The Exocrine Chemistry of the Parasitic Wasp Sphecophaga orientalis and Its Host Vespa orientalis: A Case of Chemical Deception?
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
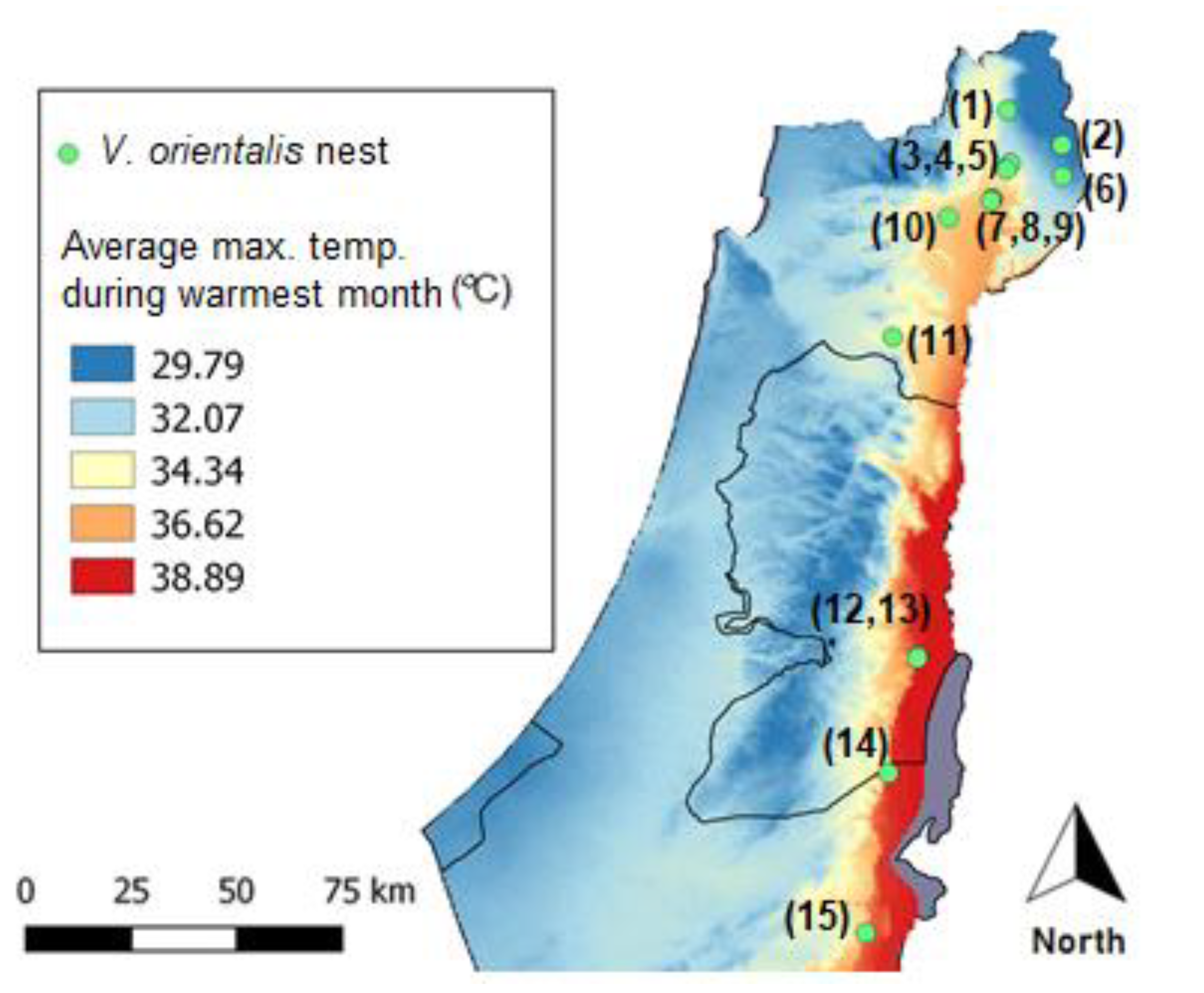
2.1. Collection of Hornets
2.2. Parasitization Analysis
2.3. Cuticular Hydrocarbon Analysis
2.4. Statistical Analysis
3. Results
3.1. Parasitization Analysis
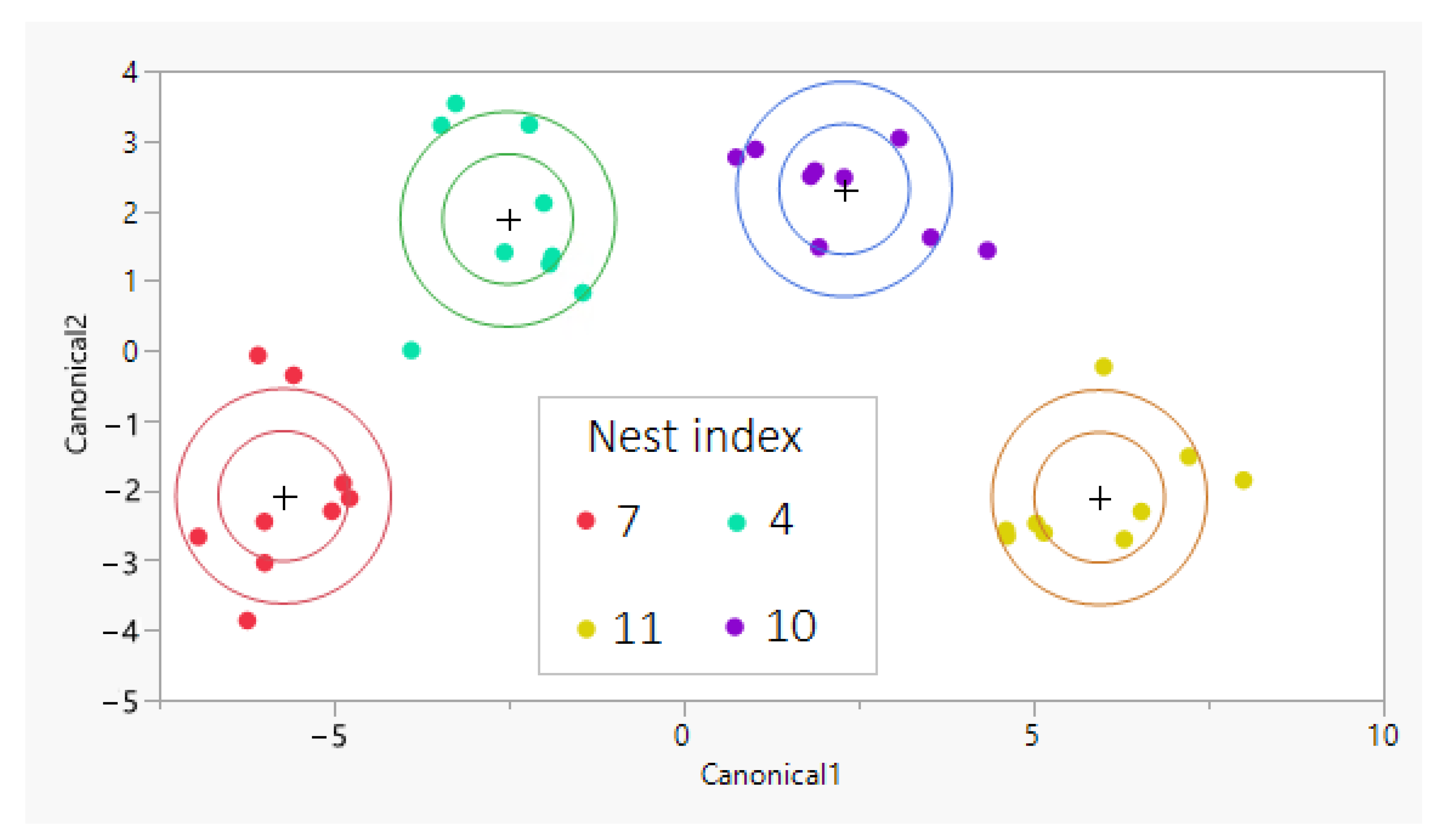
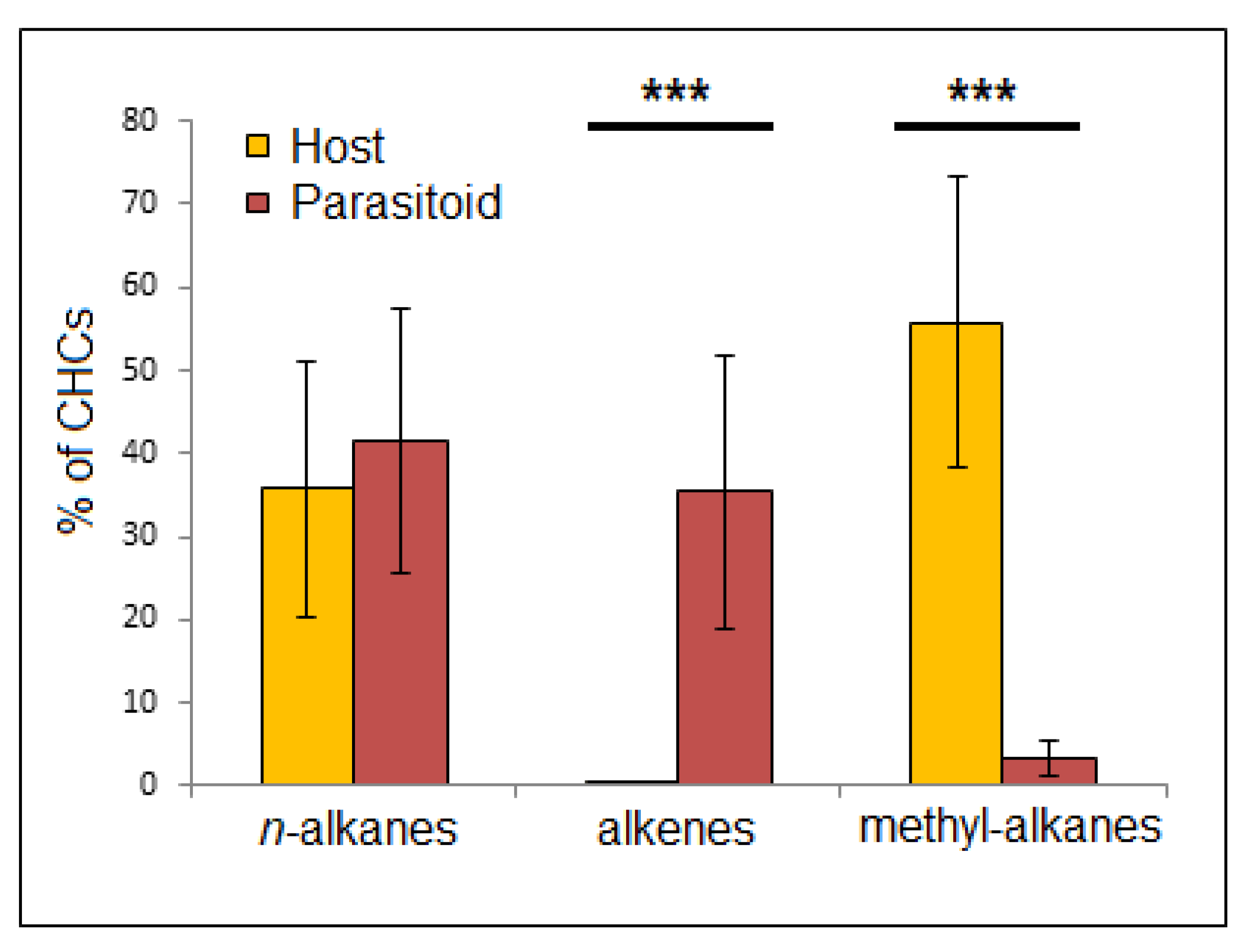
3.2. Cuticular Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richard, F.J.; Hunt, J. Intracolony chemical communication in social insects. Insectes Soc. 2013, 60, 275–291. [Google Scholar] [CrossRef]
- Lorenzi, M.C.; d’Ettorre, P. Nestmate recognition in social insects: What does it mean being chemical insignificant? Front. Ecol. Evol. 2019, 7, 488. [Google Scholar] [CrossRef]
- Dani, F.R. Cuticular lipids as semiochemicals in paper wasps and other social insects. Ann. Zool. Fenn. 2006, 43, 500–514. [Google Scholar]
- Lenoir, A.; Fresneau, D.; Errard, C.; Hefetz, A. Individuality and colonial identity in ants: The emergence of the social representation concept. In Information Processing in Social Insects; Springer: Berlin/Heidelberg, Germany, 1999; pp. 219–237. [Google Scholar]
- Blomquist, G.J.; Bagnères, A.G. Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- d’Ettorre, P.; Mondy, N.; Lenoir, A.; Errard, C. Blending in with the crowd: Social parasites integrate into their host colonies using a flexible chemical signature. Proc. R. Soc. B. 2002, 269, 1911–1918. [Google Scholar] [CrossRef]
