Impact of 2.45 GHz Microwave Irradiation on the Fruit Fly, Drosophila melanogaster
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Direct Exposure to Microwaves under Resonance Condition
2.3. Direct Exposure to Single Traveling Microwaves under no Reflection
2.4. Direct Exposure to Traveling Microwaves with Reflections Condition
2.5. Genotoxicity Test
2.6. Behavior
2.7. Paramagnetic Components
2.8. Permittivity
2.9. Statistics
3. Results
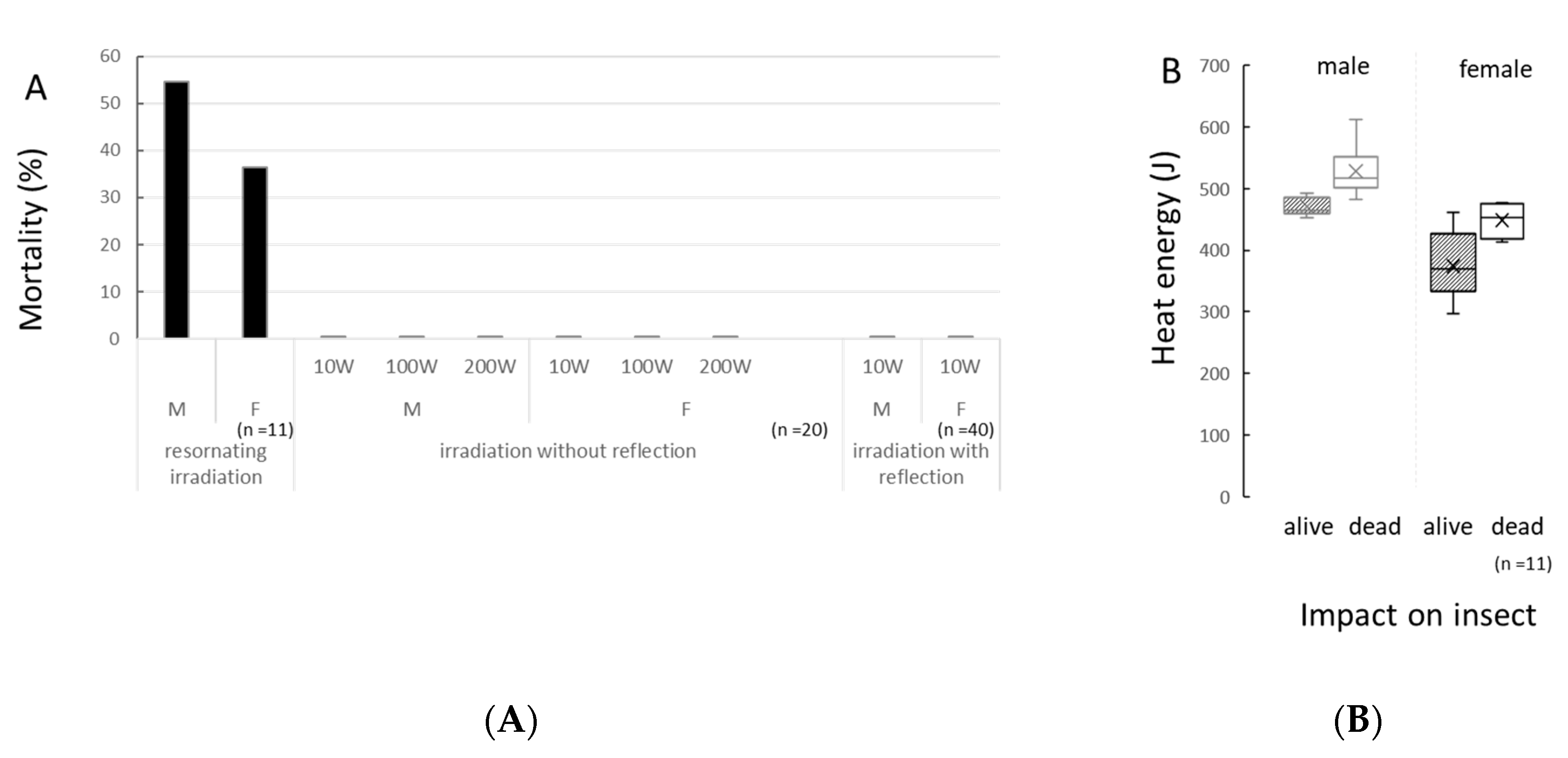
3.1. Effect of Standing Waves under Resonance Condition
3.2. Effects of Single Way Travelling Microwaves
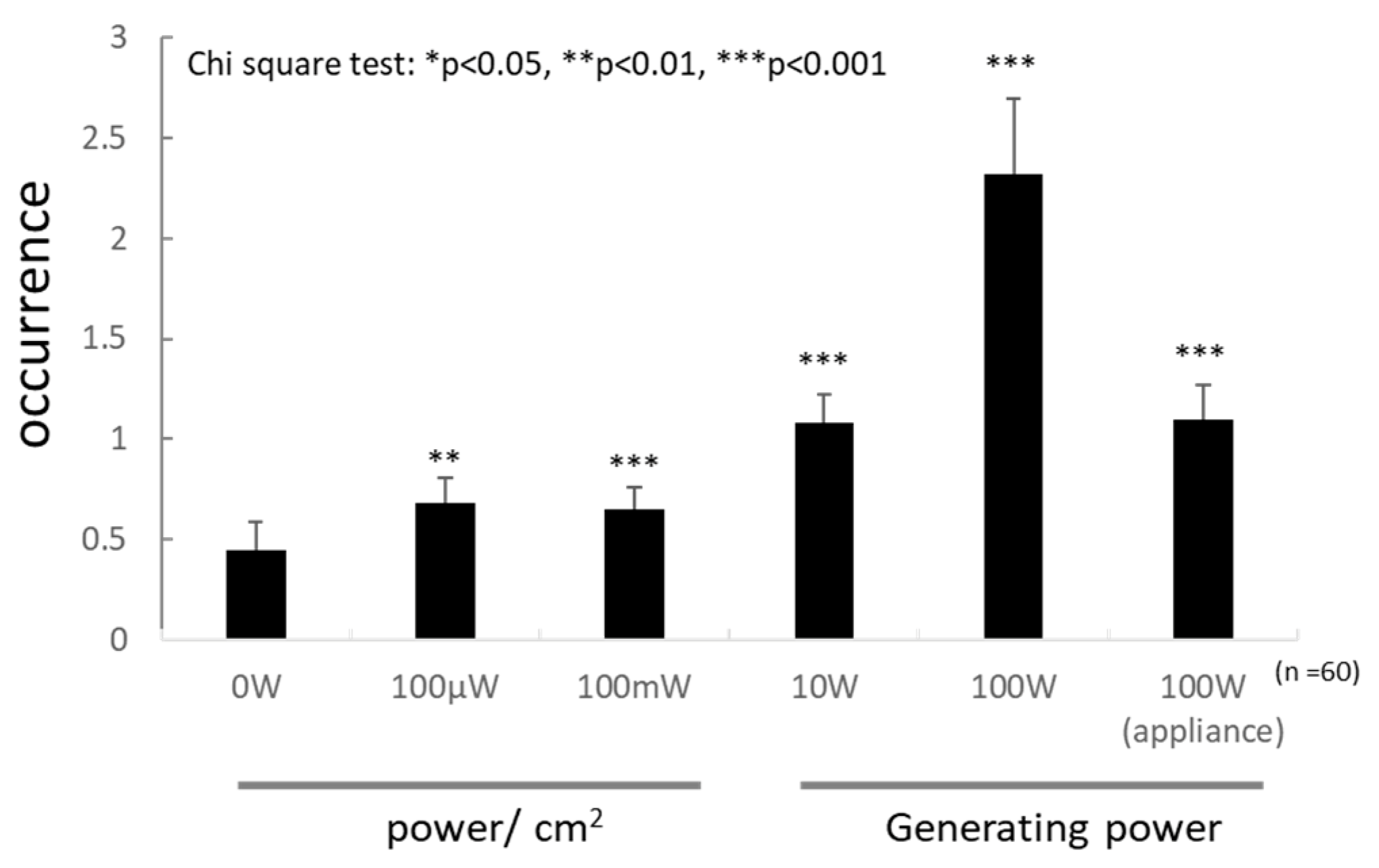
3.3. Effects of Multiway Travelling Microwaves
3.4. Genotoxicity
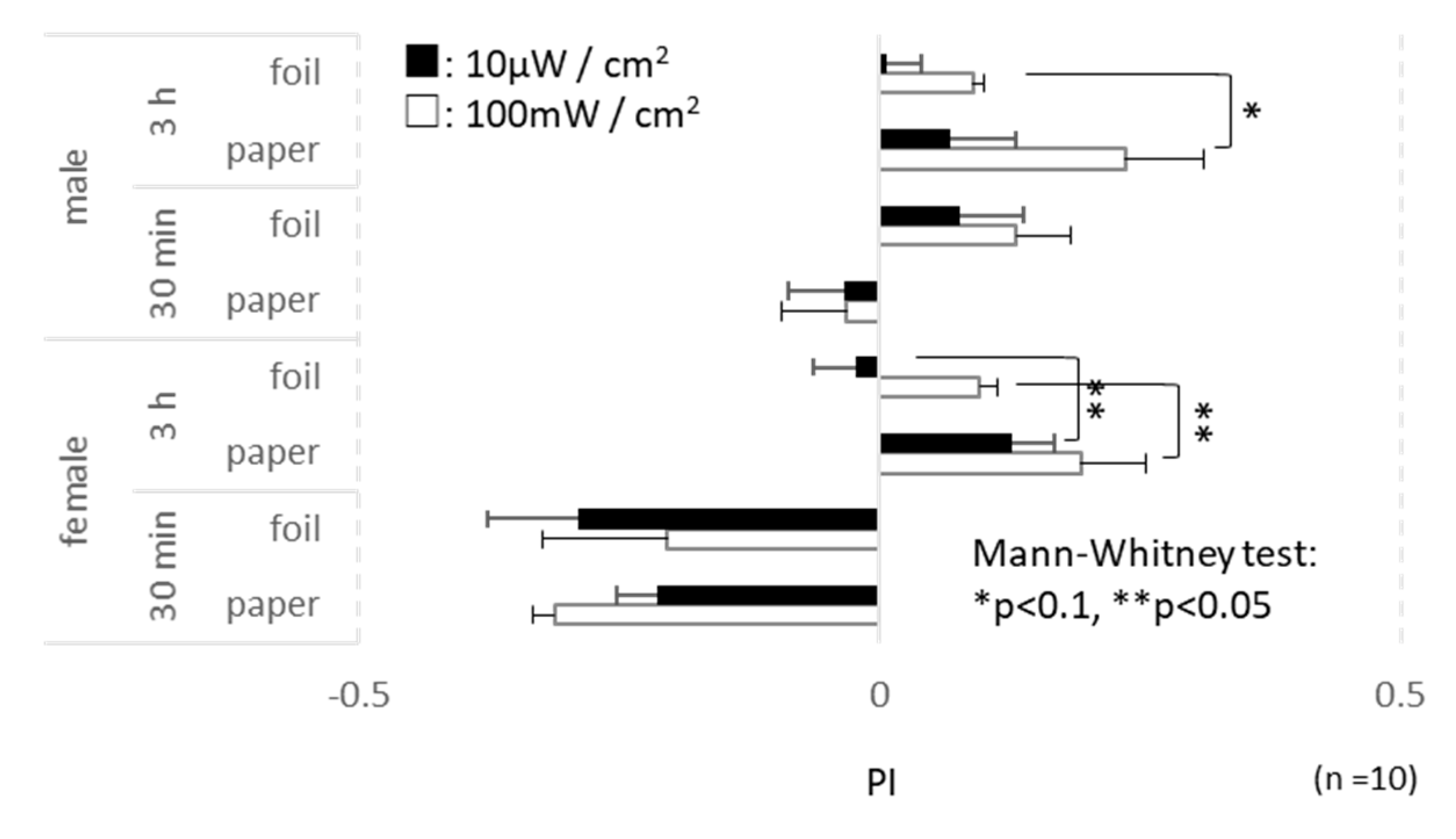
3.5. Behavior
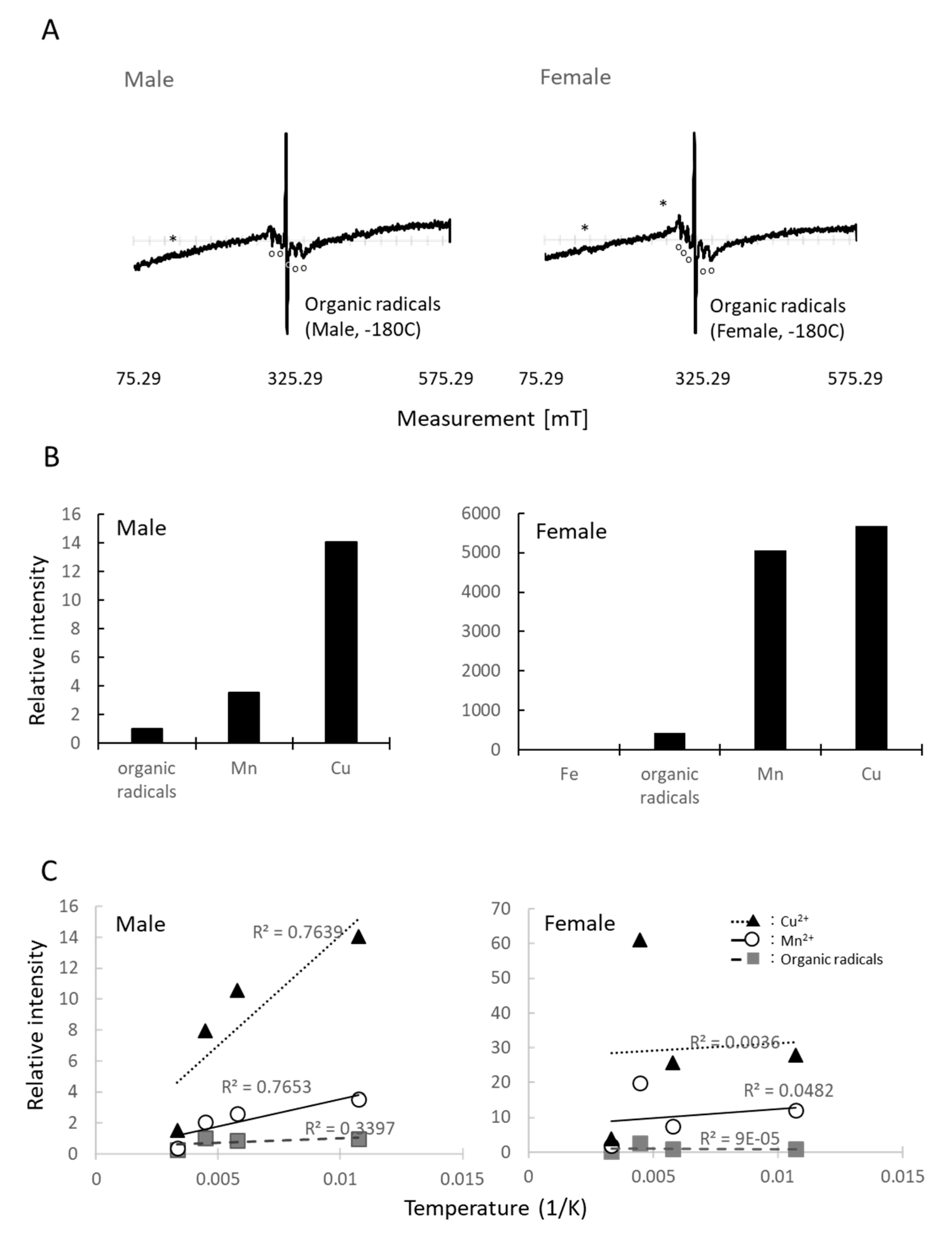
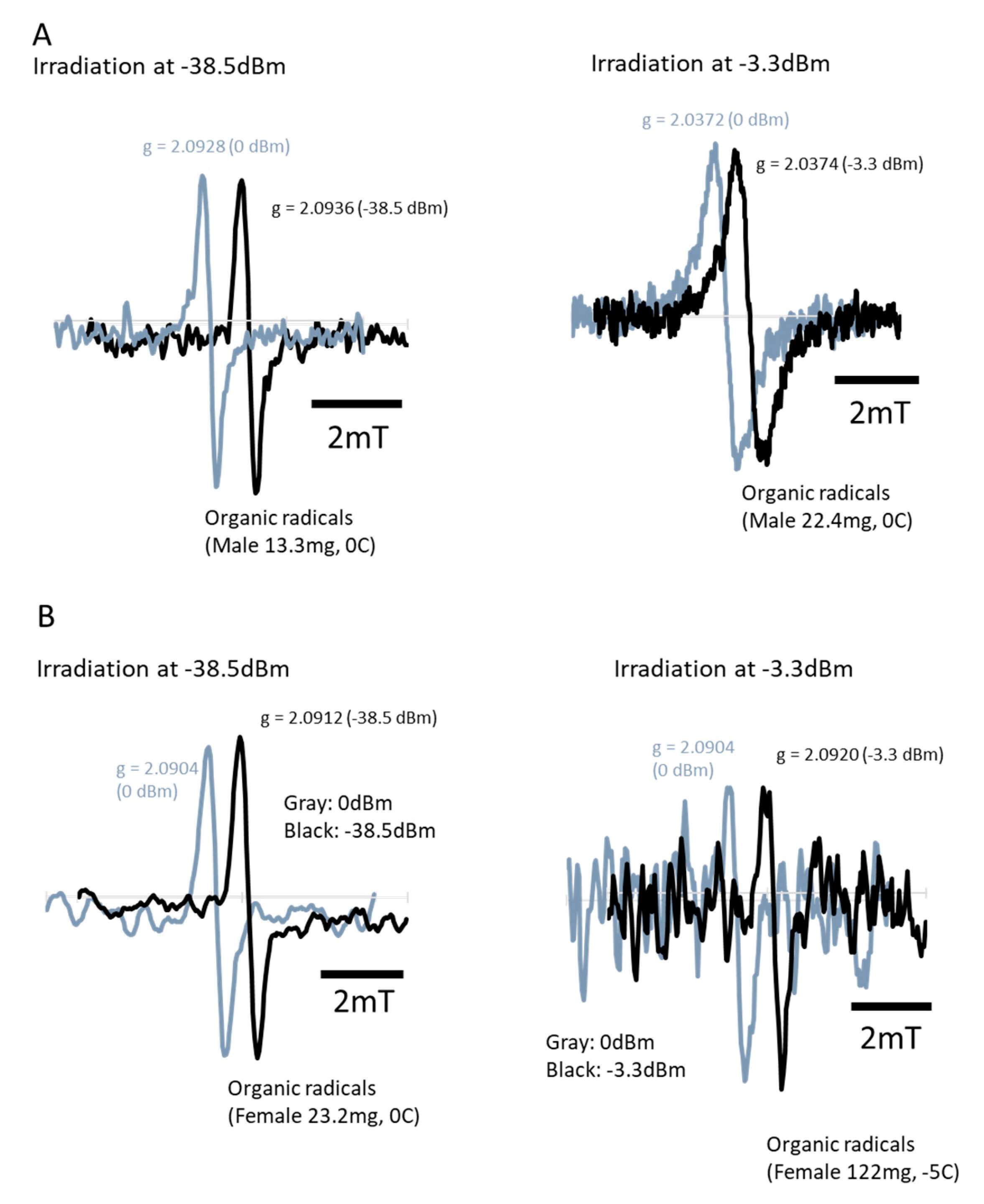
3.6. ESR Measurements
3.7. Permittivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Özorak, A.; Nazıroğlu, M.; Çelik, Ö.; Yüksel, M.; Özçelik, D.; Özkaya, M.O.; Çetin, H.; Kahya, M.C.; Kose, S.A. Wi-Fi (2.45 GHz)- and mobile phone (900 and 1800 MHz)-induced risks on oxidative stress and elements in kidney and testis of rats during pregnancy and the development of offspring. Biol. Trace Elem. Res. 2013, 156, 221–229. [Google Scholar] [CrossRef] [PubMed]
- IARC Monograohs. Non-Ionizing radiation, part 2: Radiofrequency electromagnetic fields. In IARC Monograohes on the Evaluation of Carcinogenetic Risks to Humans; International Agency for Research on Cancer: Lyon, France; WHO Press: Lyon, France, 2013; Volume 102. [Google Scholar]
