Specific and Spillover Effects on Vectors Following Infection of Two RNA Viruses in Pepper Plants
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Aphids and Thrips Rearing
2.2. CMV and TSWV Maintenance
2.3. CMV and TSWV Titer Estimation in Pepper Plants
2.4. Myzus Persicae Settling Assay
2.5. Myzus Persicae Fitness
2.6. Frankliniella Fusca Fecundity
2.7. Frankliniella Fusca Feeding
2.8. Statistical Analyses
3. Results
3.1. CMV and TSWV Symptoms and Titer Estimation in Pepper Plants
3.2. Myzus Persicae Settling Assay
3.3. Myzus Persicae Fitness
3.4. Frankliniella Fusca Fecundity
3.5. Frankliniella Fusca Feeding
4. Discussions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harris, K.; Smith, O.; Duffus, J. Virus Insect Plant Interactions, 1st ed.; Academic Press: San Diego, CA, USA, 2001; ISBN 9780123276810. [Google Scholar]
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479–480, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Hogenhout, S.A.; Ammar, E.-D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Michalakis, Y.; Munster, M.; Blanc, S. Plant feeding by insect vectors can affect life cycle, population genetics and evolution of plant viruses. Funct. Ecol. 2013, 27, 610–622. [Google Scholar] [CrossRef]
- McElhany, P.; Real, L.A.; Power, A.G. Vector preference and disease dynamics: A study of barley yellow dwarf virus. Ecology 1995, 76, 444–457. [Google Scholar] [CrossRef]
- Sisterson, M.S. Effects of insect-vector preference for healthy or infected plants on pathogen spread: Insights from a model. J. Econ. Entomol. 2008, 101, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stafford, C.A.; Walker, G.P.; Ullman, D.E. Infection with a plant virus modifies vector feeding behavior. Proc. Natl. Acad. Sci. USA. 2011, 108, 9350–9355. [Google Scholar] [CrossRef] [PubMed]
- Ingwell, L.L.; Eigenbrode, S.D.; Bosque-Pérez, N.A. Plant viruses alter insect behavior to enhance their spread. Sci. Rep. 2012, 2, 578. [Google Scholar] [CrossRef]
- Shrestha, A.; Srinivasan, R.; Riley, D.G.; Culbreath, A.K. Direct and indirect effects of a thrips-transmitted Tospovirus on the preference and fitness of its vector, Frankliniella fusca. Entomol. Exp. Appl. 2012, 145, 260–271. [Google Scholar] [CrossRef]
- Moreno-Delafuente, A.; Garzo, E.; Moreno, A.; Fereres, A. A plant virus manipulates the behavior of its whitefly vector to enhance its transmission efficiency and spread. PLoS One 2013, 8, e61543. [Google Scholar] [CrossRef]
