The Microbiome of Neotropical Water Striders and Its Potential Role in Codiversification
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Water Strider Species
2.3. DNA Extraction and Amplification
2.4. Library Preparation
2.5. Data Analysis
2.5.1. Bacterial Diversity and Community Composition
2.5.2. Exploring Phylogenetic Associations between Water Striders and Associated Microbiome
3. Results
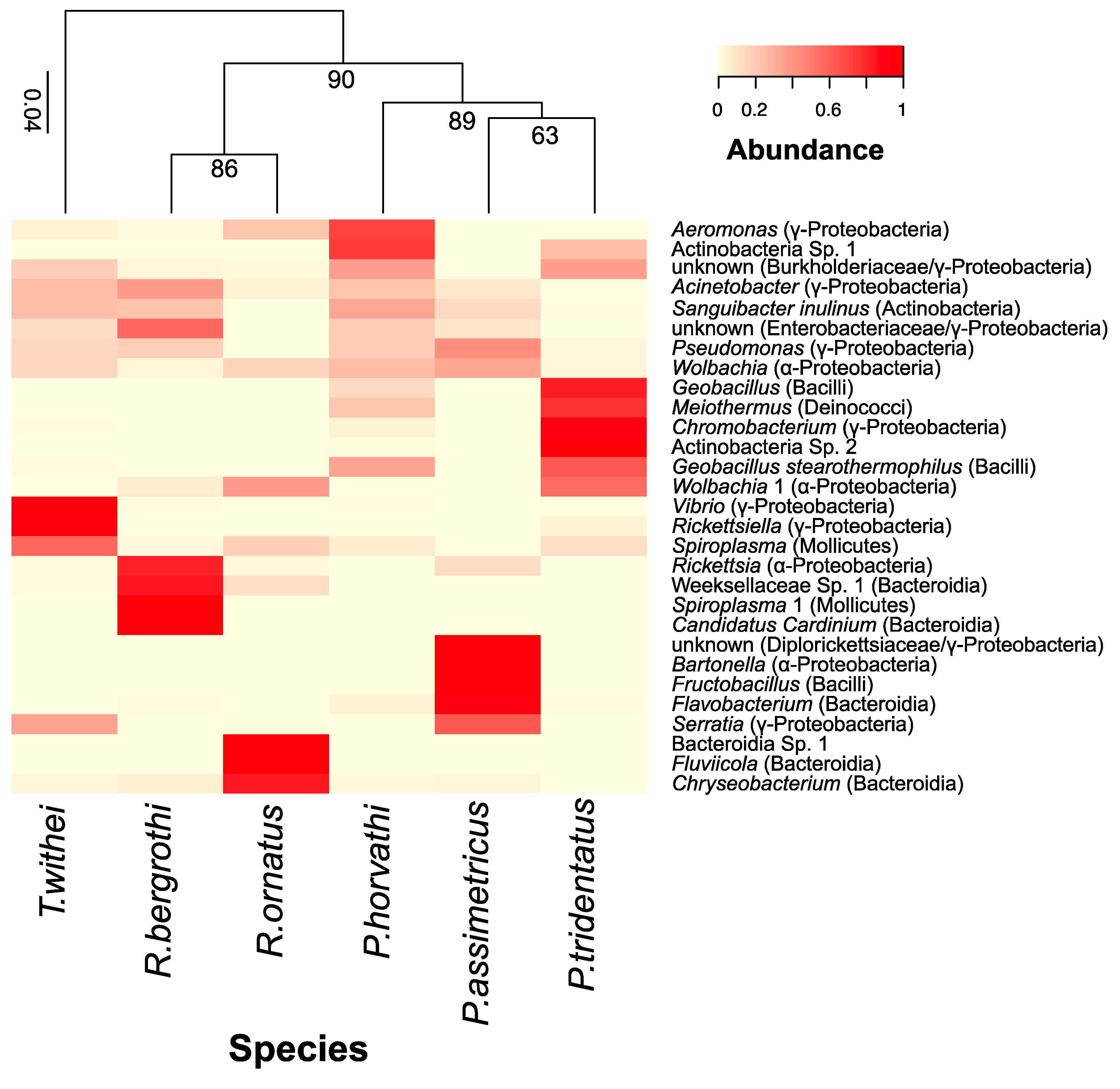
3.1. Community Composition and Diversity of Water Strider Microbiomes
3.2. Phylogenetic Associations
4. Discussion
4.1. Bacterial Diversity and Core Microbiome of Water Striders
4.2. Codiversification between Water Striders and Their Microbiome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fukatsu, T.; Hosokawa, T. Capsule-transmitted gut symbiotic bacterium of the Japanese common plataspid stinkbug, Megacopta punctatissima. Appl. Environ. Microbiol. 2002, 68, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and Virus Protection in Insects. Science 2008, 322, 702. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Schmid-Hempel, P. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc. Natl. Acad. Sci. USA 2011, 108, 19288–19292. [Google Scholar] [CrossRef]
- Motta, E.V.S.; Raymann, K.; Moran, N.A. Glyphosate perturbs the gut microbiota of honey bees. Proc. Natl. Acad. Sci. USA 2018, 115, 10305–10310. [Google Scholar] [CrossRef]
- Teixeira, L.; Ferreira, Á.; Ashburner, M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008, 6, 2753–2763. [Google Scholar] [CrossRef]
- Coon, K.L.; Valzania, L.; McKinney, D.A.; Vogel, K.J.; Brown, M.R.; Strand, M.R. Bacteria-mediated hypoxia functions as a signal for mosquito development. Proc. Natl. Acad. Sci. USA 2017, 114, E5362–E5369. [Google Scholar] [CrossRef]
- Rennison, D.J.; Rudman, S.M.; Schluter, D. Parallel changes in gut microbiome composition and function in parallel local adaptation and speciation. bioRxiv 2019, 1–29. [Google Scholar] [CrossRef]
- Dillon, R.J.; Webster, G.; Weightman, A.J.; Dillon, V.M.; Blanford, S.; Charnley, A.K. Composition of Acridid gut bacterial communities as revealed by 16S rRNA gene analysis. J. Invertebr. Pathol. 2008, 97, 265–272. [Google Scholar] [CrossRef]
- Janson, E.M.; Stireman, J.O.; Singer, M.S.; Abbot, P. Phytophagous insect-microbe mutualisms and adaptive evolutionary diversification. Evolution 2008, 62, 997–1012. [Google Scholar] [CrossRef]
- Vavre, F.; Kremer, N. Microbial impacts on insect evolutionary diversification: From patterns to mechanisms. Curr. Opin. Insect Sci. 2014, 4, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Schuler, H.; Egan, S.P.; Hood, G.R.; Busbee, R.W.; Driscoe, A.L.; Ott, J.R. Diversity and distribution of Wolbachia in relation to geography, host plant affiliation and life cycle of a heterogonic gall wasp. BMC Evol. Biol. 2018, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Poinsot, D.; Charlat, S.; Merçot, H. On the mechanism of Wolbachia-induced cytoplasmic incompatibility: Confronting the models with the facts. BioEssays 2003, 25, 259–265. [Google Scholar] [CrossRef]
