Temperature-Dependent Functional Response of Harmonia axyridis (Coleoptera: Coccinellidae) on the Eggs of Spodoptera litura (Lepidoptera: Noctuidae) in Laboratory
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Cultures
2.1.1. Mass Rearing of H. axyridis
2.1.2. Mass Rearing of S. litura
2.2. Functional Response
2.3. Data Analysis
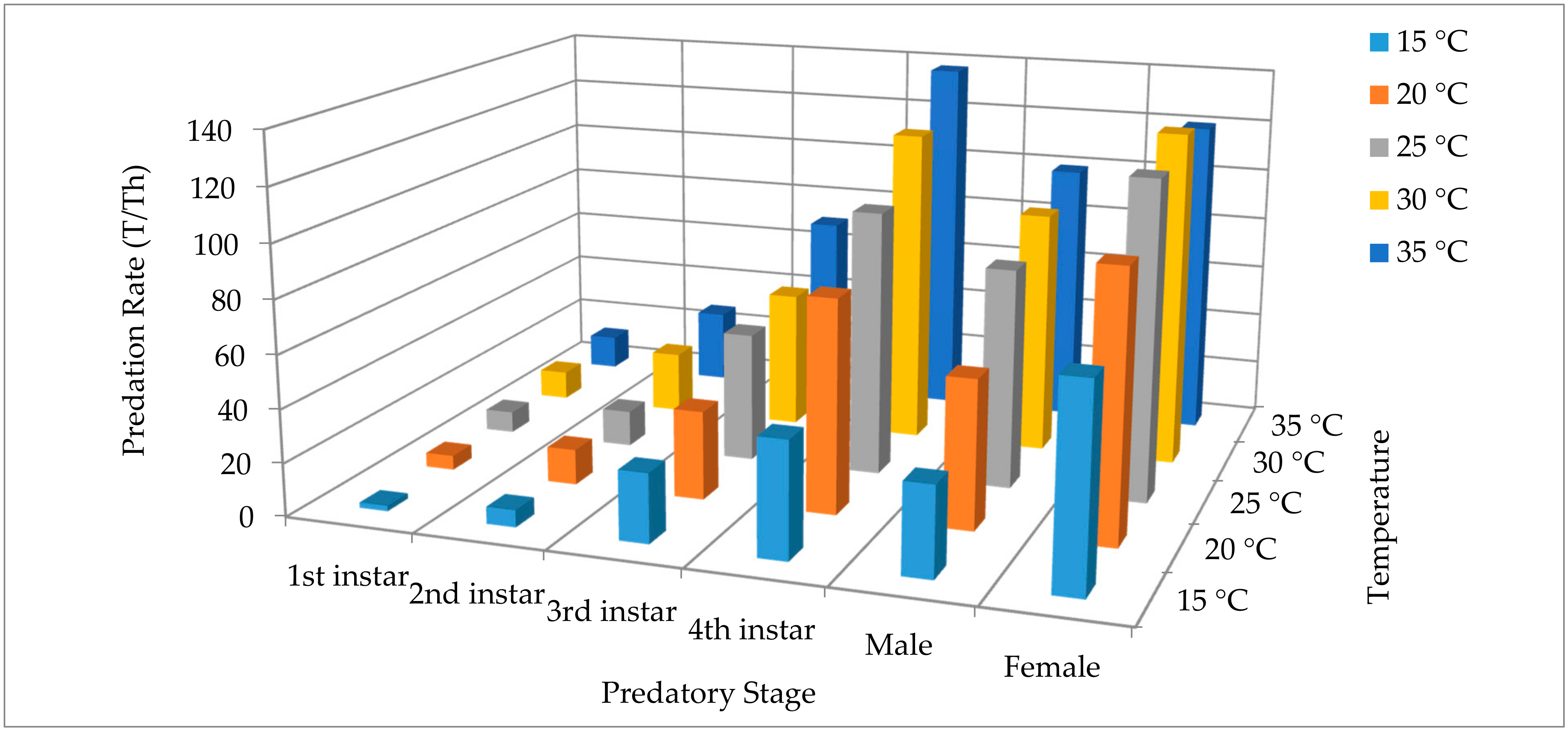
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, Z.; Chen, Z.; Xu, Z. Potential of trap crops for integrated management of the tropical armyworm. Spodoptera litura in tobacco. J. Insect Sci. 2010, 10, 1–11. [Google Scholar] [CrossRef]
- Ahmad, M.; Ghaffar, A.; Rafiq, M. Host plants of leaf worm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in Pakistan. Asian J. Agric. Biol. 2013, 1, 23–28. [Google Scholar]
- Ahmad, M.; Arif, M.I.; Ahmad, M. Occurrence of insecticide resistance in field populations of Spodoptera litura (Lepidoptera: Noctuidae) in Pakistan. Crop Prot. 2007, 26, 809–817. [Google Scholar] [CrossRef]
- Lee, K.P.; Raubenheimer, D.; Behmer, S.T.; Simpson, S.J. A correlation between macronutrient balancing and insect host-plant range: Evidence from the specialist caterpillar Spodoptera exempta (Walker). J. Insect Physiol. 2003, 49, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.I.; Arshad, M.; Afzal, M.; Khalid, S.; Saleem, M.; Mustafa, I.; Iftikhar, Y.; Molina-Ochoa, J.; Foster, J.E. Incidence of Spodoptera litura (Lepidoptera: Noctuidae) and its feeding potential on various citrus (Sapindales: Rutaceae) cultivars in the Sargodha Region of Pakistan. Fla. Entomol. 2016, 99, 192–195. [Google Scholar] [CrossRef]
- Shah, F.M.; Razaq, M.; Ali, Q.; Ali, A.; Shad, S.A.; Aslam, M.; Hardy, I.C.W. Action threshold development in cabbage pest management using synthetic and botanical insecticides. Entomol. Gen. 2020, 40, 157–172. [Google Scholar] [CrossRef]
- Shah, F.M.; Razaq, M.; Ali, Q.; Shad, S.A.; Aslam, M.; Hardy, I.C.W. Field evaluation of synthetic and neem-derived alternative insecticides in developing action thresholds against cauliflower pests. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Tuan, S.J.; Li, N.J.; Yeh, C.C.; Tang, L.-C.; Chi, H. Effects of green manure cover crops on Spodoptera litura (Lepidoptera: Noctuidae) populations. J. Econ. Entomol. 2014, 107, 897–905. [Google Scholar] [CrossRef]
- Hole, U.; Jadhav, S.; Teli, V. Bio-efficacy of Insecticides against Spodoptera litura (Fab.) infesting Soybean. Ann. Plant Protect. Sci. 2009, 17, 322–324. [Google Scholar]
- Razaq, M.; Shah, F.M.; Ahmad, S.; Afzal, M. Pest Management for Agronomic Crops. In Agronomic Crops; Hasanuzzaman, M., Ed.; Springer: Singapore, 2019; pp. 365–384. [Google Scholar]
- Caldas, E.D. Toxicological Aspects of Pesticides. In Sustainable Agrochemistry; Vaz, S., Jr., Ed.; Springer: Cham, Switzerland, 2019; pp. 275–305. [Google Scholar]
- Wiklund, K.; Dich, J.; Holm, L. Risk of malignant lymphoma in Swedish pesticide appliers. Br. J. Cancer 1987, 56, 505–508. [Google Scholar] [CrossRef]
- Huang, J.; Hu, R.; Pray, C.; Qiao, F.; Rozelle, S. Biotechnology as an alternative to chemical pesticides: A case study of Bt cotton in China. J. Agric. Econ. 2003, 29, 55–67. [Google Scholar] [CrossRef]
- Jin, S.; Zhou, J.; Ye, J. Adoption of HACCP system in the Chinese food industry: A comparative analysis. Food Control 2008, 19, 823–828. [Google Scholar] [CrossRef]
- Pu, J.; Wang, Z.; Chung, H. Climate change and the genetics of insecticide resistance. J. Pest Manag. Sci. 2020, 76, 846–852. [Google Scholar] [CrossRef]
- Kalauni, D.; Joshi, A. Pesticides Import, Use, Consumption and Residue Status among Food Crops in Nepal: A Review. Big Data Agric. 2019, 1, 21–25. [Google Scholar] [CrossRef]
- Arthropod Pesticide Resistance Database Home Page. Available online: http://www.pesticideresistance.org (accessed on 6 December 2018).
- Cheema, H.K.; Kang, B.; Jindal, V.; Kaur, S.; Gupta, V. Biochemical mechanisms and molecular analysis of fenvalerate resistant population of Spodoptera litura (Fabricius). J. Crop Prot. 2020, 127, 104951. [Google Scholar] [CrossRef]
- Gore, J.; Adamczyk, J.J., Jr. Laboratory selection for beet armyworm (Lepidoptera: Noctuidae) resistance to methoxyfenozide. Fla. Entomol. 2004, 87, 450–453. [Google Scholar] [CrossRef]