- Rajabaskar, D.; Bosque-Pérez, N.A.; Eigenbrode, S.D. Preference by a virus vector for infected plants is reversed after virus acquisition. Virus Res. 2014, 186, 32–37. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA. 2010, 107, 3600–3605. [Google Scholar] [CrossRef] [PubMed]
- Bosque-Pérez, N.A.; Eigenbrode, S.D. The influence of virus-induced changes in plants on aphid vectors: Insights from luteovirus pathosystems. Virus Res. 2011, 159, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.E.; Bosque-Pérez, N.A.; Eigenbrode, S.D.; De Moraes, C.M.; Mescher, M.C. Transmission mechanisms shape pathogen effects on host-vector interactions: Evidence from plant viruses. Funct. Ecol. 2012, 26, 1162–1175. [Google Scholar] [CrossRef]
- Chen, G.; Pan, H.; Xie, W.; Wang, S.; Wu, Q.; Fang, Y.; Shi, X.; Zhang, Y. Virus infection of a weed increases vector attraction to and vector fitness on the weed. Sci. Rep. 2013, 3, 2253. [Google Scholar] [CrossRef]
- Zhao, P.; Yao, X.; Cai, C.; Li, R.; Du, J.; Sun, Y.; Wang, M.; Zou, Z.; Wang, Q.; Kliebenstein, D.J.; et al. Viruses mobilize plant immunity to deter nonvector insect herbivores. Sci. Adv. 2019, 5, eaav9801. [Google Scholar] [CrossRef]
- Fang, Y.; Jiao, X.; Xie, W.; Wang, S.; Wu, Q.; Shi, X.; Chen, G.; Su, Q.; Yang, X.; Pan, H.; et al. Tomato yellow leaf curl virus alters the host preferences of its vector Bemisia tabaci. Sci. Rep. 2013, 3, 2876. [Google Scholar] [CrossRef]
- Liu, B.; Preisser, E.L.; Chu, D.; Pan, H.; Xie, W.; Wang, S.; Wu, Q.; Zhou, X.; Zhang, Y. Multiple forms of vector manipulation by a plant-infecting virus: Bemisia tabaci and tomato yellow leaf curl virus. J. Virol. 2013, 87, 4929–4937. [Google Scholar] [CrossRef]
- Syller, J. Biological and molecular events associated with simultaneous transmission of plant viruses by invertebrate and fungal vectors. Mol. Plant Pathol. 2014, 15, 417–426. [Google Scholar] [CrossRef]
- Peñaflor, M.F.G.V.; Mauck, K.E.; Alves, K.J.; De Moraes, C.M.; Mescher, M.C. Effects of single and mixed infections of bean pod mottle virus and soybean mosaic virus on host-plant chemistry and host–vector interactions. Funct. Ecol. 2016, 30, 1648–1659. [Google Scholar] [CrossRef]
- Wang, H.; Xu, D.; Pu, L.; Zhou, G. Southern rice black-streaked dwarf virus alters insect vectors’ host orientation preferences to enhance spread and increase rice ragged stunt virus co-infection. Phytopathology 2014, 104, 196–201. [Google Scholar] [CrossRef]