- Kikuchi, Y. Endosymbiotic Bacteria in Insects: Their Diversity and Culturability. Microbes Environ. 2009, 24, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.J.; Hunter, M.S.; Zchori-Fein, E. The emerging diversity of Rickettsia. Proc. R. Soc. B Biol. Sci. 2006, 273, 2097–2106. [Google Scholar] [CrossRef]
- Moran, N.A.; McCutcheon, J.P.; Nakabachi, A. Genomics and Evolution of Heritable Bacterial Symbionts. Annu. Rev. Genet. 2008, 42, 165–190. [Google Scholar] [CrossRef]
- Zchori-Fein, E.; Perlman, S.J. Distribution of the bacterial symbiont. Mol. Ecol. 2004, 2009–2016. [Google Scholar] [CrossRef]
- Poff, K.E.; Stever, H.; Reil, J.B.; Seabourn, P.; Ching, A.J.; Aoki, S.; Logan, M.; Michalski, J.R.; Santamaria, J.; Adams, J.W.; et al. The native Hawaiian insect microbiome initiative: A critical perspective for Hawaiian insect evolution. Insects 2017, 8, 130. [Google Scholar] [CrossRef]
- Shropshire, J.D.; Bordenstein, S.R. Speciation by symbiosis: The microbiome and behavior. MBio 2016, 7. [Google Scholar] [CrossRef]
- Brucker, R.M.; Bordenstein, S.R. Speciation by symbiosis. Trends Ecol. Evol. 2012, 27, 443–451. [Google Scholar] [CrossRef]
- Lundgren, J.G.; Lehman, R.M.; Chee-sanford, J. Bacterial Communities within Digestive Tracts of Ground Beetles (Coleoptera: Carabidae). Ann. Entomol. Soc. Am. 2007, 100, 275–282. [Google Scholar] [CrossRef]
- Cheng, L. Marine Insects; Scripps Institution of Oceanography, University of California: La Jolla, CA, USA, 2005. [Google Scholar]
- Stonedahl, G.M.; Lattin, J.D. The Gerridae or Water Striders of Oregon and Washington (Hemiptera: Heteroptera). Tech. Bull. Agric. Exp. Station. Oregon State Univ. 1982, 144, 1–36. [Google Scholar]
- Izabella, O.; Pawel, B.; Piotr, J.; Lee, S.D. Diet of Water Striders (Gerris lacustris L. 1758) in a Rice Field Near Seoul, Korea. J. Asia. Pac. Entomol. 2007, 10, 85–88. [Google Scholar] [CrossRef]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B. An Introduction to Aquatic Insects of North America, 4th ed.; Kendall/Hunt Publishing Company: Dubuque, IA, USA, 2008. [Google Scholar]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B.; Novak, J.A.; Higgins, M.J.; Wessell, K.J.; Lessard, J.L. Development and application of a macroinvertebrate functional-group approach in the bioassessment of remmant river oxbows in southwest Florida. J. N. Am. Benthol. Soc. 2002, 21, 290–310. [Google Scholar] [CrossRef]
- Nummelin, M. Waterstriders (Het: Gerridae) as predators of hatching mosquitoes. Bicovas 1988, 1, 121–125. [Google Scholar]
- Pacheco, B. Diversidad Taxonómica y Distribución de los Chinches Patinadores (Hemiptera: Gerridae) en Costa Rica. Licentiate Thesis, Universidad de Costa Rica, San Pedro, Costa Rica, 2012. [Google Scholar]
