Evaluation of Suppressed Mite Reproduction (SMR) Reveals Potential for Varroa Resistance in European Honey Bees (Apis mellifera L.)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Bee Colonies and Sampling Strategy
2.2. Evaluation of Mite Non-Reproduction
2.3. Simulation Analyses
2.4. Statistical Analyses
3. Results
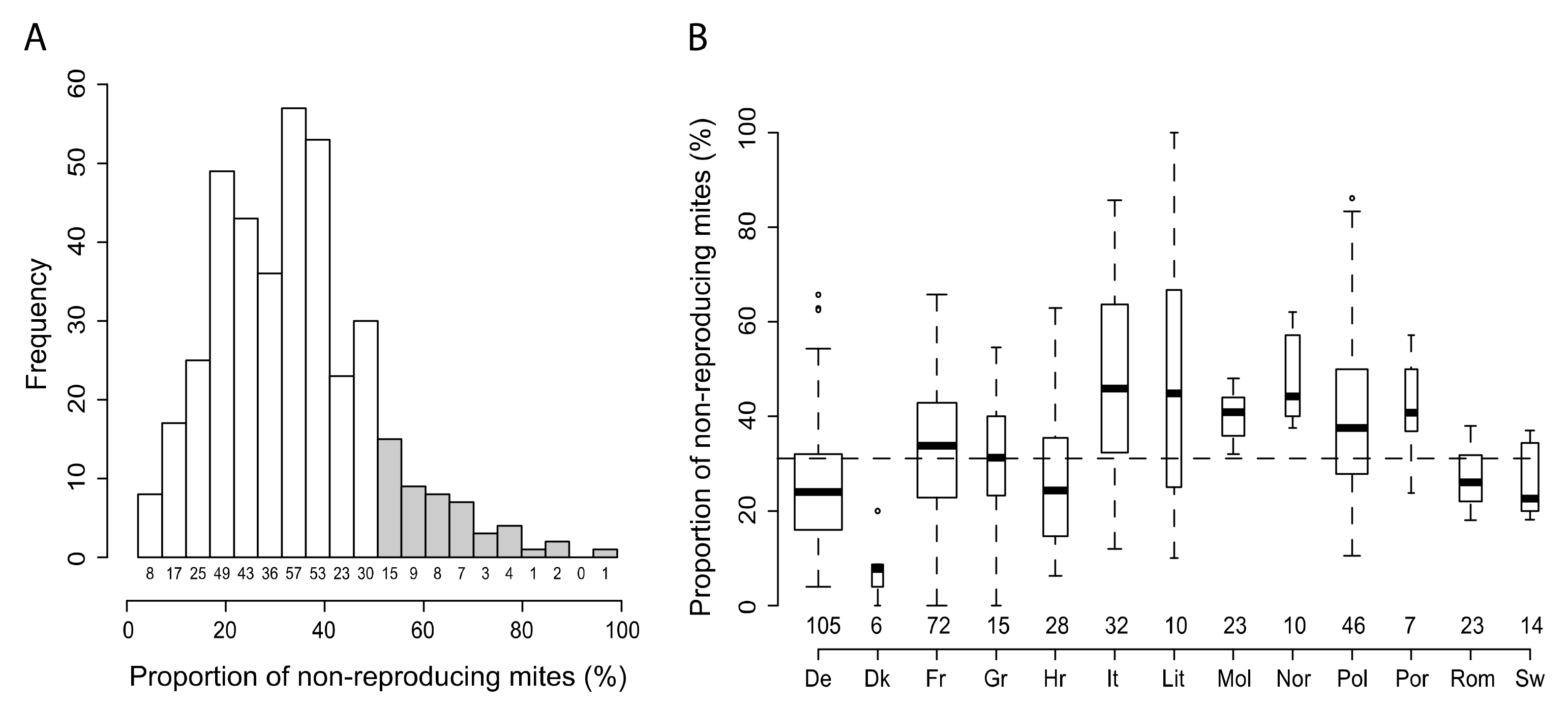
3.1. Variability of SMR in Different European Countries
3.2. Protocol Improvement to Estimate a Reliable SMR Score
3.3. Variability of SMR in Different Honey Bee Genotypes
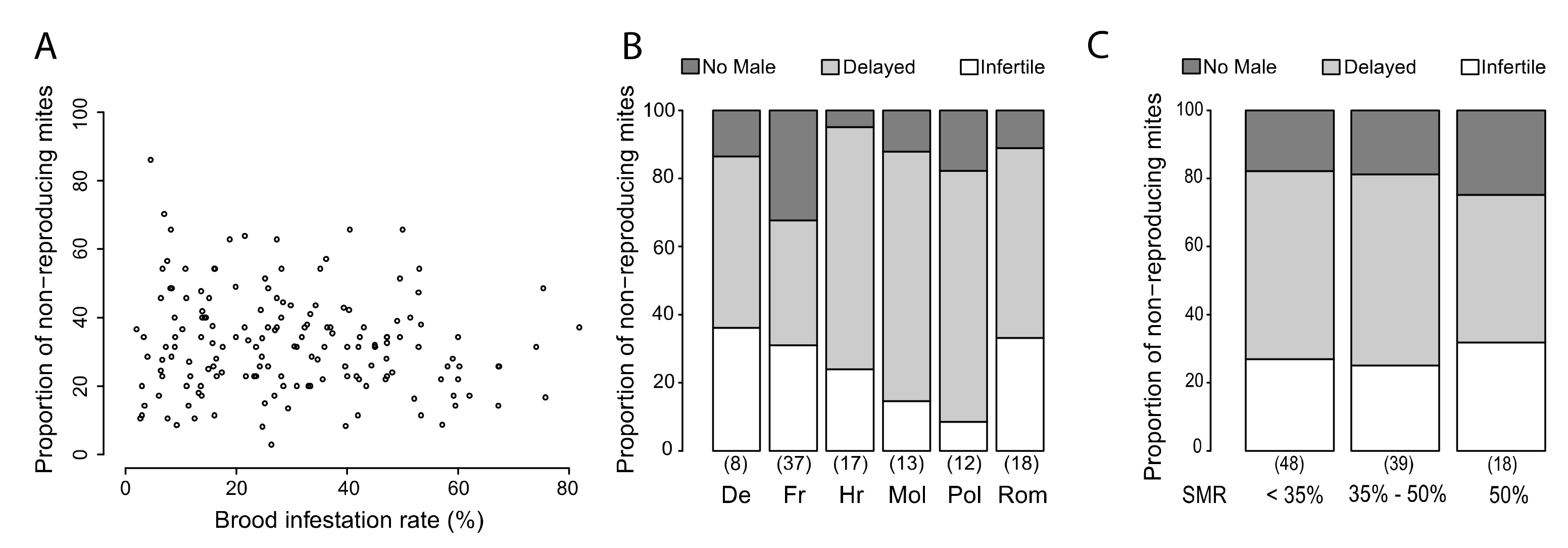
3.4. Putative Mechanisms for SMR
4. Discussion
4.1. SMR Trait in European Colonies
4.2. Factors Affecting Measurement of SMR
4.3. Triggers for SMR
4.4. Comparing SMR to Other Means of Selection for Varroa Resistance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Chen, C.; Niu, Q.; Qi, W.; Yuan, C.; Su, S.; Liu, S.; Zhang, Y.; Zhang, X.; Ji, T.; et al. Survey results of honey bee (Apis mellifera) colony losses in China (2010–2013). J. Apic. Res. 2016, 55, 29–37. [Google Scholar] [CrossRef]
- Maggi, M.; Antúnez, K.; Invernizzi, C.; Aldea, P.; Vargas, M.; Negri, P.; Brasesco, C.; De Jong, D.; Message, D.; Teixeira, E.W.; et al. Honeybee health in South America. Apidologie 2016, 47, 835–854. [Google Scholar] [CrossRef] [Green Version]
- Morawetz, L.; Köglberger, H.; Griesbacher, A.; Derakhshifar, I.; Crailsheim, K.; Brodschneider, R. Health status of honey bee colonies (Apis mellifera) and disease-related risk factors for colony losses in Austria. PLoS ONE 2019, 14, e0219293. [Google Scholar] [CrossRef] [PubMed]
- Genersch, E. Honey bee pathology: Current threats to honey bees and beekeeping. Appl. Microbiol. Biotechnol. 2010, 87, 87–97. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, D.; Méroc, E.; Nguyen, B.K.; Roelandt, S.; Roels, S.; Van der Stede, Y.; Tonnersen, T.; Kryger, P.; Jaarma, K.; Kuus, M.; et al. Risk indicators affecting honeybee colony survival in Europe: One year of surveillance. Apidologie 2016, 47, 348–378. [Google Scholar] [CrossRef] [Green Version]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Le Conte, Y.; Ellis, M.; Ritter, W. Varroa mites and honey bee health: Can Varroa explain part of the colony losses? Apidologie 2010, 41, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Jacques, A.; Laurent, M.; Consortium, E.; Ribière-Chabert, M.; Saussac, M.; Bougeard, S.; Budge, G.E.; Hendrikx, P.; Chauzat, M.-P. A pan-European epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. PLoS ONE 2017, 12, e0172591. [Google Scholar] [CrossRef] [Green Version]
- Milani, N. The resistance of Varroa jacobsoni Oud. to acaricides. Apidologie 1999, 30, 229–234. [Google Scholar] [CrossRef]