- Ayasse, M.; Paxton, R. Brood protection in social insects. In Chemoecology of Insect Eggs and Egg Deposition; Hilker, M., Meiners, T., Eds.; Blackwell: Berlin, Germany, 2002; Volume 117, p. 148. [Google Scholar]
- Jeanne, R.L. Chemical defense of brood by a social wasp. Science 1970, 168, 1465–1466. [Google Scholar] [CrossRef]
- Foitzik, S.; DeHeer, C.J.; Hunjan, D.N.; Herbers, J.M. Coevolution in host–parasite systems: Behavioural strategies of slave–making ants and their hosts. Proc. R. Soc. B. 2001, 268, 1139–1146. [Google Scholar] [CrossRef]
- Wilson, E.O. Leptothorax duloticus and the beginnings of slavery in ants. Evolution 1975, 108–119. [Google Scholar] [CrossRef]
- Lenoir, A.; d’Ettorre, P.; Errard, C.; Hefetz, A. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 2001, 46, 573–599. [Google Scholar] [CrossRef]
- Cremer, S.; Pull, C.D.; Fürst, M.A. Social immunity: Emergence and evolution of colony-level disease protection. Annu. Rev. Entomol. 2018, 63, 105–123. [Google Scholar] [CrossRef]
- Moritz, R.; Crewe, R. Air ventilation in nests of two African stingless bees Trigona denoiti and Trigona gribodoi. Experientia 1988, 44, 1024–1027. [Google Scholar] [CrossRef]
- Franks, N.R.; Mallon, E.B.; Bray, H.E.; Hamilton, M.J.; Mischler, T.C. Strategies for choosing between alternatives with different attributes: Exemplified by house-hunting ants. Anim. Behav. 2003, 65, 215–223. [Google Scholar] [CrossRef]