- Chen, B.; Teh, B.S.; Sun, C.; Hu, S.; Lu, X.; Boland, W.; Shao, Y. Biodiversity and Activity of the Gut Microbiota across the Life History of the Insect Herbivore Spodoptera littoralis. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Mizrahi-Man, O.; Davenport, E.R.; Gilad, Y. Taxonomic Classification of Bacterial 16S rRNA Genes Using Short Sequencing Reads: Evaluation of Effective Study Designs. PLoS ONE 2013, 8, e53608. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 1–17. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.A.; Holmes, S.P. DADA2: High resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 4–5. [Google Scholar] [CrossRef]
- Suenami, S.; Konishi Nobu, M.; Miyazaki, R. Community analysis of gut microbiota in hornets, the largest eusocial wasps, Vespa mandarinia and V. simillima. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, B.; Smith, C.B.; Thomas, C.M.; Esson, K.; Marin, R.; Preston, C.M.; Birch, J.M.; Scholin, C.A.; Huntemann, M.; Clum, A.; et al. Microbial metagenomes and metatranscriptomes during a coastal phytoplankton bloom. Sci. Data 2019, 6, 1–7. [Google Scholar] [CrossRef]
- R Development Core. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008. [Google Scholar]
- Callahan, B.J.; Sankaran, K.; Fukuyama, J.A.; McMurdie, P.J.; Holmes, S.P. Bioconductor workflow for microbiome data analysis: From raw reads to community analyses. F1000Research 2016, 5, 1492. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Horikoshi, M.; Li, W. Ggfortify: Unified interface to visualize statistical results of popular r packages. R J. 2016, 8, 478–489. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.0-10. 2017. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 18 December 2017).
- Wilkinson, L. Exact and approximate area-proportional circular Venn and Euler diagrams. IEEE Trans. Vis. Comput. Graph. 2012, 18, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.B.; Black, M.; Hoeh, W.; Lutz, R. DNA primers for amplification of mitochondrial Cytochrome c Oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Meyer, C.P. Molecular systematics of cowries (Gastropoda: Cypraeidae) and diversification patterns in the tropics. Biol. J. Linn. Soc. 2003, 79, 401–459. [Google Scholar] [CrossRef]
- De León, L.F.; Cornejo, A.; Gavilán, R.G.; Aguilar, C. Hidden biodiversity in Neotropical streams: DNA barcoding uncovers high endemicity of freshwater macroinvertebrates at small spatial scales. PLoS ONE 2020, 15, e0231683. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Liaw, W.H.A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; Schwartz, M.; et al. Package “gplots”: Various R programming tools for plotting data. R Packag. Version 2.17.0. 2016, pp. 1–68. Available online: https://cran.r-project.org/package=gplots (accessed on 28 August 2020).