- Milani, N.; Vedova, G.D. Decline in the proportion of mites resistant to fluvalinate in a population of Varroa destructor not treated with pyrethroids. Apidologie 2002, 33, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Elzen, P.J.; Westervelt, D. Detection of coumaphos resistance in Varroa destructor in Florida. Am. Bee J. 2002, 142, 291–292. [Google Scholar]
- González-Cabrera, J.; Bumann, H.; Rodríguez-Vargas, S.; Kennedy, P.J.; Krieger, K.; Altreuther, G.; Hertel, A.; Hertlein, G.; Nauen, R.; Williamson, M.S. A single mutation is driving resistance to pyrethroids in European populations of the parasitic mite, Varroa destructor. J. Pest. Sci. 2018, 91, 1137–1144. [Google Scholar] [CrossRef]
- Locke, B.; Forsgren, E.; Fries, I.; de Miranda, J.R. Acaricide treatment affects viral dynamics in Varroa destructor-infested honey bee colonies via both host physiology and mite control. Appl. Environ. Microbiol. 2012, 78, 227–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Mattos, I.M.; Soares, A.E.; Tarpy, D.R. Effects of synthetic acaricides on honey bee grooming behavior against the parasitic Varroa destructor mite. Apidologie 2017, 48, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Bajuk, B.P.; Babnik, K.; Snoj, T.; Milčinski, L.; Ocepek, M.P.; Škof, M.; Jenčič, V.; Filazi, A.; Štajnbaher, D.; Kobal, S. Coumaphos residues in honey, bee brood, and beeswax after Varroa treatment. Apidologie 2017, 48, 588–598. [Google Scholar] [CrossRef]
- Reybroeck, W. Residues of antibiotics and chemotherapeutics in honey. J. Apic. Res. 2018, 57, 97–112. [Google Scholar] [CrossRef]
- Le Conte, Y.; De Vaublanc, G.; Crauser, D.; Jeanne, F.; Rousselle, J.-C.; Bécard, J.-M. Honey bee colonies that have survived Varroa destructor. Apidologie 2007, 38, 566–572. [Google Scholar] [CrossRef] [Green Version]
- Fries, I.; Imdorf, A.; Rosenkranz, P. Survival of mite infested (Varroa destructor) honey bee (Apis mellifera) colonies in a Nordic climate. Apidologie 2006, 37, 564–570. [Google Scholar] [CrossRef] [Green Version]
- Seeley, T.D. Honey bees of the Arnot Forest: A population of feral colonies persisting with Varroa destructor in the northeastern United States. Apidologie 2007, 38, 19–29. [Google Scholar] [CrossRef]
- Oddie, M.A.; Dahle, B.; Neumann, P. Norwegian honey bees surviving Varroa destructor mite infestations by means of natural selection. PeerJ 2017, 5, e3956. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.J.; Hawkins, G.P.; Brettell, L.E.; Reece, N.; Correia-Oliveira, M.E.; Allsopp, M.H. Varroa destructor reproduction and cell re-capping in mite-resistant Apis mellifera populations. Apidologie 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Rinderer, T.E.; Harris, J.W.; Hunt, G.J.; De Guzman, L.I. Breeding for resistance to Varroa destructor in North America. Apidologie 2010, 41, 409–424. [Google Scholar] [CrossRef] [Green Version]