- Committee on the Possible Effects of Electromagnetic Fields on Biologic Systems. Possible Health Effects of Exposure to RESIDENTIAL ELECTRIC AND MAGNETIC FIELDS; National Academy Press: Washington, DC, USA, 1997. [Google Scholar]
- Helinski, M.E.H.; Parker, A.G.; Knols, B.G. Radiation biology of mosquitoes. Malar. J. 2009, 8, S6. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, T.; Parker, A.G.; Jessup, A.; Pereira, R.; Orozco-Davila, D.; Islam, A.; Dammalage, T.; Walder, J.M.M.A.F.; Mastrangelo, T.; Parker, A.G.; et al. New Generation of X Ray Irradiators for Insect Sterilization. J. Econ. Entomol. 2010, 103, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, A.; Kashimura, K.; Mitani, T.; Shinohara, N.; Yoshimura, T. Influence of Powerful Microwaves on the Termite Coptotermes Formosanus -Impact of Powerful Microwaves on Insects in Processing and Properties of Advanced Ceramics and Composites VI/; Singh, J.P., Ed.; (Ceramic Transactions, v. 249); Wiley-American Ceramic Society: Hoboken, NJ, USA, 2014; pp. 367–374. [Google Scholar]
- Yanagawa, A.; Kajiwara, A.; Nakajima, H.; Desmond-Le Quemener, E.; Steyer, J.-P.; Lewis, V.; Mitani, T. Physical assessments of termites (Termitidae) under 2.45 GHz microwave irradiation. Sci. Rep. 2020, 10, 5197. [Google Scholar] [CrossRef]
- de Oliverira, J.F.; Alves, O.C.; Esquivel, D.M.S.; Wajnberg, E. Ingested and biomineralized magnetic material in the prey Neocapritermes opacus termite: FMR characterization. J. Magn. Reson. 2008, 191, 112–119. [Google Scholar] [CrossRef]