- Gautam, S.; Gadhave, K.R.; Buck, J.W.; Dutta, B.; Coolong, T.; Adkins, S.; Srinivasan, R. Virus-virus interactions in a plant host and in a hemipteran vector: Implications for vector fitness and virus epidemics. Virus Res. 2020, 286, 198069. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.B.; López-Moya, J.J. When Viruses Play Team Sports: Mixed Infections in Plants. Phytopathology 2020, 110, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Vance, V.B. Replication of potato virus X RNA is altered in coinfections with potato virus Y. Virology 1991, 182, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Mukasa, S.B.; Rubaihayo, P.R.; Valkonen, J.P.T. Interactions between a crinivirus, an ipomovirus and a potyvirus in coinfected sweetpotato plants. Plant Pathol. 2006, 55, 458–467. [Google Scholar] [CrossRef]
- Domingo-Calap, M.L.; Moreno, A.B.; Díaz Pendón, J.A.; Moreno, A.; Fereres, A.; López-Moya, J.J. Assessing the impact on virus transmission and insect vector behavior of a viral mixed infection in melon. Phytopathology 2020, 110, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Salvaudon, L.; De Moraes, C.M.; Mescher, M.C. Outcomes of co-infection by two potyviruses: Implications for the evolution of manipulative strategies. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122959. [Google Scholar] [CrossRef]
- Srinivasan, R.; Alvarez, J.M. Effect of Mixed viral infections (potato virus Y-potato leafroll virus) on biology and preference of vectors Myzus persicae and Macrosiphum euphorbiae (Hemiptera: Aphididae). J. Econ. Entomol. 2007, 100, 646–655. [Google Scholar] [CrossRef]
- Wintermantel, W.M.; Cortez, A.A.; Anchieta, A.G.; Gulati-Sakhuja, A.; Hladky, L.L. Co-Infection by two criniviruses alters accumulation of each virus in a host-specific manner and influences efficiency of virus transmission. Phytopathology 2008, 98, 1340–1345. [Google Scholar] [CrossRef]
- Benner, C.P.; Kuhn, C.W.; Demski, J.W.; Dobson, J.W.; Colditz, P.; Nutter, F.W. Identification and incidence of pepper viruses in northeastern Georgia. Plant Dis. 1985, 69, 999–1001. [Google Scholar]
- Gitaitis, R.D.; Chalfant, R.B. Epidemiology of tomato spotted wilt in pepper and tomato in southern Georgia. Plant Dis. 1998, 82, 752–756. [Google Scholar] [CrossRef]
- Zitter, T.A.; Murphy, J.F. Cucumber mosaic virus. Plant Heal. Instr. 2009. [Google Scholar] [CrossRef]
- Riley, D.G.; Joseph, S.V.; Srinivasan, R.; Diffie, S. Thrips vectors of tospoviruses. J. Integr. Pest Manag. 2011, 2, I1–I10. [Google Scholar] [CrossRef]
- Whitfield, A.E.; Ullman, D.E.; German, T.L. Tospovirus-thrips interactions. Annu. Rev. Phytopathol. 2005, 43, 459–489. [Google Scholar] [CrossRef] [PubMed]