- Büchler, R.; Berg, S.; Le Conte, Y. Breeding for resistance to Varroa destructor in Europe. Apidologie 2010, 41, 393–408. [Google Scholar] [CrossRef] [Green Version]
- Mondet, F.; Beaurepaire, A.; McAfee, A.; Locke, B.; Alaux, C.; Blanchard, S.; Danka, B.; Le Conte, Y. Honey bee survival mechanisms against the parasite Varroa destructor: A systematic review of phenotypic and genomic research efforts. Int. J. Parasitol. 2020, 50, 433–447. [Google Scholar] [CrossRef]
- Leclercq, G.; Pannebakker, B.; Gengler, N.; Nguyen, B.K.; Francis, F. Drawbacks and benefits of hygienic behavior in honey bees (Apis mellifera L.): A review. J. Apic. Res. 2017, 56, 366–375. [Google Scholar] [CrossRef] [Green Version]
- Horns, F.; Hood, M.E. The evolution of disease resistance and tolerance in spatially structured populations. Ecol. Evol. 2012, 2, 1705–1711. [Google Scholar] [CrossRef]
- Locke, B.; Conte, Y.L.; Crauser, D.; Fries, I. Host adaptations reduce the reproductive success of Varroa destructor in two distinct European honey bee populations. Ecol. Evol. 2012, 2, 1144–1150. [Google Scholar] [CrossRef]
- Locke, B. Inheritance of reduced Varroa mite reproductive success in reciprocal crosses of mite-resistant and mite-susceptible honey bees (Apis mellifera). Apidologie 2016, 47, 583–588. [Google Scholar] [CrossRef]
- Harbo, J.R.; Harris, J.W. Selecting honey bees for resistance to Varroa jacobsoni. Apidologie 1999, 30, 183–196. [Google Scholar] [CrossRef]
- Harbo, J.R.; Harris, J.W. Heritability in Honey Bees (Hymenoptera: Apidae) of Characteristics Associated with Resistance to Varroa jacobsoni (Mesostigmata: Varroidae). J. Econ. Entomol. 1999, 92, 261–265. [Google Scholar] [CrossRef] [Green Version]
- Danka, R.G.; Harris, J.W.; Dodds, G.E. Selection of VSH-derived “Pol-line” honey bees and evaluation of their Varroa-resistance characteristics. Apidologie 2016, 47, 483–490. [Google Scholar] [CrossRef]
- Ibrahim, A.; Reuter, G.S.; Spivak, M. Field trial of honey bee colonies bred for mechanisms of resistance against Varroa destructor. Apidologie 2007, 38, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Ward, K.; Danka, R.; Ward, R. Comparative performance of two mite-resistant stocks of honey bees (Hymenoptera: Apidae) in alabama beekeeping operations. J. Econ. Entomol. 2008, 101, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.W.; Danka, R.G.; Villa, J.D. Changes in infestation, cell cap condition, and reproductive status of Varroa destructor (Mesostigmata: Varroidae) in brood exposed to honey bees with Varroa sensitive hygiene. Ann. Entomol. Soc. Am. 2012, 105, 512–518. [Google Scholar] [CrossRef]
