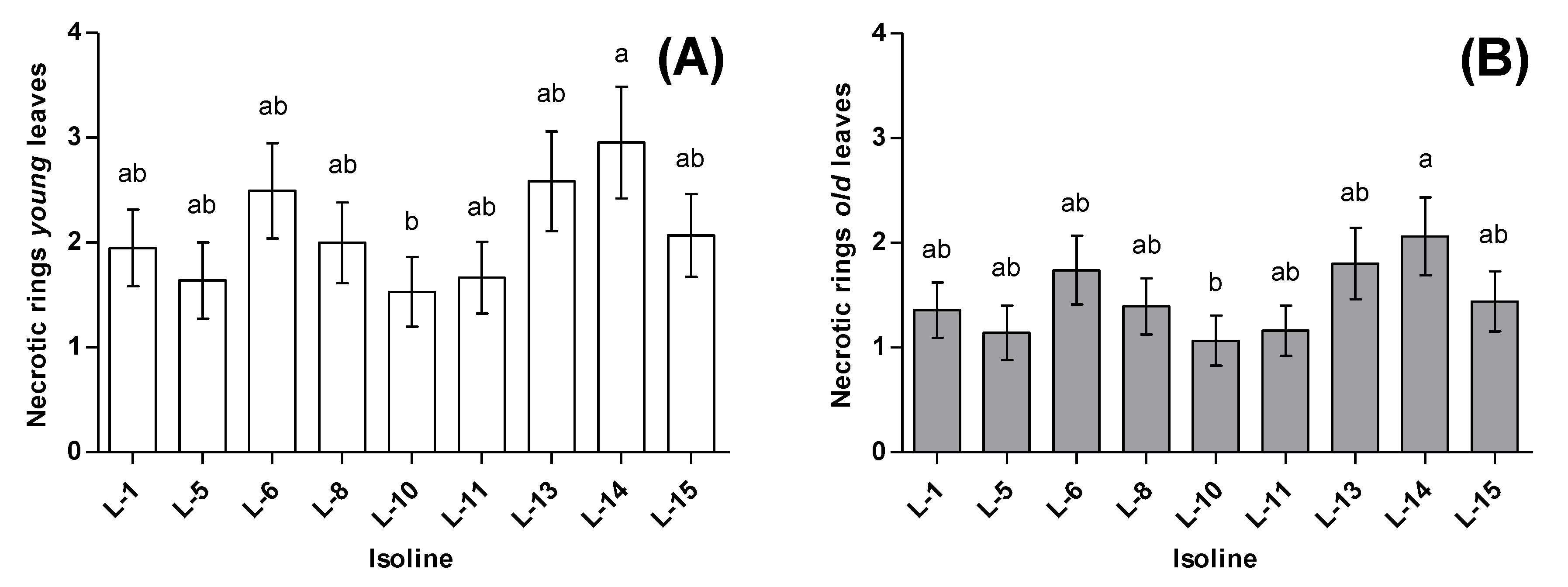
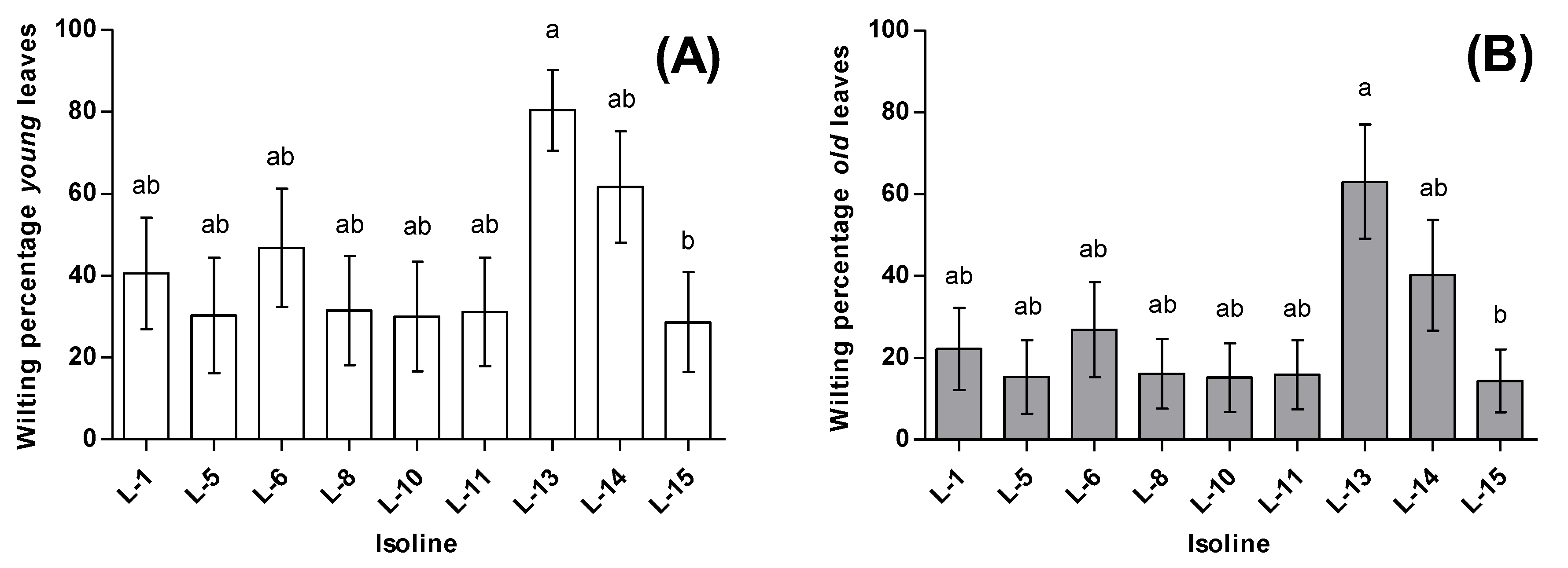
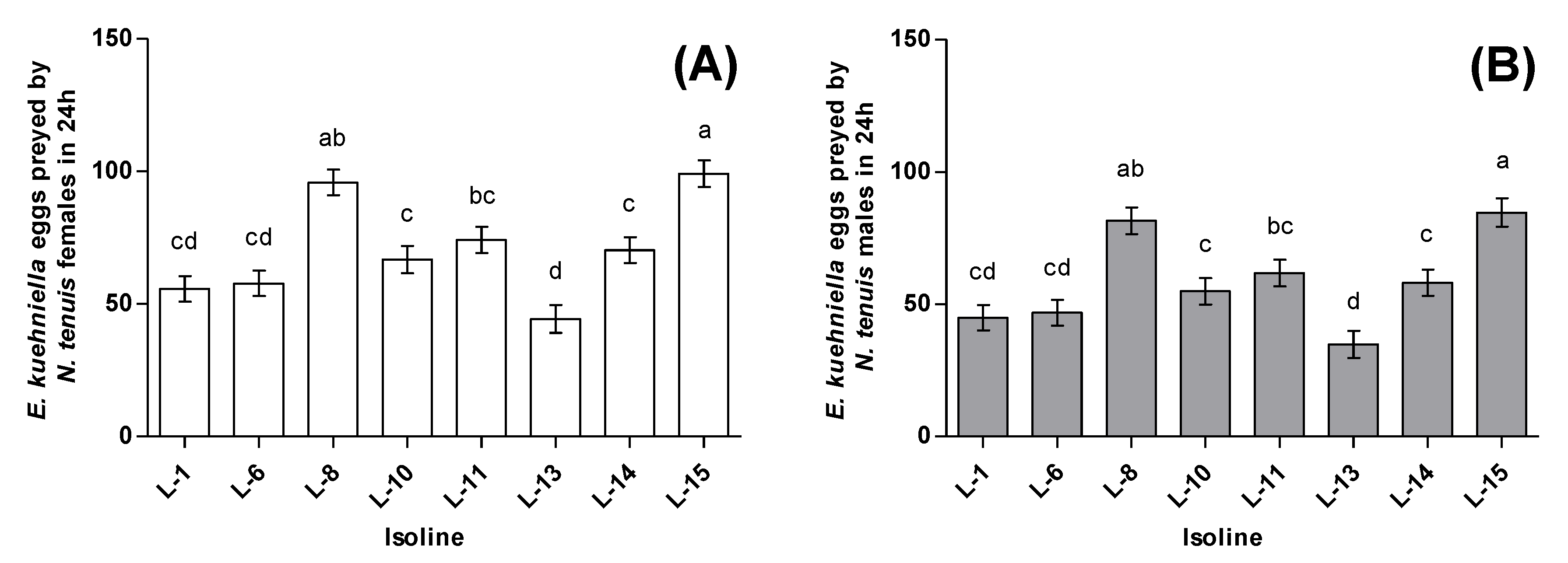
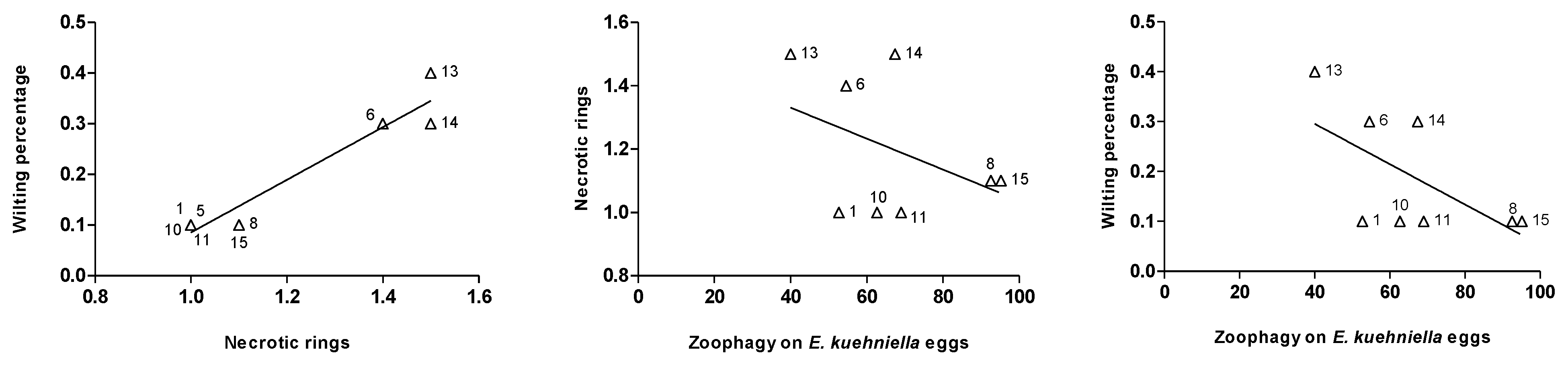
3. Results
3.1. Phytophagy
3.2. Zoophagy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mcgregor, R.R.; Gillespie, D.R.; Quiring, D.M.J.; Foisy, M.R.J. Potential use of Dicyphus hesperus Knight (Heteroptera: Miridae) for biological control of pests of greenhouse tomatoes. Biol. Control 1999, 110, 104–110. [Google Scholar] [CrossRef]
- Alomar, O.; Riudavets, J.; Castañe, C. Macrolophus caliginosus in the biological control of Bemisia tabaci on greenhouse melons. Biol. Control 2006, 36, 154–162. [Google Scholar] [CrossRef]
- Calvo, F.J.; Bolckmans, K.; Belda, J.E. Release rate for a pre-plant application of Nesidiocoris tenuis for Bemisia tabaci control in tomato. BioControl 2012, 57, 809–817. [Google Scholar] [CrossRef]
- Urbaneja, A.; González-Cabrera, J.; Arnó, J.; Gabarra, R. Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag. Sci. 2012, 68, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Zappalà, L.; Biondi, A.; Alma, A.; Al-Jboory, I.J.; Arnò, J.; Bayram, A.; Chailleux, A.; El-Arnaouty, A.; Gerling, D.; Guenaoui, Y.; et al. Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies. J. Pest Sci. 2013, 86, 635–647. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Urbaneja, A. The zoophytophagous predator Nesidiocoris tenuis: A successful but controversial biocontrol agent in tomato crops. In Advances in Insect Control and Resistance Management; Horowitz, A.R., Ishaaya, I., Eds.; Springer: Cham, Switzerland, 2016; pp. 9–26. [Google Scholar]
- Pérez-Hedo, M.; Suay, R.; Alonso, M.; Ruocco, M.; Giorgini, M.; Poncet, C.; Urbaneja, A. Resilience and robustness of IPM in protected horticulture in the face of potential invasive pests. Crop Prot. 2017, 97, 19–127. [Google Scholar] [CrossRef]
- Van Lenteren, J.C.; Alomar, O.; Ravensberg, W.J.; Urbaneja, A. Biological control agents for control of pests in greenhouses. In Integrated Pest and Disease Management in Greenhouse Crops; Gullino, M.L., Nicot, P.C., Albajes, R., Eds.; Springer: Cham, Switzerland, 2020; pp. 409–439. [Google Scholar]