- Fuchs, S.; Tautz, J. Colony defence and natural enemies. In Honeybees of Asia; Hepburn, H.R., Radloff, S.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 369–395. [Google Scholar]
- Shorter, J.; Rueppell, O. A review on self-destructive defense behaviors in social insects. Insectes Soc. 2012, 59, 1–10. [Google Scholar] [CrossRef]
- Parmentier, T.; De Laender, F.; Wenseleers, T.; Bonte, D. Prudent behavior rather than chemical deception enables a parasite to exploit its ant host. Behav. Ecol. 2018, 29, 1225–1233. [Google Scholar] [CrossRef]
- Hölldobler, B.; Kwapich, C.L. Amphotis marginata (Coleoptera: Nitidulidae) a highwayman of the ant Lasius fuliginosus. PLoS ONE 2017, 12, e0180847. [Google Scholar] [CrossRef]
- Eggleton, P.; Belshaw, R. Insect parasitoids: An evolutionary overview. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1992, 337, 1–20. [Google Scholar]
- Field, R.; Darby, S. Host specificity of the parasitoid, Sphecophaga vesparum (Curtis) (Hymenoptera: Ichneumonidae), a potential biological control agent of the social wasps, Vespula germanica (Fabricius) and V. vulgaris (Linnaeus) (Hymenoptera: Vespidae) in Australia. N. Z. J. Zool. 1991, 18, 193–197. [Google Scholar] [CrossRef]
- Oi, C.A.; Brown, R.L.; Stevens, I.; Wenseleers, T. Hydrocarbon ignatures of the ectoparasitoid Sphecophaga vesparum shows wasp host dependency. Insects 2020, 11, 268. [Google Scholar] [CrossRef]
- Donovan, B. Life cycle of Sphecophaga vesparum (Curtis) (Hymenoptera: Ichneumonidae), a parasitoid of some vespid wasps. N. Z. J. Zool. 1991, 18, 181–192. [Google Scholar] [CrossRef]