- Dhaliwal, G.; Jindal, V.; Dhawan, A. Insect pest problems and crop losses: Changing trends. Indian J. Ecol. 2010, 37, 1–7. [Google Scholar]
- Ahmad, M.; Mehmood, R. Monitoring of resistance to new chemistry insecticides in Spodoptera litura (Lepidoptera: Noctuidae) in Pakistan. J. Econ. Entomol. 2015, 108, 1279–1288. [Google Scholar] [CrossRef]
- Zhou, Z. A review on control of tobacco caterpillar, Spodoptera litura. Chin. Bull. Entomol. 2009, 46, 354–361. [Google Scholar]
- Juen, A.; Hogendoorn, K.; Ma, G.; Schmidt, O.; Keller, M.A. Analysing the diets of invertebrate predators using terminal restriction fragments. J. Pest Sci. 2012, 85, 89–100. [Google Scholar] [CrossRef]
- Ali, S.; Li, S.; Jaleel, W.; Khan, M.M.; Wang, J.; Zhou, X. Using a Two-Sex Life Table Tool to Calculate the Fitness of Orius strigicollis as a Predator of Pectinophora gossypiella. Insects 2020, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Obrycki, J.J.; Harwood, J.D.; Kring, T.J.; O’Neil, R.J. Aphidophagy by Coccinellidae: Application of biological control in agroecosystems. Biol. Control 2009, 51, 244–254. [Google Scholar] [CrossRef]
- Holling, C. Principles of insect predation. Annu. Rev. Entomol. 1961, 6, 163–182. [Google Scholar] [CrossRef]
- Murdoch, W.W.; Oaten, A. Predation and population stability. Adv. Ecol. Res. 1975, 9, 1–131. [Google Scholar]
- Solomon, M. The natural control of animal populations. J. Anim. Ecol. 1949, 18, 1–35. [Google Scholar] [CrossRef]
- Fernández-Arhex, V.; Corley, J.C. The functional response of Lbalia leucospoides (Hymenoptera: Ibaliidae), a parasitoid of Sirex noctilio (Hymenoptera: Siricidae). Biocontrol Sci. Technol. 2005, 15, 207–212. [Google Scholar] [CrossRef]
- Van Lenteren, J.; Hemerik, L.; Lins, J.C.; Bueno, V.H. Functional responses of three neotropical mirid predators to eggs of Tuta absoluta on tomato. Insects 2016, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Cédola, C.V.; Sánchez, N.E.; Liljesthröm, G.G. Effect of tomato leaf hairiness on functional and numerical response of Neoseiulus californicus (Acari: Phytoseiidae). Exp. Appl. Acarol. 2001, 25, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Koveos, D.S.; Broufas, G.D. Functional response of Euseius finlandicus and Amblyseius andersoni to Panonychus ulmi on apple and peach leaves in the laboratory. Exp. Appl. Acarol. 2000, 24, 247–256. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, N.; Siddiqui, A.; Tripathi, C. Prey-predator relationship between Lipaphis erysimi Kalt. (Hom., Aphididae) and Coccinella septempunctata L. (Col., Coccinellidae). II. Effect of host plants on the functional response of the predator. J. Appl. Entomol. 1999, 123, 591–601. [Google Scholar] [CrossRef]
- Madadi, H.; Enkegaard, A.; Brodsgaard, H.; Kharrazi-Pakdel, A.; Mohaghegh, J.; Ashouri, A. Host plant effects on the functional response of Neoseiulus cucumeris to onion thrips larvae. J. Appl. Entomol. 2007, 131, 728–733. [Google Scholar] [CrossRef]
- Skirvin, D.; Fenlon, J. Plant species modifies the functional response of Phytoseiulus persimilis (Acari: Phytoseiidae) to Tetranychus urticae (Acari: Tetranychidae): Implications for biological control. Bull. Entomol. Res. 2001, 91, 61–67. [Google Scholar] [PubMed]
- Shah, M.A.; Khan, A. Functional response-a function of predator and prey species. Bioscan 2013, 8, 751–758. [Google Scholar]
- Castagnoli, M.; Simoni, S. Effect of long-term feeding history on functional and numerical response of Neoseiulus californicus (Acari: Phytoseiidae). Exp. Appl. Acarol. 1999, 23, 217–234. [Google Scholar] [CrossRef]
- Donnelly, B.E.; Phillips, T.W. Functional response of Xylocoris flavipes (Hemiptera: Anthocoridae)-effects of prey species and habitat. Environ. Entomol. 2001, 30, 617–624. [Google Scholar] [CrossRef]
- Hoddle, M.S. The effect of prey species and environmental complexity on the functional response of Franklinothrips orizabensis: A test of the fractal foraging model. Ecol. Entomol. 2003, 28, 309–318. [Google Scholar] [CrossRef]
- Farhadi, R.; Allahyari, H.; Juliano, S.A. Functional response of larval and adult stages of Hippodamia variegata (Coleoptera: Coccinellidae) to different densities of Aphis fabae (Hemiptera: Aphididae). Environ. Entomol. 2010, 39, 1586–1592. [Google Scholar] [CrossRef]