- Dumont, F.; Lucas, E.; Réale, D. Evidence of genetic basis of zoophagy and nymphal developmental time in isogroup lines of the zoophytophagous mullein bug, Campylomma verbasci. BioControl 2016, 61, 425–435. [Google Scholar] [CrossRef]
- Dumont, F.; Aubry, O.; Lucas, E. From Evolutionary aspects of zoophytophagy to biological control. Front. Ecol. Evol. 2018, 6, 221. [Google Scholar] [CrossRef] [Green Version]
- Calvo, J.; Urbaneja, A. Nesidiocoris tenuis un aliado para el control biológico de mosca blanca. Hortic. Int. 2004, 44, 20–25. [Google Scholar] [CrossRef]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Ruescas, D.C.; Tabone, E.; Frandon, J.; et al. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Cabello, T.; Gallego, J.R.; Fernandez, F.J.; Gamez, M.; Vila, E.; Del Pino, M.; Hernandez-Suarez, E. Biological control strategies for the South American Tomato Moth (Lepidoptera: Gelechiidae) in greenhouse tomatoes. J. Econ. Entomol. 2013, 105, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Mollá, O.; Biondi, A.; Alonso-Valiente, M.; Urbaneja, A. A comparative life history study of two mirid bugs preying on Tuta absoluta and Ephestia kuehniella eggs on tomato crops: Implications for biological control. BioControl 2014, 59, 175–183. [Google Scholar] [CrossRef]
- Sanchez, J.A.; La-Spina, M.; Lacasa, A. Numerical response of Nesidiocoris tenuis (Hemiptera: Miridae) preying on Tuta absoluta (Lepidoptera: Gelechiidae) in tomato crops. Eur. J. Entomol. 2014, 111, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Calvo, J.; Bolckmans, K.; Stansly, P.A.; Urbaneja, A. Predation by Nesidiocoris tenuis on Bemisia tabaci and injury to tomato. BioControl 2009, 54, 237–246. [Google Scholar] [CrossRef]
- Arnó, J.; Castañé, C.; Riudavets, J.; Gabarra, R. Risk of damage to tomato crops by the generalist zoophytophagous predator Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae). Bull. Entomol. Res. 2010, 100, 105–115. [Google Scholar] [CrossRef]