- Oddie, M.; Büchler, R.; Dahle, B.; Kovacic, M.; Le Conte, Y.; Locke, B.; de Miranda, J.R.; Mondet, F.; Neumann, P. Rapid parallel evolution overcomes global honey bee parasite. Sci. Rep. 2018, 8, 7704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagoner, K.M.; Spivak, M.; Rueppell, O. Brood affects hygienic behavior in the honey bee (Hymenoptera: Apidae). J. Econ. Entomol. 2018, 111, 2520–2530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conlon, B.H.; Frey, E.; Rosenkranz, P.; Locke, B.; Moritz, R.F.A.; Routtu, J. The role of epistatic interactions underpinning resistance to parasitic Varroa mites in haploid honey bee (Apis mellifera) drones. J. Evol. Biol. 2018, 31, 801–809. [Google Scholar] [CrossRef]
- Frey, E.; Odemer, R.; Blum, T.; Rosenkranz, P. Activation and interruption of the reproduction of Varroa destructor is triggered by host signals (Apis mellifera). J. Invertebr. Pathol. 2013, 113, 56–62. [Google Scholar] [CrossRef]
- Techer, M.A.; Rane, R.V.; Grau, M.L.; Roberts, J.M.; Sullivan, S.T.; Liachko, I.; Childers, A.K.; Evans, J.D.; Mikheyev, A.S. Divergent evolutionary trajectories following speciation in two ectoparasitic honey bee mites. Commun. Biol. 2019, 2, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Beaurepaire, A.; Sann, C.; Arredondo, D.; Mondet, F.; Le Conte, Y. Behavioral Genetics of the Interactions between Apis mellifera and Varroa destructor. Insects 2019, 10, 299. [Google Scholar] [CrossRef] [Green Version]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Wauquiez, Q.; Tannahill, C.; Frey, E.; Ziegelmann, B.; et al. Standard methods for Varroa research. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef] [Green Version]
- Büchler, R.; Costa, C.; Mondet, F.; Kezic, N.; Kovacic, M. RNSBB SMR Recapping Protocol. Available online: www.beebreeding.net/wp-content/uploads/2017/11/RNSBB_SMR-recapping_protocol_2017_09_11.pdf (accessed on 1 September 2020).
- Danka, R.G.; Harris, J.W.; Villa, J.D. Expression of Varroa Sensitive Hygiene (VSH) in commercial VSH honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 2011, 104, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Villa, J.D.; Danka, R.G.; Harris, J.W. Simplified methods of evaluating colonies for levels of Varroa Sensitive Hygiene (VSH). J. Apic. Res. 2009, 48, 162–167. [Google Scholar] [CrossRef]