- Sharma, P.; Verma, S.; Chandel, R.; Shah, M.; Gavkare, O. Functional response of Harmonia dimidiata (fab.) to melon aphid, Aphis gossypii Glover under laboratory conditions. Phytoparasitica 2017, 45, 373–379. [Google Scholar] [CrossRef]
- Jalali, M.A.; Tirry, L.; De Clercq, P. Effect of temperature on the functional response of Adalia bipunctata to Myzus persicae. BioControl 2010, 55, 261–269. [Google Scholar] [CrossRef]
- McCaffrey, J.; Horsburgh, R. Functional response of Orius insidiosus (Hemiptera: Anthocoridae) to the European red mite, Panonychus ulmi (Acari: Tetranychidae), at different constant temperatures. Environ. Entomol. 1986, 15, 532–535. [Google Scholar] [CrossRef]
- Clercq, D. Functional response of the predators Podisus maculiventris (Say) and Podisus nigrispinus (Dallas) (Het., Pentatomidae) to the beet armyworm, Spodoptera exigua (Hübner) (Lep., Noctuidae): Effect of temperature. J. Appl. Entomol. 2001, 125, 131–134. [Google Scholar]
- Skirvin, D.J.; Fenlon, J.S. The effect of temperature on the functional response of Phytoseiulus persimilis (Acari: Phytoseiidae). Exp. Appl. Acarol. 2003, 31, 37. [Google Scholar] [CrossRef]
- Koch, R. The multicolored Asian lady beetle, Harmonia axyridis: A review of its biology, uses in biological control, and non-target impacts. Insect Sci. 2003, 3. [Google Scholar] [CrossRef]
- Roy, H.E.; Brown, P.M.; Adriaens, T.; Berkvens, N.; Borges, I.; Clusella-Trullas, S.; Comont, R.F.; De Clercq, P.; Eschen, R.; Estoup, A. The harlequin ladybird, Harmonia axyridis: Global perspectives on invasion history and ecology. Biol. Invasions 2016, 18, 997–1044. [Google Scholar] [CrossRef]
- Tedders, W.; Schaefer, P. Release and establishment of Harmonia axyridis (Coleoptera: Coccinellidae) in the southeastern United States. Entomol. News 1994, 105, 228–243. [Google Scholar]
- Koch, R.L.; Hutchison, W.D.; Venette, R.; Heimpel, G.E. Susceptibility of immature monarch butterfly, Danaus plexippus (Lepidoptera: Nymphalidae: Danainae), to predation by Harmonia axyridis (Coleoptera: Coccinellidae). Biol. Control 2003, 28, 265–270. [Google Scholar] [CrossRef]
- Luo, H. Functional response of Harmonia axyridis to the density of Rhopalosiphum prunifoliae. Nat. Enem. Insects 1987, 9, 84–87. [Google Scholar]
- Hu, Y.; Wang, Z.; Ning, C.; Pi, Z.; Gao, G. The functional response of Harmonia (Leis) axyridis to their prey of Cinara sp. Nat. Enemies Insects 1989, 11, 164–168. [Google Scholar]
- He, J.; Ma, E.; Shen, Y.; Chen, W.; Sun, X. Observations of the biological characteristics of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). J. Shanghai Agric. Coll. 1994, 12, 119–124. [Google Scholar]
- Seo, M.; Youn, Y. The Asian ladybird, Harmonia axyridis, as biological control agents: I. Predacious behavior and feeding ability. Kor. J. App. Entomol. 2000, 39, 59–71. [Google Scholar]
- Ul Haq, R.; Khan, J.; Ali, G. Rearing of Spodoptera litura (Fabricius) on different artificial diets and its parasitization with Trichogramma chilonis (Ishii). Pak. J. Zool. 2015, 47, 169–175. [Google Scholar]
- Ayyub, M.B.; Nawaz, A.; Arif, M.J.; Amrao, L. Individual and combined impact of nuclear polyhedrosis virus and spinosad to control the tropical armyworm, Spodoptera litura (Fabricius)(Lepidoptera: Noctuidae), in cotton in Pakistan. Egypt. J. Biol. Pest Control 2019, 29, 67. [Google Scholar] [CrossRef]
- Hassanpour, M.; Mohaghegh, J.; Iranipour, S.; Nouri-Ganbalani, G.; Enkegaard, A. Functional response of Chrysoperla carnea (Neuroptera: Chrysopidae) to Helicoverpa armigera (Lepidoptera: Noctuidae): Effect of prey and predator stages. Insect Sci. 2011, 18, 217–224. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Juliano, S.A. Nonlinear curve fitting: Predation and functional response curves. In Design and Analysis of Ecological Experiments; Scheiner, S.M., Gurevitch, J., Eds.; Chapman and Hall: New York, NY, USA, 2001; Volume 2, pp. 178–196. [Google Scholar]
- Rogers, D. Random search and insect population models. J. Anim. Ecol. 1972, 1, 369–383. [Google Scholar] [CrossRef]
- Pritchard, D.; Paterson, R.; Bovy, H.; Barrios-O’Neill, D. Frair: An R package for fitting and comparing consumer functional responses. Methods Ecol. Evol. 2017, 8, 1528–1534. [Google Scholar] [CrossRef]
- R core Team. R: A Language and Environment for Statistical Computing; Version 4.00; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 24 May 2020).