- Arnó, J.; Castañé, C.; Riudavets, J.; Roig, J.; Gabarra, R. Characterization of damage to tomato plants produced by the zoophytophagous predator Nesidiocoris tenuis. IOBC/ WPRS Bull 2006, 29, 249–254. [Google Scholar]
- Sánchez, J.A.; Lacasa, A. Impact of the zoophytophagous plant bug Nesidiocoris tenuis (Heteroptera: Miridae) on tomato yield. J. Econ. Entomol. 2008, 101, 1864–1870. [Google Scholar] [CrossRef]
- Cano, M.; Vila, E.; Janssen, D.; Bretones, G.; Salvador, E.; Lara, L.; Tellez, M.M. Selection of refuges for Nesidiocoris tenuis (Het.: Miridae) and Orius laevigatus (Het.: Anthocoridae): Virus reservoir risk assessment. IOBC/wprs Bull 2009, 49, 281–286. [Google Scholar]
- Leung, K.; Ras, E.; Ferguson, K.B.; Ariëns, S.; Babendreier, D.B.; Bijma, P.; Bourtzis, K.; Brodeur, J.; Bruins, M.; Centurión, A.; et al. Next-generation biological control: The need for integrating genetics and genomics. Biol. Rev. 2020. [Google Scholar] [CrossRef]
- Bielza, P.; Balanza, V.; Cifuentes, D.; Mendoza, J.E. Challenges facing arthropod biological control: Identifying traits for genetic improvement of predators in protected crops. Pest Manag. Sci. 2020. [Google Scholar] [CrossRef]
- Le Hesran, S.; Ras, E.; Wajnberg, E.; Beukeboom, L.W. Next generation biological control—An introduction. Entomol. Exp. Appl. 2019, 167, 579–583. [Google Scholar] [CrossRef] [Green Version]
- Kruitwagen, A.; Beukeboom, L.W.; Wertheim, B. Optimization of native biocontrol agents, with parasitoids of the invasive pest Drosophila suzukii as an example. Evol. Appl. 2018, 11, 1473–1497. [Google Scholar] [CrossRef] [PubMed]
- Lommen, S.T.E.; de Jong, P.W.; Pannebakker, B.A. It is time to bridge the gap between exploring and exploiting: Prospects for utilizing intraspecific genetic variation to optimize arthropods for augmentative pest control—A review. Entomol. Exp. Appl. 2017, 162, 108–123. [Google Scholar] [CrossRef]
- Hoy, M.A. Use of genetic improvement in biological control. Agric. Ecosyst. Environ. 1986, 15, 109–119. [Google Scholar] [CrossRef]