- Hardell, L.; Sage, C. Biological effects from electromagnetic field exposure and public exposure standards. Biomed. Pharmacother. 2007, 62, 104–109. [Google Scholar] [CrossRef]
- Paul, S.; Meng, L.Q.; Berger, S.; Grampp, G.; Matysik, J.; Wang, X.J. The flavin-tryptophan dyad F10T as a cryptochrome model compound: Synthesis and photochemistry. Chemphotochem 2017, 1, 12–16. [Google Scholar] [CrossRef]
- Maeda, K.; Henbest, K.B.; Cintolesi, F.; Kuprov, I.; Rodgers, C.T.; Liddell, P.A.; Gust, D.; Timmel, C.R.; Hore, P.J. Chemical compass model of avian magnetoreception. Nature 2008, 453, 387–390. [Google Scholar] [CrossRef]
- Solov’yov, I.A.; Schulten, K. Reaction Kinetics and Mechanism of Magnetic Field Effects in Cryptochrome. J. Phys. Chem. B 2012, 116, 1089–1099. [Google Scholar] [CrossRef]
- Gould, J.L.; Kirschvink, J.L.; Deffeyes, K.S.; Brines, M.L. Orientation of demagnetized bees. J. Exp. Biol. 1980, 86, 1–8. [Google Scholar]
- Vácha, M.; Kvíčalová, M.; Půžová, T. Radio frequency magnetic fields disrupt magnetoreception in American cockroach. J. Exp. Biol. 2009, 212, 3473–3477. [Google Scholar] [CrossRef] [PubMed]
- Vácha, M.; Kvíčalová, M.; Půžová, T. American cockroaches prefer four cardinal geomagnetic positions at rest. Behaviour 2010, 147, 425–440. [Google Scholar]
- Rickli, M.; Leuthold, R.H. Homing in harvester termites: Evidence of magnetic orientation. Ethology 1988, 77, 209–216. [Google Scholar] [CrossRef]
- Wajnberg, E.; Acosta-Avalos, D.; Alves, O.C.; de Oliveira, J.F.; Srygley, R.B.; Esquivel, M.S. Magnetoreception in eusocial insects: An update. J. R. Soc. Interface 2010, 7, S207–S225. [Google Scholar] [CrossRef] [PubMed]