- Hassell, M. The Spatial and Temporal Dynamics of Host-Parasitoid Interactions; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Wu, P.; Zhang, J.; Haseeb, M.; Yan, S.; Kanga, L. Functional responses and intraspecific competition in the ladybird Harmonia axyridis (Coleoptera: Coccinellidae) provided with Melanaphis sacchari (Homoptera: Aphididae) as prey. Eur. J. Entomol. 2018, 115, 232–241. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, T.J. Functional response of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) to Aphis gossypii Glover (Homoptera: Aphididae) in the laboratory. Biol. Control 2004, 31, 306–310. [Google Scholar] [CrossRef]
- Moura, R.; Cabral, S.; Soares, A.O. Does pirimicarb affect the voracity of the euriphagous predator, Coccinella undecimpunctata L. (Coleoptera: Coccinellidae)? Biol. Control 2006, 38, 363–368. [Google Scholar] [CrossRef]
- Timms, J.; Oliver, T.; Straw, N.; Leather, S. The effects of host plant on the coccinellid functional response: Is the conifer specialist Aphidecta obliterata (L.) (Coleoptera: Coccinellidae) better adapted to spruce than the generalist Adalia bipunctata (L.) (Coleoptera: Coccinellidae)? Biol. Control 2008, 47, 273–281. [Google Scholar] [CrossRef]
- Mogi, M. Predation response of the larvae of Harmonia axyridis Pallas (Coccinellidae) to different prey density. Jpn. J. Appl. Entomol. Zool. 1969, 13, 9–16. [Google Scholar] [CrossRef]
- Hu, G. Functional Response of larvae of three ladybirds, Adonia variegata, Coccinella septempunctata and C. transversogutata to the aphid Schizaphis graminum (Homoptera; Aphididae). Nat. Enemies Insects 1992, 14, 180–185. [Google Scholar]
- Wu, H.; Cheng, X.; Zou, Y.; Wei, C.; Lu, F.; Ma, F. Predatism of Harmonia axyridis adults on different ranges of starvation to Myzus persicae. J. Anhui Agric. Coll. 2000, 27, 348–351. [Google Scholar]
- Deng, J.; Tan, Z.; Shan, Q.; Wu, X.; Liu, J. Functional Responses and density Interference Effect in Harmonis axyridis Pallas A Predator to Myzus nicotianae (Blackman). J. Southwest Agric Univ. 2002, 24, 433–435. [Google Scholar]
- McCoull, C.; Swain, R.; Barnes, R. Effect of temperature on the functional response and components of attack rate in Naucoris congrex Stål (Hemiptera: Naucoridae). Aust. J. Entomol. 1998, 37, 323–327. [Google Scholar] [CrossRef]
- De Clercq, P.; Degheele, D. Quality of predatory bugs of the genus Podisus (Heteroptera: Pentatomidae) reared on natural and artificial diets. In Proceedings of the 7th Workshop of the IOBC Global Working Group ‘Quality Control of Mass Reared Arthropods’, Rimini, Italy, 13–16 September 1993; pp. 129–142. [Google Scholar]
- Thompson, D.J. Towards a realistic predator-prey model: The effect of temperature on the functional response and life history of larvae of the damselfly, Ischnura elegans. J. Anim. Ecol. 1978, 47, 757–767. [Google Scholar] [CrossRef]
- Gresens, S.E.; Cothran, M.L.; Thorp, J.H. The influence of temperature on the functional response of the dragonfly Celithemis fasciata (Odonata: Libellulidae). Oecologia 1982, 53, 281–284. [Google Scholar] [CrossRef]
- Bailey, P. The effect of water temperature on the functional response of the water stick insect Ranatra dispar (Heteroptera: Nepidae). Aust. J. Entomol. 1989, 14, 381–386. [Google Scholar] [CrossRef]