- Balanza, V.; Mendoza, J.E.; Bielza, P. Variation in susceptibility and selection for resistance to imidacloprid and thiamethoxam in Mediterranean populations of Orius laevigatus. Entomol. Exp. Appl. 2019, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Lirakis, M.; Magalhães, S. Does experimental evolution produce better biological control agents? A critical review of the evidence. Entomol. Exp. Appl. 2019, 167, 584–597. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, K.B.; Chattington, S.R.; Plouvier, W.N.; Pannebakker, B.A. Genetic variation of traits in natural enemies relevant for biological control: A systematic review. Preprints 2020. [Google Scholar] [CrossRef]
- Beukeboom, L.W.; Zwaan, B.J. Genetics. In Insects as Natural Enemies; Jervis, M., Ed.; Springer: Dordrecht, The Netherlands, 2005; pp. 167–218. [Google Scholar]
- Ferguson, K.B.; Visser, S.; Dalíková, M.; Provazníková, I.; Urbaneja, A.; Pérez-Hedo, M.; Marec, F.; Werren, J.H.; Zwaan, B.J.; Pannebakker, B.A.; et al. Jekyll or Hyde? The genome (and more) of Nesidiocoris tenuis, a zoophytophagous predatory bug that is both a biological control agent and a pest. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Urbaneja, A.; Instituto Valenciano de Investigaciones Agrarias, Moncada, Valencia, Spain. Personal communication, 2016.
- Parsons, P.A. Isofemale strains and evolutionary strategies in natural populations. In Evolutionary Biology; Hecht, M.K., Steere, W.C., Wallace, B., Eds.; Springer: Boston, MA, USA, 1980; Volume 113, pp. 175–217. [Google Scholar]
- Wajnberg, E. Measuring genetic variation in natural enemies used for biological control. In Genetics, Eolution and Biological Control; Ehler, L.E., Sforza, R., Mateille, T., Eds.; CABI Publishing: Oxon, UK, 2004; pp. 19–37. [Google Scholar]
- David, J.R.; Gibert, P.; Legout, H.; Pétavy, G.; Capy, P.; Moreteau, B. Isofemale lines in Drosophila: An empirical approach to quantitative trait analysis in natural populations. Heredity 2005, 94, 3–12. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Parsons, P.A. The analysis of quantitative variation in natural populations with isofemale strains. Genet. Sel. Evol. 1988, 1, 87–98. [Google Scholar] [CrossRef]