- Maher, B.A. Magnetite biomineralization in termites. Proc. R. Soc. Lond. B 1998, 265, 733–737. [Google Scholar] [CrossRef]
- Alves, O.C.; Wajnberg, E.; de Oliverira, J.F.; Esquivel, D.M.S. Magnetic material arrangement in oriented termites: A magnetic resonance study. J. Magn. Reson. 2004, 168, 246–251. [Google Scholar] [CrossRef]
- Gegear, R.J.; Casselman, A.; Waddell, S.; Reppert, S.M. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature 2008, 454, 1014–1018. [Google Scholar] [CrossRef]
- Gegear, R.J.; Foley, L.E.; Casselman, A.; Reppert, S.M. Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism. Nature 2010, 463, 804–808. [Google Scholar] [CrossRef]
- Berndt, A.; Kottke, T.; Breitkreuz, H.; Dvorsky, R.; Hennig, S.; Alexander, M.; Wolf, E. A novel photoreaction mechanism for the circadian blue light photoreceptor Drosophila cryptochrome. J. Biol. Chem. 2007, 282, 13011–13021. [Google Scholar] [CrossRef]
- Vinayak, P.; Coupar, J.; Hughes, S.E.; Fozdar, P.; Kilby, J.; Garren, E.; Yoshii, T.; Hirsh, J. Exquisite Light Sensitivity of Drosophila melanogaster Cryptochrome. PLoS Genet. 2013, 9, e1003615. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection. ICNIRP Guidelines for limiting exposure to time-varying electric, magnetic and electromagnetic fields (up to 300 GHZ). Health Phys. 1998, 74, 494–522. [Google Scholar]
- Fujikawa, K. Genotoxic potency in Drosophila melanogaster of selected aromatic amines and polycyclic aromatic hydrocarbons as assayed in the DNA repair test. Mutat. Res. 1993, 290, 175–182. [Google Scholar] [CrossRef]