- Anderson, M.T.; Kiesecker, J.M.; Chivers, D.P.; Blaustein, A.R. The direct and indirect effects of temperature on a predator prey relationship. Can. J. Zool. 2001, 79, 1834–1841. [Google Scholar]
- Gotoh, T.; Nozawa, M.; Yamaguchi, K. Prey consumption and functional response of three acarophagous species to eggs of the two-spotted spider mite in the laboratory. Appl. Entomol. Zool. 2004, 39, 97–105. [Google Scholar] [CrossRef]
- Xue, Y.; Bahlai, C.A.; Frewin, A.; Sears, M.; Schaafsma, A.; Hallett, R.H. Predation by Coccinella septempunctata and Harmonia axyridis (Coleoptera: Coccinellidae) on Aphis glycines (Homoptera: Aphididae). Environ. Entomol. 2009, 38, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Holling, C.S. Some characteristics of simple types of predation and parasitism. J. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Seko, T.; Miura, K. Functional response of the lady beetle Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) on the aphid Myzus persicae (Sulzer) (Homoptera: Aphididae). Appl. Entomol. Zool. 2008, 43, 341–345. [Google Scholar] [CrossRef]
- Hodek, I. Diapause/dormancy. In Ecology and Behaviour of the Ladybird Beetles (Coccinellidae); Hodek, I., van Emden, H.F., Honk, A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; pp. 275–342. [Google Scholar]
- Athan, R.; Guldal, H. Prey density-dependent feeding activity and life history of Scymnus subvillosus Goeze. (Coleoptera: Coccinellidae). Phytoparasitica 2009, 37, 35–41. [Google Scholar] [CrossRef]
- Zarghami, S.; Mossadegh, M.S.; Kocheili, F.; Allahyari, H.; Rasekh, A. Functional responses of Nephus arcuatus Kapur (Coleoptera: Coccinellidae), the most important predator of spherical mealybug Nipaecoccus viridis (Newstead). Psyche A J. Entomol. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Ganjisaffar, F.; Perring, T.M. Prey stage preference and functional response of the predatory mite Galendromus flumenis to Oligonychus pratensis. Biol. Control 2015, 82, 40–45. [Google Scholar] [CrossRef]
- Aljetlawi, A.A.; Sparrevik, E.; Leonardsson, K. Prey–predator size-dependent functional response: Derivation and rescaling to the real world. J. Anim Ecol. 2004, 73, 239–252. [Google Scholar] [CrossRef]
- Price, P.W. Resource-driven terrestrial interaction webs. Ecol. Res. 2002, 17, 241–247. [Google Scholar] [CrossRef]
- Uiterwaal, S.F.; DeLong, J.P. Multiple factors, including arena size, shape the functional responses of ladybird beetles. J. Appl. Ecol. 2018, 55, 2429–2438. [Google Scholar] [CrossRef]
- Englund, G.; Öhlund, G.; Hein, C.L.; Diehl, S. Temperature dependence of the functional response. Ecol. Lett. 2011, 14, 914–921. [Google Scholar] [CrossRef]
- Vasseur, D.A.; McCann, K.S. A mechanistic approach for modeling temperature-dependent consumer-resource dynamics. Am. Nat. 2005, 166, 184–198. [Google Scholar] [CrossRef]
- Vucic-Pestic, O.; Ehnes, R.B.; Rall, B.C.; Brose, U. Warming up the system: Higher predator feeding rates but lower energetic efficiencies. Glob. Chang. Biol. 2011, 17, 1301–1310. [Google Scholar] [CrossRef]
- Sentis, A.; Hemptinne, J.-L.; Brodeur, J. Using functional response modeling to investigate the effect of temperature on predator feeding rate and energetic efficiency. Oecologia 2012, 169, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Kareiva, P. The spatial dimension in pest-enemy interactions. In Critical Issues in Biological Control; Mackauer, M., Ehler, L.E., Roland, J., Eds.; Intercept: Andover, MA, USA, 1990; pp. 213–227. [Google Scholar]