- Perdikis, D.; Lucas, E.; Garantonakis, N.; Giatropoulos, A.; Kitsis, P.; Maselou, D.; Panagakis, S.; Paraskevopoulos, A.; Lykouressis, D.; Fantinou, A. Intraguild predation and sublethal interactions between two zoophytophagous mirids, Macrolophus pygmaeus and Nesidiocoris tenuis. Biol. Control 2014, 70, 35–41. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F. Introduction to Quantitative Genetics; Addison Wesley Longman Limited: Essex, UK, 1996. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2009. [Google Scholar]
- Houle, D. Comparing evolvability and variability of quantitative traits. Genetics 1992, 130, 195–204. [Google Scholar] [PubMed]
- Castañé, C.; Arnó, J.; Gabarra, R.; Alomar, O. Plant damage to vegetable crops by zoophytophagous mirid predators. Biol. Control 2011, 59, 22–29. [Google Scholar] [CrossRef]
- Wheeler, A.G. Biology of the Plant Bugs (Hemiptera: Miridae): Pests, Predators, Opportunists; Cornell University Press: Ithaca, NY, USA, 2001. [Google Scholar]
- Thompson, J.N.; Pellmyr, O. Evolution of oviposition behavior and host preference in Lepidoptera. Annu. Rev. Entomol. 1991, 36, 65–89. [Google Scholar] [CrossRef]
- Hedrick, A.V.; Riechert, S.E. Genetically-based variation between two spider populations in foraging behavior. Oecologia 1989, 80, 533–539. [Google Scholar] [CrossRef]
- Merilä, J.; Sheldon, B.C.; Kruuk, L.E.B. Explaining stasis: Microevolutionary studies in natural populations. Genetica 2001, 112–113, 199–222. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Roff, D.A. Natural selection and the heritability of fitness components. Heredity 1987, 59, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Mitter, C.; Futuyma, D.J. An evolutionary–genetic view of host-plant utilization by insects. In Variable Plants and Herbivores in Natural and Managed Systems; Denno, R.F., McClure, M.S., Eds.; Academic Press: New York, NY, USA, 1983; pp. 427–459. [Google Scholar]
- Urbaneja, A.; Tapia, G.; Stansly, P. Influence of host plant and prey availability on developmental time and surviorship of Nesidiocoris tenius (Het.: Miridae). Biocontrol Sci. Technol. 2005, 15, 513–518. [Google Scholar] [CrossRef]