- Pirone, T.P.; Harris, K.F. Nonpersistent transmission of plant viruses by aphids. Annu. Rev. Phytopathol. 1977, 15, 55–73. [Google Scholar] [CrossRef]
- Maris, P.C.; Joosten, N.N.; Goldbach, R.W.; Peters, D. Tomato spotted wilt virus infection improves host suitability for its vector Frankliniella occidentalis. Phytopathology 2004, 94, 706–711. [Google Scholar] [CrossRef]
- Gadhave, K.R.; Dutta, B.; Coolong, T.; Srinivasan, R. A non-persistent aphid-transmitted Potyvirus differentially alters the vector and non-vector biology through host plant quality manipulation. Sci. Rep. 2019, 9, 2503. [Google Scholar] [CrossRef]
- Munger, F. A method for rearing citrus thrips in the laboratory. J. Econ. Entomol. 1942, 35, 373–375. [Google Scholar] [CrossRef]
- Mandal, B.; Csinos, A.S.; Martinez-Ochoa, N.; Pappu, H.R. A rapid and efficient inoculation method for tomato spotted wilt tospovirus. J. Virol. Methods 2008, 149, 195–198. [Google Scholar] [CrossRef]
- Shrestha, A.; Sundaraj, S.; Culbreath, A.K.; Riley, D.G.; Abney, M.R.; Srinivasan, R. Effects of thrips density, mode of inoculation, and plant age on tomato spotted wilt virus transmission in peanut plants. Environ. Entomol. 2015, 44, 136–143. [Google Scholar] [CrossRef]
- Clark, M.F.; Adams, A.N. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef]
- Castle, S.J.; Mowry, T.M.; Berger, P.H. Differential settling by Myzus persicae (Homoptera: Aphididae) on various virus infected host plants. Ann. Entomol. Soc. Am. 1998, 91, 661–667. [Google Scholar] [CrossRef]
- Simonet, D.E.; Pienkowski, R.L. Sampling and distribution of potato leafhopper eggs in alfalfa stems. Ann. Entomol. Soc. Am. 1977, 70, 933–936. [Google Scholar] [CrossRef]
- Sundaraj, S.; Srinivasan, R.; Culbreath, A.K.; Riley, D.G.; Pappu, H.R. Host plant resistance against tomato spotted wilt virus in peanut (Arachis hypogaea) and its impact on susceptibility to the virus, virus population genetics, and vector feeding behavior and survival. Phytopathology 2014, 104, 202–210. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Searle, S.R.; Speed, F.M.; Milliken, G.A. Population marginal means in the linear model: An alternative to least squares means. Am. Stat. 1980, 34, 216–221. [Google Scholar] [CrossRef]
- Chen, G.; Su, Q.; Shi, X.; Pan, H.; Jiao, X.; Zhang, Y. Persistently transmitted viruses restrict the transmission of other viruses by affecting their vectors. Front. Physiol. 2018, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Crespo, O.; Robles, C.; Ruiz, L.; Janssen, D. Antagonism of cucumber green mottle mosaic virus against tomato leaf curl New Delhi virus in zucchini and cucumber. Ann. Appl. Biol. 2020, 176, 147–157. [Google Scholar] [CrossRef]