- Zamani, A.; Talebi, A.; Fathipour, Y.; Baniameri, V. Temperature-dependent functional response of two aphid parasitoids, Aphidius colemani and Aphidius matricariae (Hymenoptera: Aphidiidae), on the cotton aphid. J. Pest Sci. 2006, 79, 183–188. [Google Scholar] [CrossRef]
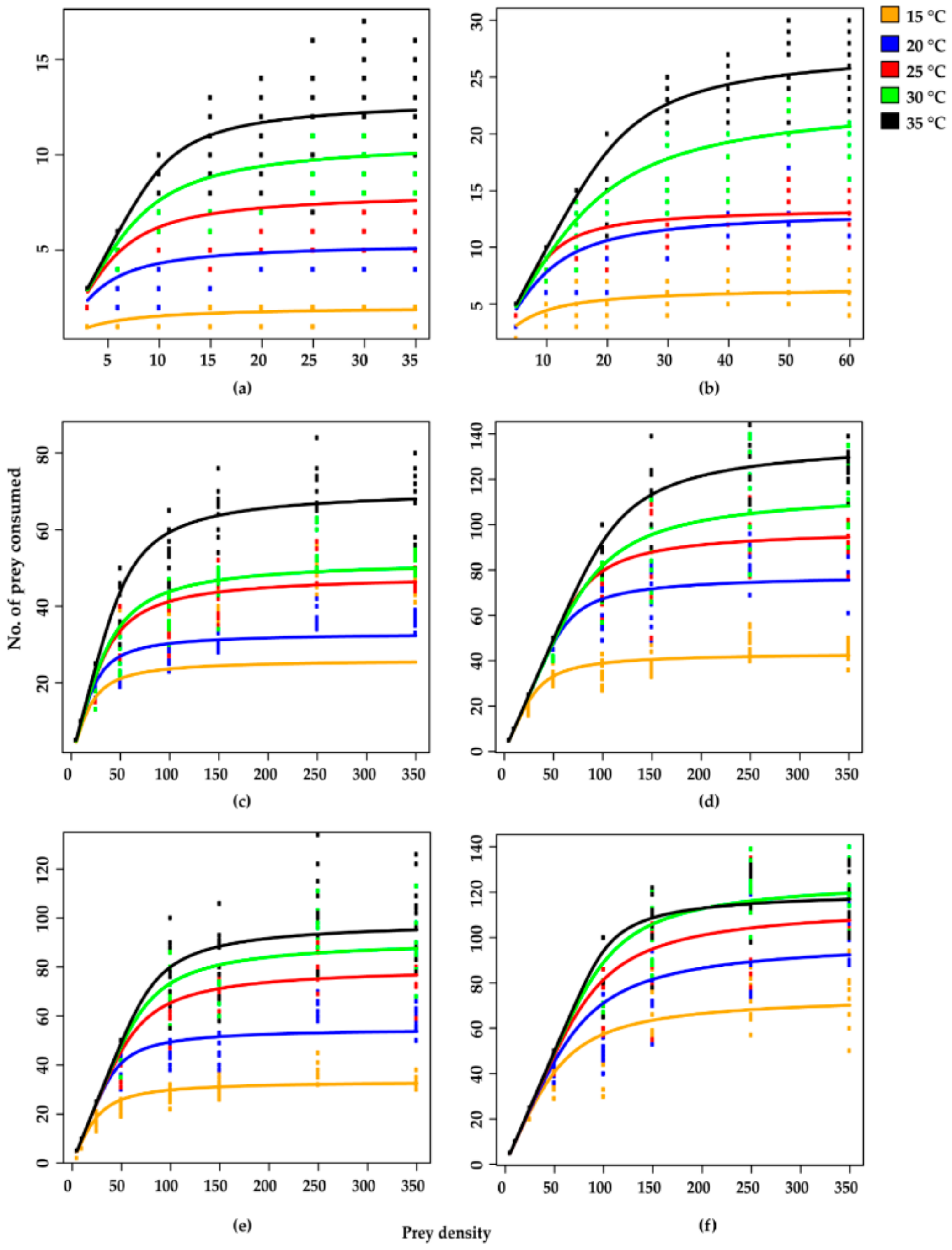
| Predator Stage | Temperature | Mean | ||||
|---|---|---|---|---|---|---|
| 15 °C | 20 °C | 25 °C | 30 °C | 35 °C | ||
| 1st instar | 1.61 ± 0.12 a/A | 4.39 ± 0.37 a/B | 6.35 ± 0.62 a/BC | 8.04 ± 0.94 a/C | 9.78 ± 1.31 a/C | 6.03 ± 1.42 |
| 2nd instar | 5.23 ± 0.39 b/A | 10.14 ± 1.00 ab/B | 11.08 ± 1.12 a/B | 14.95 ± 2.07 ab/BC | 18.34 ± 2.79 ab/C | 11.95 ± 2.23 |
| 3rd instar * | 18.89 ± 3.19 c/* | 23.78 ± 4.19 bc/* | 31.21 ± 6.28 b/* | 33.03 ± 6.83 bc/* | 43.05 ± 9.50 bc/* | 29.99 ± 4.14 |
| 4th instar * | 29.50 ± 5.57 cd/* | 47.69 ± 10.93 c/* | 56.24 ± 13.90 b/* | 60.11 ± 15.45 c/* | 68.71 ± 18.49 c/* | 52.45 ± 6.66 |
| Male * | 23.16 ± 4.07 cd/* | 36.56 ± 7.57 c/* | 47.10 ± 10.81 b/* | 52.18 ± 12.52 c/* | 56.58 ± 14.06 c/* | 43.12 ± 6.00 |
| Female * | 42.70 ± 9.90 d/* | 52.91 ± 13.18 c/* | 59.95 ± 15.39 b/* | 65.60 ± 17.46 c/* | 65.78 ± 17.21 c/* | 57.39 ± 4.36 |
| Mean | 20.18 ± 6.25 | 29.24 ± 8.10 | 35.32 ± 9.36 | 38.98 ± 9.83 | 43.70 ± 10.12 | |
| Predator Stage | Temperature | Mean | ||||
|---|---|---|---|---|---|---|
| 15 °C | 20 °C | 25 °C | 30 °C | 35 °C | ||
| 1st instar | 0.14 ± 0.03 a/A | 0.36 ± 0.08 */AB | 0.49 ± 0.10 b/B | 0.59 ± 0.09 b/B | 0.68 ± 0.09 b/B | 0.45 ± 0.10 |
| 2nd instar | 0.28 ± 0.07 ab/A | 0.50 ± 0.09 */AB | 0.55 ± 0.10 ab/C | 0.66 ± 0.08 ab/C | 0.77 ± 0.08 b/C | 0.55 ± 0.08 |
| 3rd instar | 0.43 ± 0.13 ab/* | 0.50 ± 0.13 */* | 0.56 ± 0.13 */* | 0.58 ± 0.13 */* | 0.67 ± 0.12 */* | 0.55 ± 0.04 |
| 4th instar | 0.56 ± 0.13 b/* | 0.70 ± 0.12 */* | 0.75 ± 0.11 */* | 0.77 ± 0.10 */* | 0.82 ± 0.09 */* | 0.72 ± 0.04 |
| Male | 0.48 ± 0.13 b/* | 0.62 ± 0.13 */* | 0.69 ± 0.11 */* | 0.73 ± 0.11 */* | 0.75 ± 0.11 */* | 0.65 ± 0.05 |
| Female | 0.65 ± 0.12 b/* | 0.72 ± 0.11 */* | 0.77 ± 0.10 */* | 0.80 ± 0.09 */* | 0.81 ± 0.10 */* | 0.75 ± 0.03 |
| Mean | 0.42 ± 0.08 | 0.57 ± 0.06 | 0.64 ± 0.05 | 0.69 ± 0.04 | 0.75 ± 0.03 | |
| Temperatures | Growth Stages | Parameters | Estimates | S.E. | Z-Value | Pr (z) |
|---|---|---|---|---|---|---|
| 15 °C | 1st instar | Intercept | −0.074 | 0.60 | −0.124 | 0.9017 |
| Linear | −0.242 | 0.12 | −1.929 | 0.0537 | ||
| 2nd instar | Intercept | 1.47 | 4.10 × 10−01 | 3.595 | <0.05 | |
| Linear | −2.08 × 10−01 | 4.73 × 10−02 | −4.405 | 1.06 × 10−05 | ||
| 3rd instar | Intercept | 1.61 | 1.38 × 10−01 | 11.67 | <2 × 10−16 | |
| Linear | −4.76 × 10−02 | 3.13 × 10−03 | −15.20 | <2 × 10−16 | ||
| 4th instar | Intercept | 3.21 | 1.77 × 10−01 | 18.10 | <2 × 10−16 | |
| Linear | −5.98 × 10−02 | 3.48 × 10−03 | −17.18 | <2 × 10−16 | ||
| Male | Intercept | 2.09 | 1.46 × 10−01 | 14.32 | <2 × 10−16 | |
| Linear | −4.85 × 10−02 | 3.14 × 10−03 | −15.44 | <2 × 10−16 | ||
| Female | Intercept | 3.59 | 2.01 × 10−01 | 17.86 | <2 × 10−16 | |
| Linear | −5.36 × 10−02 | 3.66 × 10−03 | −14.64 | <2 × 10−16 | ||
| 20 °C | 1st instar | Intercept | 2.40 | 5.65 × 10−01 | 4.256 | <2 × 10−16 |
| Linear | −4.16 × 10−01 | 1.05 × 10−01 | −3.964 | <2 × 10−16 | ||