- Havron, A.; Margalith, Y. Parasitization of Vespa orientalis nests by Sphecophaga vesparum curtis in Southern Israel (Hymenoptera: Vespidae, Ichneumonidae). Phytoparasitica 1995, 23, 19–25. [Google Scholar] [CrossRef]
- Donovan, B.J. Description of Sphecophaga orientalis sp. n. (Hymenoptera: Ichneumonidae: Cryptinae), a potential parasitoid of Vespula spp. (Hymenoptera: Vespidae: Vespinae) in New Zealand. N. Z. Entomol. 2002, 25, 3–15. [Google Scholar] [CrossRef]
- Dvořák, L. Oriental Hornet Vespa orientalis Linnaeus, 1771 found in Mexico (Hymenoptera, Vespidae, Vespinae). Entomol. Prob. 2006, 36, 80. [Google Scholar]
- Ishay, J. Comb bulding by the oriental hornet (Vespa orientalis). Anim. Behav. 1976, 24, 72–83. [Google Scholar] [CrossRef]
- Ishay, J.; Ikan, R. Food exchange between adults and larvae in Vespa orientalis F. Anim. Behav. 1968, 16, 298–303. [Google Scholar] [CrossRef]
- Carpenter, J.; Kojima, J.; Villemant, C. Phylogeny of hornets: A total evidence approach (Hymenoptera, Vespidae, Vespinae, Vespa). J. Hymenopt. Res. 2013, 32, 1–15. [Google Scholar]
- Glaiim, M.K. Hunting behavior of the oriental hornet, Vespa orientalis L., and defense behavior of the honey bee Apis melifera L., in Iraq. Bull. Iraq Nat. Hist. Mus. 2009, 10, 17–30. [Google Scholar]
- Taha, A.A. Effect of some climatic factors on the seasonal activity of oriental wasp, Vespa orientalis L. attackting honeybee colonies in Dakahlia governorate, Egypt. Egypt. J. Agric. Res. 2014, 92, 43–51. [Google Scholar]