- Mascia, T.; Cillo, F.; Fanelli, V.; Finetti-Sialer, M.M.; De Stradis, A.; Palukaitis, P.; Gallitelli, D. Characterization of the interactions between cucumber mosaic virus and potato virus Y in mixed infections in tomato. Mol. Plant-Microbe Interact. 2010, 23, 1514–1524. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, K.C.; Gaba, V.; Wong, S.M.; Palukaitis, P.; Gal-On, A. Breakage of resistance to cucumber mosaic virus by co-infection with zucchini yellow mosaic virus: Enhancement of CMV accumulation independent of symptom expression. Arch. Virol. 2004, 149, 379–396. [Google Scholar] [CrossRef]
- Zeng, R.; Liaq, Q.; Feng, J.; Li, D.; Chen, J. Synergy between cucumber mosaic virus and zucchini yellow mosaic virus on Cucurbitaceae hosts tested by real-time reverse transcription-polymerase chain reaction. Acta Biochim. Biophys. Sin. (Shanghai) 2007, 39, 431–437. [Google Scholar] [CrossRef][Green Version]
- Fukuzawa, N.; Itchoda, N.; Ishihara, T.; Goto, K.; Masuta, C.; Matsumura, T. HC-Pro, a potyvirus RNA silencing suppressor, cancels cycling of cucumber mosaic virus in Nicotiana benthamiana plants. Virus Genes 2010, 40, 440–446. [Google Scholar] [CrossRef] [PubMed]
- García-Cano, E.; Resende, R.O.; Fernández-Muñoz, R.; Moriones, E. Synergistic interaction between tomato chlorosis virus and tomato spotted wilt virus results in breakdown of resistance in tomato. Phytopathology 2006, 96, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Boquel, S.; Giordanengo, P.; Ameline, A. Divergent effects of PVY-infected potato plant on aphids. Eur. J. Plant Pathol. 2011, 129, 507–510. [Google Scholar] [CrossRef]
- Jiménez, J.; Webster, C.G.; Moreno, A.; Almeida, R.P.P.; Blanc, S.; Fereres, A.; Uzest, M. Fasting alters aphid probing behaviour but does not universally increase the transmission rate of non-circulative viruses. J. Gen. Virol. 2017, 98, 3111–3121. [Google Scholar] [CrossRef] [PubMed]
- Ziebell, H.; Murphy, A.M.; Groen, S.C.; Tungadi, T.; Westwood, J.H.; Lewsey, M.G.; Moulin, M.; Kleczkowski, A.; Smith, A.G.; Stevens, M.; et al. Cucumber mosaic virus and its 2b RNA silencing suppressor modify plant-aphid interactions in tobacco. Sci. Rep. 2011, 1, 187. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Gao, Y.; Yan, S.; Tang, X.; Zhou, X.; Zhang, D.; Liu, Y. Aphid performance changes with plant defense mediated by cucumber mosaic virus titer. Virol. J. 2016, 13, 70. [Google Scholar] [CrossRef]