- Asano, M.; Sakaguchi, M.; Tanaka, S.; Kashimura, K.; Mitani, T.; Kawase, M.; Matsumura, H.; Yamaguchi, T.; Fujita, Y.; Tabuse, K. Effects of Normothermic Conditioned Microwave Irradiation on Cultured Cells Using an Irradiation System with Semiconductor Oscillator and Thermo-regulatory Applicator. Sci. Rep. 2017, 7, 41244. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.O.; Bartley, P.G., Jr.; Lawrence, K.C. Measuring RF and Microwave Permittivities of Adult Rice Weevils. IEEE Trans. Instrum. Meas. 1997, 46, 941–946. [Google Scholar] [CrossRef]
- Wang, Y.; Wig, T.D.; Tang, J.; Hallberg, L.M. Dielectric properties of foods relevant to RF and microwave pasteurization and sterilization. J. Food Eng. 2003, 57, 257–268. [Google Scholar] [CrossRef]
- Nakai, K.; Mitani, T.; Yoshimura, T.; Shinohara, N.; Tsunoda, K.; Imamura, Y. Effects of Microwave lrradiation on the Drywood Termite Incisitermes minor (Hagen). Jpn. J. Environ. Entomol. Zool. 2009, 20, 171–184. [Google Scholar]
- Nelson, S.O.; Bartley, P.G., Jr.; Lawrence, K.C. RF and microwave dielectric properties of stored-grain insects and their implications for potential insect control. Trans. ASAE 1998, 41, 685–692. [Google Scholar] [CrossRef]
- Nelson, S.O. RF and microwave permittivities of insects and some applications. In Proceedings of the URSI EMTS International Symposium on Electromagnetic Theory 2004, Pisa, Italy, 23–27 May 2004; pp. 1224–1226. [Google Scholar]