- De Puysseleyr, V.; De Man, S.; Höfte, M.; De Clercq, P. Plantless rearing of the zoophytophagous bug Nesidiocoris tenuis. BioControl 2013, 58, 205–213. [Google Scholar] [CrossRef]
- Nachappa, P.; Margolies, D.C.; Nechols, J.R.; Morgan, T.J. Response of a complex foraging phenotype to artificial selection on its component traits. Evol. Ecol. 2010, 24, 631–655. [Google Scholar] [CrossRef]
- Sánchez, J.A. Zoophytophagy in the plantbug Nesidiocoris tenuis. Agric. For. Entomol. 2008, 10, 75–80. [Google Scholar] [CrossRef]
| Fixed Effects | Random Effects | Akaike Information Criterion (AIC) | ||
|---|---|---|---|---|
| Necrotic Rings | Wilting | Zoophagy | ||
| Leaf + Mortality | Block + Line | 457.21 | 304.56 | |
| Leaf + Mortality | Line | 493.52 | 314.91 | |
| Leaf + Mortality | Block | 463.03 | 310.01 | |
| Leaf | Block + Line | 451.19 | 308.96 | |
| Mortality | Block + Line | 468.64 | 321.92 | |
| Sex | Line | 714.31 | ||
| Sex | 769.89 | |||
| Parameter | n | Mean ± SE * | VG | VE | H2 | CVG (%) |
|---|---|---|---|---|---|---|
| Necrotic rings | 240 | 1.177 ± 0.044 | 0.070 | 0.444 | 0.16 | 22.54 |
| Wilting | 240 | 1.254 ± 0.032 | 0.039 | 0.217 | 0.18 | 15.77 |
| Zoophagy | 193 | 7.961 ± 0.131 | 1.190 | 3.231 | 0.37 | 13.70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinchilla-Ramírez, M.; Pérez-Hedo, M.; Pannebakker, B.A.; Urbaneja, A. Genetic Variation in the Feeding Behavior of Isofemale Lines of Nesidiocoris tenuis. Insects 2020, 11, 513. https://doi.org/10.3390/insects11080513
Chinchilla-Ramírez M, Pérez-Hedo M, Pannebakker BA, Urbaneja A. Genetic Variation in the Feeding Behavior of Isofemale Lines of Nesidiocoris tenuis. Insects. 2020; 11(8):513. https://doi.org/10.3390/insects11080513
Chicago/Turabian StyleChinchilla-Ramírez, Milena, Meritxell Pérez-Hedo, Bart A. Pannebakker, and Alberto Urbaneja. 2020. "Genetic Variation in the Feeding Behavior of Isofemale Lines of Nesidiocoris tenuis" Insects 11, no. 8: 513. https://doi.org/10.3390/insects11080513