- Azambuja, P.; Feder, D.; Garcia, E.S. Isolation of Serratia marcescens in the midgut of Rhodnius prolixus: Impact on the establishment of the parasite Trypanosoma cruzi in the vector. Exp. Parasitol. 2004, 107, 89–96. [Google Scholar] [CrossRef]
- Montenegro, H.; Solferini, V.N.; Klaczko, L.B.; Hurst, G.D.D. Male-killing Spiroplasma naturally infecting Drosophila melanogaster. Insect Mol. Biol. 2005, 14, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Minard, G.; Tran, F.H.; Raharimalala, F.N.; Hellard, E.; Ravelonandro, P.; Mavingui, P.; Valiente Moro, C. Prevalence, genomic and metabolic profiles of Acinetobacter and Asaia associated with field-caught Aedes albopictus from Madagascar. FEMS Microbiol. Ecol. 2013, 83, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Sazama, E.J.; Bosch, M.J.; Shouldis, C.S.; Ouellette, S.P.; Wesner, J.S. Incidence of Wolbachia in aquatic insects. Ecol. Evol. 2017, 7, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Kolasa, M.; Kubisz, D.; Mazur, M.A.; Ścibior, R.; Kajtoch, Ł. Wolbachia prevalence and diversity in selected riverine predatory beetles (Bembidiini and Paederini). Bull. Insectol. 2018, 71, 193–200. [Google Scholar]
- González, C.T.; Saltonstall, K.; Fernández-marín, H. Garden microbiomes of Apterostigma dentigerum and Apterostigma pilosum fungus-growing ants (Hymenoptera: Formicidae ). J. Microbiol. 2019, 57. [Google Scholar] [CrossRef]
- Abbott, D.W.; Boraston, A.B. Structural Biology of Pectin Degradation by Enterobacteriaceae. Microbiol. Mol. Biol. Rev. 2008, 72, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Esemu, S.N.; Dong, X.; Kfusi, A.J.; Hartley, C.S.; Ndip, R.N.; Ndip, L.M.; Darby, A.C.; Post, R.J.; Makepeace, B.L. Aquatic Hemiptera in Southwest Cameroon: Biodiversity of Potential Reservoirs of Mycobacterium ulcerans and multiple Wolbachia sequence types revealed by metagenomics. Diversity 2019, 11, 225. [Google Scholar] [CrossRef]
- Endo, A.; Tanizawa, Y.; Tanaka, N.; Maeno, S.; Kumar, H.; Shiwa, Y.; Okada, S.; Yoshikawa, H.; Dicks, L.; Nakagawa, J.; et al. Comparative genomics of Fructobacillus spp. and Leuconostoc spp. reveals niche-specific evolution of Fructobacillus spp. BMC Genom. 2015, 16. [Google Scholar] [CrossRef]
- Maddaloni, M.; Hoffman, C.; Pascual, D.W. Paratransgenesis feasibility in the honeybee (Apis mellifera) using Fructobacillus fructosus commensal. J. Appl. Microbiol. 2014, 117, 1572–1584. [Google Scholar] [CrossRef]
- Endo, A.; Maeno, S.; Tanizawa, Y.; Kneifel, W.; Arita, M.; Dicks, L.; Salminen, S. Fructophilic lactic acid bacteria, a unique group of fructose-fermenting microbes. Appl. Environ. Microbiol. 2018, 84, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. Fructophilic lactic acid bacteria inhabit fructose-rich niches in nature. Microb. Ecol. Health Dis. 2012, 23. [Google Scholar] [CrossRef] [PubMed]
- Janashia, I.; Choiset, Y.; Rabesona, H.; Hwanhlem, N.; Bakuradze, N.; Chanishvili, N.; Haertlé, T. Protection of honeybee Apis mellifera by its endogenous and exogenous lactic flora against bacterial infections. Ann. Agrar. Sci. 2016, 14, 177–181. [Google Scholar] [CrossRef]
- Su, Q.; Zhou, X.; Zhang, Y. Symbiont-mediated functions in insect hosts. Commun. Integr. Biol. 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- Bouchon, D.; Cordaux, R.; Grève, P. Rickettsiella, Intracellular Pathogens of Arthropods. In Manipulative Tenants; Zchori-Fein, E., Bourtzis, K., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 127–148. [Google Scholar]