- Mai, H.Q.; Mo, H.Y.; Deng, J.F.; Deng, M.Q.; Mai, W.Y.; Huang, X.M.; Guo, X.; Hong, M.H. Endoscopic microwave coagulation therapy for early recurrent T1 nasopharyngeal carcinoma. Eur. J. Cancer 2009, 45, 1107–1110. [Google Scholar] [CrossRef]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Asano, M.; Tanaka, S.; Sakaguchi, M.; Matsumura, H.; Yamaguchi, T.; Fujita, Y.; Tabuse, K. Normothermic Microwave Irradiation Induces Death of HL-60 Cells through Heat-Independent Apoptosis. Sci Rep. 2017, 7, 11406. [Google Scholar] [CrossRef]
- Huizen, A.V.V.; Morton, J.M.; Kinsey, L.J.; Kannon, D.G.V.; Saad, M.A.; Birkholz, T.R.; Czajka, J.M.; Cyrus, J.; Barnes, F.S.; Beane, W.S. Weak magnetic fields alter stem cell–mediated growth. Sci. Adv. 2019, 5, eaau7201. [Google Scholar] [CrossRef] [PubMed]
- Hoang, N.; Schleicher, E.; Kacprzak, S.; Bouly, J.P.; Picot, M.; Wu, W.; Berndt, A.; Wolf, E.; Bittl, R.; Ahmad, M. Human and Drosophila Cryptochromes Are Light Activated by Flavin Photoreduction in Living Cells. PLoS Biol. 2008, 6, e200. [Google Scholar]
- Lin, J.C.; Wang, Z. Hearing of microwave pulses by humans and animals: Effects, mechanism, and thresholds. Health Phys. 2007, 92, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Egan, E.S.; Franklin, T.M.; Hilderbrand-Chae, M.J.; McNeil, G.P.; Roberts, M.A.; Schroeder, A.J.; Zhang, X.; Jackson, F.R. An extraretinally expressed insect cryptochrome with similarity to the blue light photoreceptors of mammals and plants. J. Neurosci. 1999, 19, 3665–3673. [Google Scholar] [CrossRef]