- De Vaublanc, G.; Otis, G.W.; Le Conte, Y.; Crauser, D.; Kelly, P. Comparative resistance of Canadian and French colonies of honey bees (Apis mellifera) to Varroa destructor: Influence of bee strain, mite strain, and environment. In Proceedings of the Annual North American Apicultural Research Symposium, Niagara Falls, ON, Canada, 5–6 December 2002. [Google Scholar]
- Büchler, R.; Costa, C.; Hatjina, F.; Andonov, S.; Meixner, M.D.; Le Conte, Y.; Uzunov, A.; Berg, S.; Bienkowska, M.; Bouga, M.; et al. The influence of genetic origin and its interaction with environmental effects on the survival of Apis mellifera L. colonies in Europe. J. Apic. Res. 2014, 53, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Meixner, M.D.; Kryger, P.; Costa, C. Effects of genotype, environment, and their interactions on honey bee health in Europe. Curr. Opin. Insect Sci. 2015, 10, 177–184. [Google Scholar] [CrossRef]
- Martin, S.; Holland, K.; Murray, M. Non-reproduction in the honeybee mite Varroa jacobsoni. Exp. Appl. Acarol. 1997, 21, 539–549. [Google Scholar] [CrossRef]
- Locke, B.; Fries, I. Characteristics of honey bee colonies (Apis mellifera) in Sweden surviving Varroa destructor infestation. Apidologie 2011, 42, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Eguaras, M.; Marcangeli, J.; Fernandez, N.A. Influence of ‘parasitic intensity’ on Varroa jacobsoni Oud. reproduction. J. Apic. Res. 1994, 33, 155–159. [Google Scholar] [CrossRef]
- Büchler, R. Varroa Tolerance in honey bees—Occurrence, characters and breeding. Bee World 1994, 75, 54–70. [Google Scholar] [CrossRef]
- Schöning, C.; Gisder, S.; Geiselhardt, S.; Kretschmann, I.; Bienefeld, K.; Hilker, M.; Genersch, E. Evidence for damage-dependent hygienic behaviour towards Varroa destructor-parasitised brood in the western honey bee, Apis mellifera. J. Exp. Biol. 2012, 215, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Momot, J.P.; Rothenbuhler, W.C. Behaviour genetics of nest cleaning in honeybees. VI. Interactions of age and genotype of bees, and nectar flow. J. Apic. Res. 1971, 10, 11–21. [Google Scholar] [CrossRef]
- Wielewski, P.; Arnaut de Toledo, A.V.; Martins, E.N.; Costa-Maia, F.M.; Faquinello, P.; Lino-Lourenço, D.A.; Ruvolo-Takasusuki, M.C.C.; Lopes de Oliveira, C.A.; Sereia, M.J. Relationship between hygienic behavior and Varroa destructor mites in colonies producing honey or royal jelly. Sociobiology 2012, 59, 251–274. [Google Scholar] [CrossRef]
- Perrin, J.; Boukadiri, A.; Boyard, P.; Soubelet, J.-B.; Mazoit, J.X. Hygienic behavior in honey bees and prediction of Varroa non-reproduction in single-drone inseminated (SDI) colonies. J. Apic. Res. 2020, 59, 185–192. [Google Scholar] [CrossRef]