- Abdelaziz, M.; Ibrahem, M.D.; Ibrahim, M.A.; Abu-Elala, N.M.; Abdel-moneam, D.A. Monitoring of different vibrio species affecting marine fishes in Lake Qarun and Gulf of Suez: Phenotypic and molecular characterization. Egypt. J. Aquat. Res. 2017, 43, 141–146. [Google Scholar] [CrossRef]
- Hughes, J. Population Genetic Structure in Stream Insects: What Have We Learned. In Aquatic Insects: Challenges to Populations; Lancaster, J., Briers, R.A., Eds.; CAB International: Wallingford, UK, 2008; pp. 268–288. [Google Scholar]
- Dugas, J.E.; Zurek, L.; Paster, B.J.; Keddie, B.A.; Leadbetter, E.R. Isolation and characterization of a Chryseobacterium strain from the gut of the American cockroach, Periplaneta americana. Arch. Microbiol. 2001, 175, 259–262. [Google Scholar] [CrossRef]
- Bloch, K.C.; Nadarajah, R.; Jacobs, R. Chryseobacterium meningosepticum: An emerging pathogen among immunocompromised adults. Report of 6 cases and literature review. Medicine 1997, 76, 30–41. [Google Scholar] [CrossRef]
- Pruzzo, C.; Huq, A.; Colwell, R.R.; Donelli, G. Pathogenic vibrio species in the marine and estuarine environment. Ocean. Health Pathog. Mar. Environ. 2005, 217–252. [Google Scholar] [CrossRef]
- Thompson, F.; Iida, T.; Swings, J. Biodiversity of Vibrios. Microbiol. Mol. Biol. Rev. 2004, 68, 403–431. [Google Scholar] [CrossRef]
- Morrissey, E.M.; Franklin, R.B.; Kent, A. Evolutionary history influences the salinity preference of bacterial taxa in wetland soils. Front. Microbiol. 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Y.; Liu, Y.; Qiao, F.; Chen, L.; Liu, W.T.; Du, Z.; Li, E. Response of gut microbiota to salinity change in two euryhaline aquatic animals with reverse salinity preference. Aquaculture 2016, 454, 72–80. [Google Scholar] [CrossRef]
- Gallardo, K.; Candia, J.E.; Remonsellez, F.; Escudero, L.V.; Demergasso, C.S. The ecological coherence of temperature and salinity tolerance interaction and pigmentation in a non-marine Vibrio isolated from salar de atacama. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Truong, A.; Sondossi, M.; Clark, J.B. Genetic characterization of Wolbachia from Great Salt Lake brine flies. Symbiosis 2017, 72, 95–102. [Google Scholar] [CrossRef]
- Krams, I.A.; Kecko, S.; Jõers, P.; Trakimas, G.; Elferts, D.; Krams, R.; Luoto, S.; Rantala, M.J.; Inashkina, I.; Gudrā, D.; et al. Microbiome symbionts and diet diversity incur costs on the immune system of insect larvae. J. Exp. Biol. 2017, 220, 4204–4212. [Google Scholar] [CrossRef] [PubMed]
- Ditrich, T.; Papáček, M. Differences in prey capture in semiaquatic bugs (Heteroptera: Gerromorpha). Entomol. Sci. 2016, 19, 34–41. [Google Scholar] [CrossRef]
- Falush, D.; Wirth, T.; Linz, B.; Pritchard, J.K.; Stephens, M.; Kidd, M.; Blaser, M.J.; Graham, D.Y.; Vacher, S.; Perez-perez, G.I.; et al. Traces of Human Migrations in Helicobacter pylori Populations. Science 2003, 299, 1582–1585. [Google Scholar] [CrossRef]
- Moeller, A.H.; Gomes-Neto, J.C.; Mantz, S.; Kittana, H.; Segura Munoz, R.R.; Schmaltz, R.J.; Ramer-Tait, A.E.; Nachman, M.W. Experimental Evidence for Adaptation to Species-Specific Gut Microbiota in House Mice. mSphere 2019, 4. [Google Scholar] [CrossRef]
- Kropáčková, L.; Těšický, M.; Albrecht, T.; Kubovčiak, J.; Čížková, D.; Tomášek, O.; Martin, J.F.; Bobek, L.; Králová, T.; Procházka, P.; et al. Codiversification of gastrointestinal microbiota and phylogeny in passerines is not explained by ecological divergence. Mol. Ecol. 2017, 26, 5292–5304. [Google Scholar] [CrossRef]
- Ren, T.; Kahrl, A.F.; Wu, M.; Cox, R.M. Does adaptive radiation of a host lineage promote ecological diversity of its bacterial communities? A test using gut microbiota of Anolis lizards. Mol. Ecol. 2016, 25, 4793–4804. [Google Scholar] [CrossRef]