| 2nd instar | Intercept | 3.67 | 5.15 × 10−01 | 7.126 | <2 × 10−16 | |
| Linear | −2.92 × 10−01 | 5.29 × 10−02 | −5.525 | <2 × 10−16 | ||
| 3rd instar | Intercept | 2.34 | 1.51 × 10−01 | 15.46 | <2 × 10−16 | |
| Linear | −5.31 × 10−02 | 3.21 × 10−03 | −16.54 | <2 × 10−16 | ||
| 4th instar | Intercept | 6.54 | 3.65 × 10−01 | 17.91 | <2 × 10−16 | |
| Linear | −9.11 × 10−02 | 5.95 × 10−03 | −15.30 | <2 × 10−16 | ||
| Male | Intercept | 4.43 | 2.27 × 10−01 | 19.51 | <2 × 10−16 | |
| Linear | −7.27 × 10−02 | 4.10 × 10−03 | −17.74 | <2 × 10−16 | ||
| Female | Intercept | 3.59 | 2.01 × 10−01 | 17.86 | <2 × 10−16 | |
| Linear | −5.36 × 10−02 | 3.66 × 10−03 | −14.64 | <2 × 10−16 | ||
| 25 °C | 1st instar | Intercept | 4.00 | 7.15 × 10−01 | 5.604 | 2.10 × 10−08 |
| Linear | −5.27 × 10−01 | 1.20 × 10−01 | −4.36 | 1.27 × 10−05 | ||
| 2nd instar | Intercept | 6.17 | 6.82 × 10−01 | 9.050 | <2 × 10−16 | |
| Linear | −5.05 × 10−01 | 6.59 × 10−02 | −7.66 | 1.86 × 10−14 | ||
| 3rd instar | Intercept | 2.902 | 1.68 × 10−01 | 17.27 | <2 × 10−16 | |
| Linear | −5.40 × 10−02 | 3.31 × 10−03 | −16.31 | <2 × 10−16 | ||
| 4th instar | Intercept | 7.70 | 4.80 × 10−01 | 16.02 | <2 × 10−16 | |
| Linear | −9.82 × 10−02 | 7.50 × 10−03 | −13.09 | <2 × 10−16 | ||
| Male | Intercept | 5.13 | 2.88 × 10−01 | 17.82 | <2 × 10−16 | |
| Linear | −6.92 × 10−02 | 4.85 × 10−03 | −14.27 | <2 × 10−16 | ||
| Female | Intercept | 6.41 | 4.18 × 10−01 | 15.33 | <2 × 10−16 | |
| Linear | −7.48 × 10−02 | 6.61 × 10−03 | −11.30 | <2 × 10−16 | ||
| 30 °C | 1st instar | Intercept | 5.00 | 0.88 | 5.645 | 1.65 × 10−08 |
| Linear | −0.56 | 0.14 | −4.05 | 5.00 × 10−05 | ||
| 2nd instar | Intercept | 4.76 | 6.68 × 10−01 | 7.123 | 1.05 × 10−12 | |
| Linear | −2.91 × 10−01 | 6.35 × 10−02 | −4.58 | 4.47 × 10−06 | ||
| 3rd instar | Intercept | 2.89 | 1.69 × 10−01 | 17.11 | <2 × 10−16 | |
| Linear | −5.15 × 10−02 | 3.29 × 10−03 | −15.62 | <2 × 10−16 | ||
| 4th instar | Intercept | 7.20 | 4.72 × 10−01 | 15.23 | <2 × 10−16 | |
| Linear | −8.65 × 10−02 | 7.37 × 10−03 | −11.73 | <2 × 10−16 | ||
| Male | Intercept | 7.08 | 4.20 × 10−01 | 16.85 | <2 × 10−16 | |
| Linear | −9.33 × 10−02 | 6.69 × 10−03 | −13.95 | <2 × 10−16 | ||
| Female | Intercept | 1.04 × 10+01 | 7.46 × 10−01 | 13.99 | <2 × 10−16 | |
| Linear | −1.27 × 10−01 | 1.10 × 10−02 | −11.48 | <2 × 10−16 | ||
| 35 °C | 1st instar | Intercept | 12.16 | 1.98 | 6.139 | 8.31 × 10−10 |
| Linear | −1.43 | 0.27 | −5.30 | 1.16 × 10−07 | ||
| 2nd instar | Intercept | 9.87 | 1.46 | 6.75 | 1.46 × 10−11 | |
| Linear | −5.76 × 10−01 | 1.19 × 10−01 | −4.83 | 1.36 × 10−06 | ||
| 3rd instar | Intercept | 4.48 | 2.47 × 10−01 | 18.16 | <2 × 10−16 | |
| Linear | −6.30 × 10−02 | 4.30 × 10−03 | −14.65 | <2 × 10−16 | ||
| 4th instar | Intercept | 7.12 | 6.30 × 10−01 | 11.29 | <2 × 10−16 | |
| Linear | −6.27 × 10−02 | 9.51 × 10−03 | −6.59 | 4.34 × 10−11 | ||
| Male | Intercept | 8.29 | 5.16 × 10−01 | 16.06 | <2 × 10−16 | |
| Linear | −1.07 × 10−01 | 8.00 × 10−03 | −13.39 | <2 × 10−16 | ||
| Female | Intercept | 1.27 × 10+01 | 1.03 | 12.34 | <2 × 10−16 | |
| Linear | −1.49 × 10−01 | 1.49 × 10−02 | −10.01 | <2 × 10−16 |
| Predator Stage | Temperature | ||||
|---|---|---|---|---|---|
| 15 °C | 20 °C | 25 °C | 30 °C | 35 °C | |
| 1st instar | 0.03 ± 0.016 (0.022–0.039) | 0.118 ± 0.035 (0.075–0.204) | 0.177 ± 0.04 (0.127–0.252) | 0.202 ± 0.039 (0.139–0.282) | 0.373 ± 0.089 (0.255–0.727) |