- Villa, J.D.; Danka, R.G.; Harris, J.W. Repeatability of measurements of removal of mite-infested brood to assess Varroa Sensitive Hygiene. J. Apic. Res. 2017, 56, 631–634. [Google Scholar] [CrossRef]
- Fries, I.; Camazine, S.; Sneyd, J. Population Dynamics of Varroa jacobsoni: A Model and a Review. Bee World 1994, 75, 5–28. [Google Scholar] [CrossRef]
- Guichard, M.; Neuditschko, M.; Fried, P.; Soland, G.; Dainat, B. A future resistance breeding strategy against Varroa destructor in a small population of the dark honey bee. J. Apic. Res. 2019, 58, 814–823. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.W.; Harbo, J.R. Changes in reproduction of Varroa destructor after honey bee queens were exchanged between resistant and susceptible colonies. Apidologie 2000, 31, 689–699. [Google Scholar] [CrossRef]
- Harbo, J.R.; Harris, J.W. Responses to Varroa by honey bees with different levels of Varroa Sensitive Hygiene. J. Apic. Res. 2009, 48, 156–161. [Google Scholar] [CrossRef]
- Harris, J.W.; Danka, R.G.; Villa, J.D. Honey bees (Hymenoptera: Apidae) with the trait of Varroa Sensitive Hygiene remove brood with all reproductive stages of Varroa mites (Mesostigmata: Varroidae). Ann. Entomol. Soc. Am. 2010, 103, 146–152. [Google Scholar] [CrossRef]
- Otten, C. Reproduction and population dynamics of Varroa jacobsoni Oud. in colonies of Apis mellifera L. of different origins. In Proceedings of the International Symposium of the International Federation of Beekeepers Associations, Gent, Belgium, 5–7 September 1990. [Google Scholar]
- Gregorc, A.; Adamczyk, J.; Kapun, S.; Planinc, I. Integrated Varroa control in honey bee (Apis mellifera carnica) colonies with or without brood. J. Apic. Res. 2016, 55, 253–258. [Google Scholar] [CrossRef]
- Kovačić, M. Influence of Selection on Traits of Honey Bee (Apis mellifera carnica) in Croatia. Ph.D. Thesis, Faculty of Agrobiotechnical Sciences, University of Osijek, Osijek, Croatia, 2018. [Google Scholar]
- DeGrandi-Hoffman, G.; Ahumada, F.; Zazueta, V.; Chambers, M.; Hidalgo, G.; deJong, E.W. Population growth of Varroa destructor (Acari: Varroidae) in honey bee colonies is affected by the number of foragers with mites. Exp. Appl. Acarol. 2016, 69, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Büchler, R.; Garrido, C.; Bienefeld, K.; Ehrhardt, K. Selection for Varroa tolerance: Concept and results of a long-term selection project. Apidologie 2008, 39, 598. [Google Scholar]
- Blacquière, T.; Boot, W.; Calis, J.; Moro, A.; Neumann, P.; Panziera, D. Darwinian black box selection for resistance to settled invasive Varroa destructor parasites in honey bees. Biol. Invasions 2019, 21, 2519–2528. [Google Scholar] [CrossRef] [Green Version]