- Nachappa, P.; Margolies, D.C.; Nechols, J.R.; Whitfield, A.E.; Rotenberg, D. Tomato spotted wilt virus benefits a non-vector arthropod, Tetranychus urticae, by modulating different plant responses in tomato. PLoS ONE 2013, 8, e75909. [Google Scholar] [CrossRef]
- Belliure, B.; Sabelis, M.W.; Janssen, A. Vector and virus induce plant responses that benefit a non-vector herbivore. Basic Appl. Ecol. 2010, 11, 162–169. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Evidence of local adaptation in plant virus effects on host-vector interactions. Integr. Comp. Biol. 2014, 54, 193–209. [Google Scholar] [CrossRef]
- Chang, C.L. Effect of amino acids on larvae and adults of Ceratitis capitata (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2004, 97, 529–535. [Google Scholar] [CrossRef]
- Smykal, V.; Raikhel, A.S. Nutritional control of insect reproduction. Curr. Opin. Insect Sci. 2015, 11, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Reitz, S.R.; Gao, Y.; Kirk, W.D.J.; Hoddle, M.S.; Leiss, K.A.; Funderburk, J.E. Invasion biology, ecology, and management of western flower thrips. Annu. Rev. Entomol. 2020, 65, 17–37. [Google Scholar] [CrossRef] [PubMed]



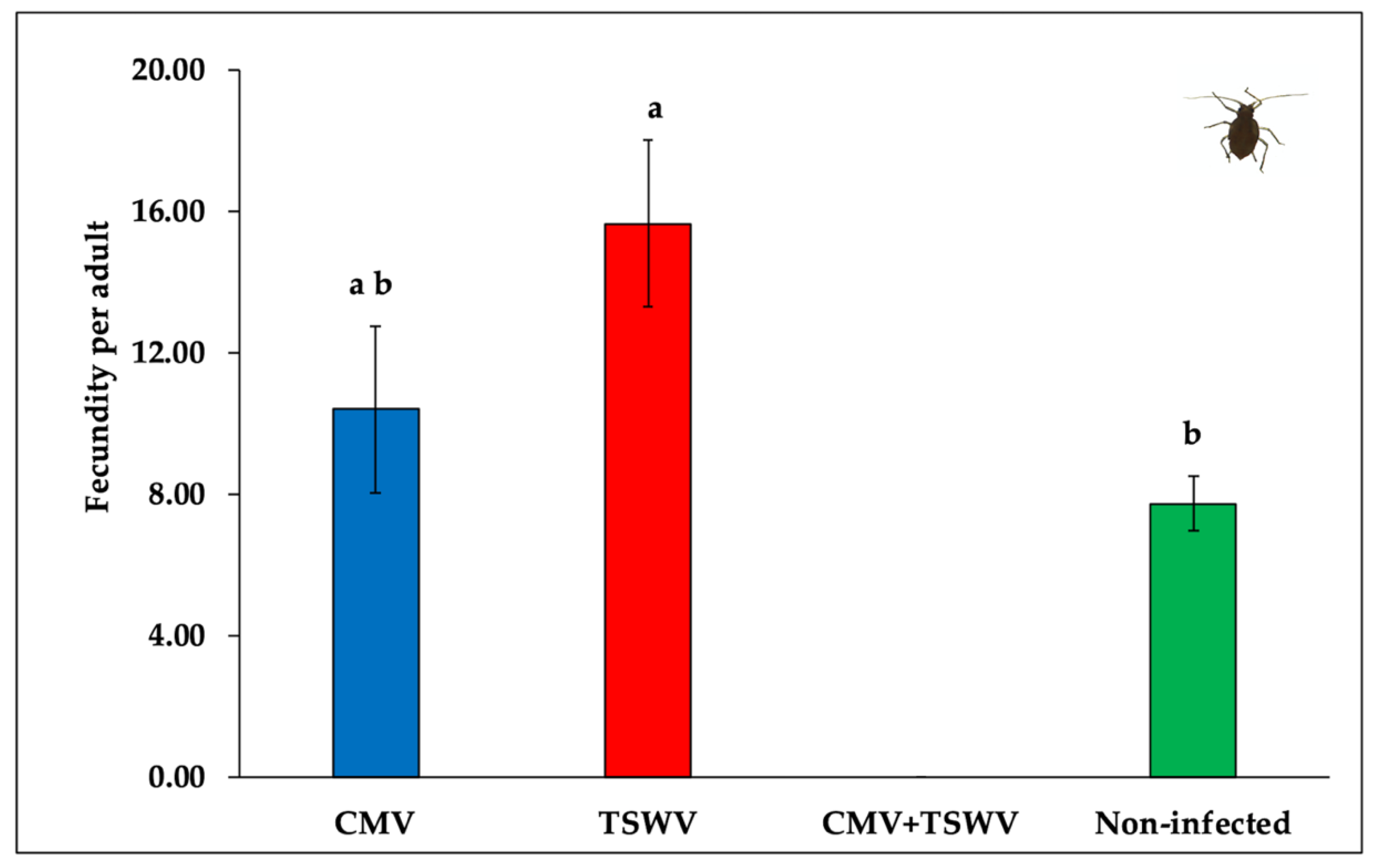
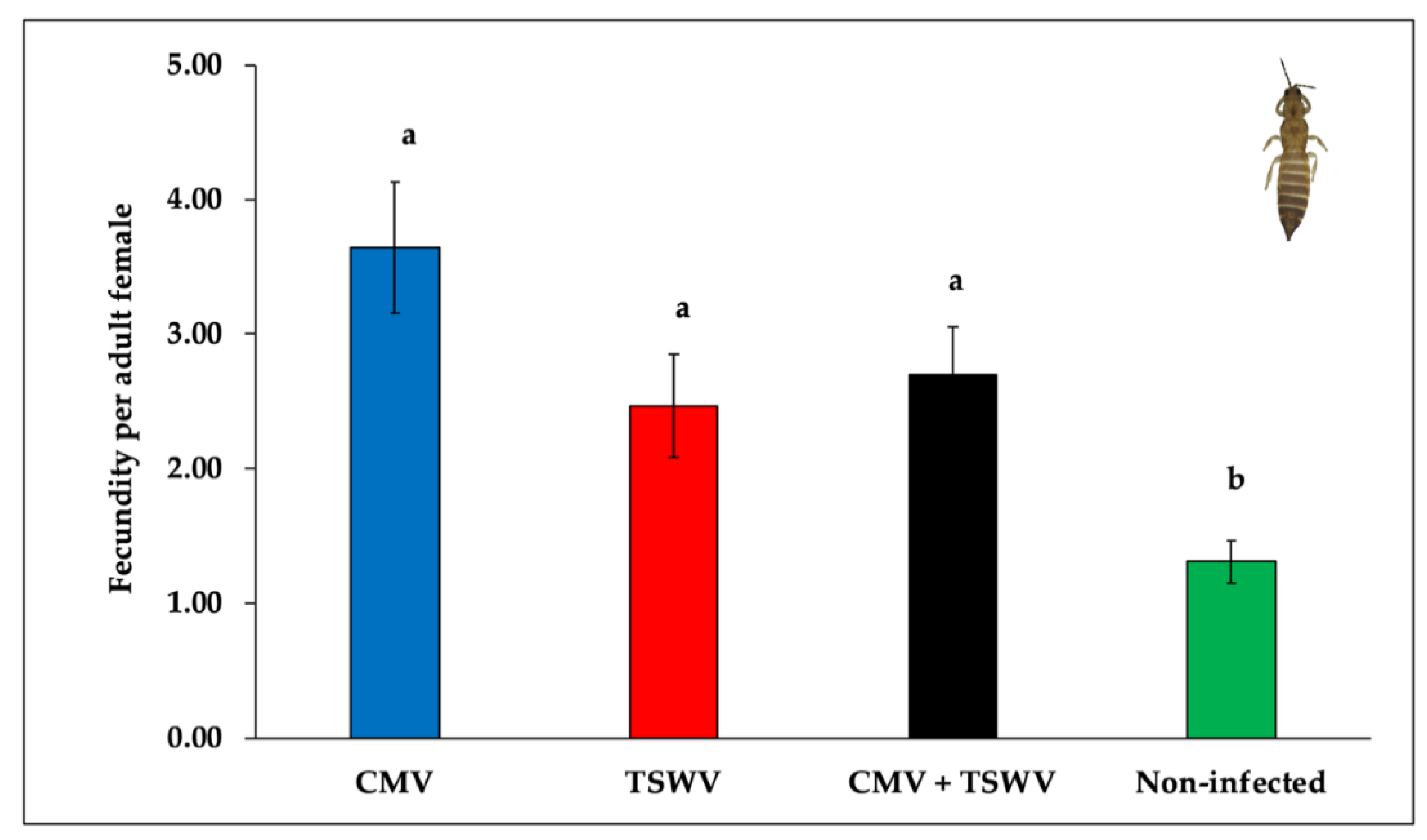

| Treatments | N a | Nymph-Adult b |
|---|---|---|
| CMV | 45 | 8 (6–9) a |
| TSWV | 23 | 9 (7–10) a |
| CMV and TSWV | 26 | 8 (7–9) a |
| Non-infected | 30 | 9 (7–9) a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam, S.; Mugerwa, H.; Sundaraj, S.; Gadhave, K.R.; Murphy, J.F.; Dutta, B.; Srinivasan, R. Specific and Spillover Effects on Vectors Following Infection of Two RNA Viruses in Pepper Plants. Insects 2020, 11, 602. https://doi.org/10.3390/insects11090602
Gautam S, Mugerwa H, Sundaraj S, Gadhave KR, Murphy JF, Dutta B, Srinivasan R. Specific and Spillover Effects on Vectors Following Infection of Two RNA Viruses in Pepper Plants. Insects. 2020; 11(9):602. https://doi.org/10.3390/insects11090602
Chicago/Turabian StyleGautam, Saurabh, Habibu Mugerwa, Sivamani Sundaraj, Kiran R. Gadhave, John F. Murphy, Bhabesh Dutta, and Rajagopalbabu Srinivasan. 2020. "Specific and Spillover Effects on Vectors Following Infection of Two RNA Viruses in Pepper Plants" Insects 11, no. 9: 602. https://doi.org/10.3390/insects11090602
APA StyleGautam, S., Mugerwa, H., Sundaraj, S., Gadhave, K. R., Murphy, J. F., Dutta, B., & Srinivasan, R. (2020). Specific and Spillover Effects on Vectors Following Infection of Two RNA Viruses in Pepper Plants. Insects, 11(9), 602. https://doi.org/10.3390/insects11090602