- Daniels, W.M.U.; Pitout, I.L.; Afullo, T.O.J.; Mabandla, M.V. The effect of electromagnetic radiation in the mobile phone range on the behaviour of the rat. Metab Brain Dis. 2009, 24, 629–641. [Google Scholar] [CrossRef]
- Graf, U.; Würgler, F.E. The somatic white-ivory eye spot test does not detect the same spectrum of genotoxic events as the wing somatic mutation and recombination test in Drosophila melanogaster. Environ. Mol. Mutagen. 1996, 27, 219–226. [Google Scholar] [CrossRef]
- Gonet, B.; Kosik-Bogacka, D.I.; Kuźna-Grygiel, W. Effects of extremely low-frequency magnetic fields on the oviposition of Drosophila melanogaster over three generations. Bio Elecro Magn. 2009, 30, 687–689. [Google Scholar]
- Panagopoulos, D.J. Effect of Microwave Exposure on the Ovarian Development of Drosophila melanogaster. Cell Biochem. Biophys. 2012, 63, 121–132. [Google Scholar] [CrossRef]
- Panagopoulos, D.J.; Karabarbounis, A.; Margaritis, L.H. Effect of GSM 900-MHz Mobile Phone Radiation on the Reproductive Capacity of Drosophila melanogaster. Electromagn. Biol. Med. 2004, 23, 29–43. [Google Scholar] [CrossRef]
- Panagopoulos, D.J.; Chavdoula, E.D.; Karabarbounis, A.; Margaritis, L.H. Comparison of bioactivity between GSM 900 MHz and DCS 1800 MHz mobile telephony radiation. Electromagn. Biol. Med. 2007, 26, 33–44. [Google Scholar] [CrossRef]
- Panagopoulos, D.J.; Margaritis, L.H. The effect of exposure duration on the biological activity of mobile telephony radiation. Mutat. Res. 2010, 699, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Kumar, S.; Behari, J. Mobile phone usage and male infertility in Wistar rat. Indian J. Expr. Biol. 2010, 47, 987–992. [Google Scholar]