- Locke, B. Natural Varroa mite-surviving Apis mellifera honeybee populations. Apidologie 2016, 47, 467–482. [Google Scholar] [CrossRef] [Green Version]




| Country | Laboratory | Genotypes | Samples (≥10 SIC) | Samples (≥35 SIC) |
|---|---|---|---|---|
| Croatia (Hr) | HPA | carnica | 12 | 7 |
| OS | carnica | 16 | 12 | |
| Denmark (Dk) | Dk | Buckfast | 6 | 2 |
| France (Fr) | INRA | Buckfast, mellifera mix, VSH | 56 | 34 |
| ITSAP | Buckfast, caucasica, carnica mix, ligustica mix | 39 | 33 | |
| Germany (De) | LLH Bee Institute | carnica | 105 | 35 |
| Greece (Gr) | HAO-API | cecropia, macedonica | 15 | 0 |
| Italy (It) | CREA-API | ligustica | 12 | 2 |
| Aspromiele | Buckfast | 20 | 0 | |
| Lithuania (Lit) | Vilnius | carnica mix | 10 | 0 |
| R. of Moldova (Mol) | IZASM | carpatica | 23 | 13 |
| Norway (Nor) | NBA | mellifera | 10 | 1 |
| Poland (Pol) | Pulawy | carnica, caucasica, mellifera | 17 | 7 |
| Olsztyn | carnica | 29 | 9 | |
| Portugal (Por) | CIMO | iberiensis | 7 | 0 |
| Romania (Rom) | ICDA | carpatica | 23 | 19 |
| Switzerland (Sw) | Liebefeld | carnica mix | 14 | 2 |
| Total | 414 | 176 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondet, F.; Parejo, M.; Meixner, M.D.; Costa, C.; Kryger, P.; Andonov, S.; Servin, B.; Basso, B.; Bieńkowska, M.; Bigio, G.; et al. Evaluation of Suppressed Mite Reproduction (SMR) Reveals Potential for Varroa Resistance in European Honey Bees (Apis mellifera L.). Insects 2020, 11, 595. https://doi.org/10.3390/insects11090595
Mondet F, Parejo M, Meixner MD, Costa C, Kryger P, Andonov S, Servin B, Basso B, Bieńkowska M, Bigio G, et al. Evaluation of Suppressed Mite Reproduction (SMR) Reveals Potential for Varroa Resistance in European Honey Bees (Apis mellifera L.). Insects. 2020; 11(9):595. https://doi.org/10.3390/insects11090595
Chicago/Turabian StyleMondet, Fanny, Melanie Parejo, Marina D. Meixner, Cecilia Costa, Per Kryger, Sreten Andonov, Bertrand Servin, Benjamin Basso, Małgorzata Bieńkowska, Gianluigi Bigio, and et al. 2020. "Evaluation of Suppressed Mite Reproduction (SMR) Reveals Potential for Varroa Resistance in European Honey Bees (Apis mellifera L.)" Insects 11, no. 9: 595. https://doi.org/10.3390/insects11090595
APA StyleMondet, F., Parejo, M., Meixner, M. D., Costa, C., Kryger, P., Andonov, S., Servin, B., Basso, B., Bieńkowska, M., Bigio, G., Căuia, E., Cebotari, V., Dahle, B., Dražić, M. M., Hatjina, F., Kovačić, M., Kretavicius, J., Lima, A. S., Panasiuk, B., ... Büchler, R. (2020). Evaluation of Suppressed Mite Reproduction (SMR) Reveals Potential for Varroa Resistance in European Honey Bees (Apis mellifera L.). Insects, 11(9), 595. https://doi.org/10.3390/insects11090595