| 2nd instar | 0.079 ± 0.022 (0.052–0.169) | 0.156 ± 0.025 (0.114–0.236) | 0.292 ± 0.06 (0.193–0.518) | 0.163 ± 0.018 (0.125–0.214) | 0.317 ± 0.04 (0.202–0.523) |
| 3rd instar | 0.12 ± 0.015 (0.089–0.172) | 0.176 ± 0.02 (0.128–0.233) | 0.155 ± 0.014 (0.112–0.225) | 0.151 ± 0.013 (0.101–0.224) | 0.233 ± 0.019 (0.164–0.330) |
| 4th instar | 0.194 ± 0.018 (0.146–0.281) | 0.344 ± 0.033 (0.234–0.549) | 0.351 ± 0.037 (0.236–0.506) | 0.246 ± 0.016 (0.178–0.380) | 0.333 ± 0.022 (0.232–0.544) |
| Male | 0.133 ± 0.013 (0.097–0.182) | 0.274 ± 0.03 (0.187–0.399) | 0.24 ± 0.018 (0.160–0.409) | 0.278 ± 0.021 (0.183–0.508) | 0.354 ± 0.037 (0.235–0.544) |
| Female | 0.155 ± 0.011 (0.117–0.215) | 0.183 ± 0.012 (0.134–0.261) | 0.243 ± 0.016 (0.176–0.372) | 0.313 ± 0.024 (0.209–0.579) | 0.546 ± 0.058 (0.270–2.912) |
| Predator Stage | Temperature | ||||
|---|---|---|---|---|---|
| 15 °C | 20 °C | 25 °C | 30 °C | 35 °C | |
| 1st instar | 11.715 ± 1.759 (10.99–12.96) | 4.448 ± 0.337 (4.019–4.845) | 2.972 ± 0.176 (2.741–3.246) | 2.216 ± 0.121 (1.982–2.399) | 1.85 ± 0.086 (1.691–2.008) |
| 2nd instar | 3.717 ± 0.276 (3.373–4.178) | 1.806 ± 0.087 (1.680–1.946) | 1.776 ± 0.073 (1.630–1.913) | 1.036 ± 0.047 (0.942–1.129) | 0.863 ± 0.031 (0.794–0.922) |
| 3rd instar | 0.92 ± 0.031 (0.867–0.996) | 0.726 ± 0.021 (0.683–0.769) | 0.498 ± 0.013 (0.462–0.538) | 0.46 ± 0.012 (0.426–0.499) | 0.339 ± 0.007 (0.322–0.359) |
| 4th instar | 0.551 ± 0.014 (0.520–0.584) | 0.308 ± 0.006 (0.289–0.332) | 0.244 ± 0.005 (0.224–0.267) | 0.208 ± 0.004 (0.188–0.230) | 0.174 ± 0.003 (0.161–0.187) |
| Male | 0.715 ± 0.021 (0.678–0.761) | 0.435 ± 0.01 (0.407–0.462) | 0.299 ± 0.006 (0.280–0.319) | 0.262 ± 0.005 (0.241–0.282) | 0.243 ± 0.005 (0.223–0266) |
| Female | 0.322 ± 0.008 (0.289–0.356) | 0.242 ± 0.005 (0.222–0.270) | 0.209 ± 0.004 (0.190–0.230) | 0.189 ± 0.004 (0.175–0.205) | 0.199 ± 0.004 (0.185–0.215) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, Y.; Shah, F.M.; Shah, M.A.; Musa Khan, M.; Rasheed, M.A.; Ur Rehman, S.; Ali, S.; Zhou, X. Temperature-Dependent Functional Response of Harmonia axyridis (Coleoptera: Coccinellidae) on the Eggs of Spodoptera litura (Lepidoptera: Noctuidae) in Laboratory. Insects 2020, 11, 583. https://doi.org/10.3390/insects11090583
Islam Y, Shah FM, Shah MA, Musa Khan M, Rasheed MA, Ur Rehman S, Ali S, Zhou X. Temperature-Dependent Functional Response of Harmonia axyridis (Coleoptera: Coccinellidae) on the Eggs of Spodoptera litura (Lepidoptera: Noctuidae) in Laboratory. Insects. 2020; 11(9):583. https://doi.org/10.3390/insects11090583
Chicago/Turabian StyleIslam, Yasir, Farhan Mahmood Shah, M. Abas Shah, Muhammad Musa Khan, Muhammad Asim Rasheed, Shakeel Ur Rehman, Shahzaib Ali, and Xingmiao Zhou. 2020. "Temperature-Dependent Functional Response of Harmonia axyridis (Coleoptera: Coccinellidae) on the Eggs of Spodoptera litura (Lepidoptera: Noctuidae) in Laboratory" Insects 11, no. 9: 583. https://doi.org/10.3390/insects11090583
APA StyleIslam, Y., Shah, F. M., Shah, M. A., Musa Khan, M., Rasheed, M. A., Ur Rehman, S., Ali, S., & Zhou, X. (2020). Temperature-Dependent Functional Response of Harmonia axyridis (Coleoptera: Coccinellidae) on the Eggs of Spodoptera litura (Lepidoptera: Noctuidae) in Laboratory. Insects, 11(9), 583. https://doi.org/10.3390/insects11090583