- Moran, N.A.; Sloan, D.B. The Hologenome Concept: Helpful or Hollow? PLoS Biol. 2015, 13, 1–10. [Google Scholar] [CrossRef]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia?—A statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, J.P.; Outreman, Y.; Mieuzet, L.; Simon, J.C. Bacterial communities associated with host-adapted populations of pea aphids revealed by deep sequencing of 16S ribosomal DNA. PLoS ONE 2015, 10, e0120664. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J.; Lu, F.; Cheng, J.-A.; Jiang, M.-X.; Way, M.O. Identification and Biological Role of the Endosymbionts Wolbachia in Rice Water Weevil (Coleoptera: Curculionidae). Environ. Entomol. 2012, 41, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Zchori-Fein, E.; Borad, C.; Harari, A.R. Oogenesis in the date stone beetle, Coccotrypes dactyliperda, depends on symbiotic bacteria. Physiol. Entomol. 2006, 31, 164–169. [Google Scholar] [CrossRef]
- Stouthamer, R.; Breeuwer, J.A.J.; Hurst, G.D.D. Wolbachia Pipientis: Microbial Manipulator of Arthropod Reproduction. Annu. Rev. Microbiol. 1999, 53, 71–102. [Google Scholar] [CrossRef]
- Hiroki, M.; Kato, Y.; Kamito, T.; Miura, K. Feminization of genetic males by a symbiotic bacterium in a butterfly, Eurema hecabe (Lepidoptera: Pieridae). Naturwissenschaften 2002, 89, 167–170. [Google Scholar] [CrossRef]
- Hurst, G.D.D.; Jigging, F.M.; Von Der Schulenburg, J.H.G.; Bertrand, D.; West, S.A.; Goriacheva, I.I.; Zakharov, I.A.; Werren, J.H.; Stouthamer, R.; Majerus, M.E.N. Male-killing Wolbachia in two species of insect. Proc. R. Soc. B Biol. Sci. 1999, 266, 735–740. [Google Scholar] [CrossRef]
- Telschow, A.; Hammerstein, P.; Werren, J.H. The effect of Wolbachia versus genetic incompatibilities on reinforcement and speciation. Evolution 2005, 59, 1607–1619. [Google Scholar] [CrossRef]
- Harumoto, T.; Fukatsu, T.; Lemaitre, B. Common and unique strategies of male killing evolved in two distinct Drosophila symbionts. Proc. R. Soc. B Biol. Sci. 2018, 285. [Google Scholar] [CrossRef]




| Site | Species | Number |
|---|---|---|
| Playa Reina lagoon | Rheumatobates bergrothi | 5 |
| Rheumatobates ornatus | 3 | |
| Telmatometra withei | 5 | |
| Río Angulito | Potamobates horvathi | 4 |
| Potamobates tridentatus | 3 | |
| Telmatometra withei | 5 | |
| Río Negro | Platygerris assimetricus | 5 |
| Potamobates horvathi | 4 | |
| Telmatometra withei | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, A.M.; Saltonstall, K.; Arias, C.F.; Chavarria, K.A.; Ramírez-Camejo, L.A.; Mejía, L.C.; De León, L.F. The Microbiome of Neotropical Water Striders and Its Potential Role in Codiversification. Insects 2020, 11, 578. https://doi.org/10.3390/insects11090578
Castillo AM, Saltonstall K, Arias CF, Chavarria KA, Ramírez-Camejo LA, Mejía LC, De León LF. The Microbiome of Neotropical Water Striders and Its Potential Role in Codiversification. Insects. 2020; 11(9):578. https://doi.org/10.3390/insects11090578
Chicago/Turabian StyleCastillo, Anakena M., Kristin Saltonstall, Carlos F. Arias, Karina A. Chavarria, Luis A. Ramírez-Camejo, Luis C. Mejía, and Luis F. De León. 2020. "The Microbiome of Neotropical Water Striders and Its Potential Role in Codiversification" Insects 11, no. 9: 578. https://doi.org/10.3390/insects11090578
APA StyleCastillo, A. M., Saltonstall, K., Arias, C. F., Chavarria, K. A., Ramírez-Camejo, L. A., Mejía, L. C., & De León, L. F. (2020). The Microbiome of Neotropical Water Striders and Its Potential Role in Codiversification. Insects, 11(9), 578. https://doi.org/10.3390/insects11090578