- Jacob, J.; Chia, L.H.L.; Boey, F.Y.C. Thermal and non-thermal interaction of microwave radiation with materials. J. Mater. Sci. 1995, 30, 5321–5327. [Google Scholar] [CrossRef]
- la Hoz, A.D.; Díaz-Ortiz, A.; Moreno, A. Review on non-thermal effects of microwave irradiation in organic synthesis. J. Microw. Power Electromagn. Energy 2007, 41, 44–64. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanagawa, A.; Tomaru, M.; Kajiwara, A.; Nakajima, H.; Quemener, E.D.-L.; Steyer, J.-P.; Mitani, T. Impact of 2.45 GHz Microwave Irradiation on the Fruit Fly, Drosophila melanogaster. Insects 2020, 11, 598. https://doi.org/10.3390/insects11090598
Yanagawa A, Tomaru M, Kajiwara A, Nakajima H, Quemener ED-L, Steyer J-P, Mitani T. Impact of 2.45 GHz Microwave Irradiation on the Fruit Fly, Drosophila melanogaster. Insects. 2020; 11(9):598. https://doi.org/10.3390/insects11090598
Chicago/Turabian StyleYanagawa, Aya, Masatoshi Tomaru, Atsushi Kajiwara, Hiroki Nakajima, Elie Desmond-Le Quemener, Jean-Philippe Steyer, and Tomohiko Mitani. 2020. "Impact of 2.45 GHz Microwave Irradiation on the Fruit Fly, Drosophila melanogaster" Insects 11, no. 9: 598. https://doi.org/10.3390/insects11090598
APA StyleYanagawa, A., Tomaru, M., Kajiwara, A., Nakajima, H., Quemener, E. D.-L., Steyer, J.-P., & Mitani, T. (2020). Impact of 2.45 GHz Microwave Irradiation on the Fruit Fly, Drosophila melanogaster. Insects, 11(9), 598. https://doi.org/10.3390/insects11090598