- Uboni, A.; Bagnères, A.-G.; Christidès, J.-P.; Lorenzi, M.C. Cleptoparasites, social parasites and a common host: Chemical insignificance for visiting host nests, chemical mimicry for living in. J. Insect Physiol. 2012, 58, 1259–1264. [Google Scholar] [CrossRef]
- Martin, S.J.; Takahashi, J.-I.; Ono, M.; Drijfhout, F.P. Is the social parasite Vespa dybowskii using chemical transparency to get her eggs accepted? J. Insect Physiol. 2008, 54, 700–707. [Google Scholar] [CrossRef]
- Dani, F.R.; Jones, G.R.; Corsi, S.; Beard, R.; Pradella, D.; Turillazzi, S. Nestmate recognition cues in the honey bee: Differential importance of cuticular alkanes and alkenes. Chem. Senses 2005, 30, 477–489. [Google Scholar] [CrossRef]
- Breed, M.D. Recognition pheromones of the honey bee. Bioscience 1998, 48, 463–470. [Google Scholar] [CrossRef]
- Dani, F.R.; Jones, G.R.; Destri, S.; Spencer, S.H.; Turillazzi, S. Deciphering the recognition signature within the cuticular chemical profile of paper wasps. Anim. Behav. 2001, 62, 165–171. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Pirk, C.W.; Crewe, R.M.; Njagi, P.G.; Gordon, I.; Torto, B. Nestmate recognition and the role of cuticular hydrocarbons in the African termite raiding ant Pachycondyla analis. J. Chem. Ecol. 2010, 36, 441–448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akino, T.; Yamamura, K.; Wakamura, S.; Yamaoka, R. Direct behavioral evidence for hydrocarbons as nestmate recognition cues in Formica japonica (Hymenoptera: Formicidae). Appl. Entomol. Zool. 2004, 39, 381–387. [Google Scholar] [CrossRef]
- Martin, S.J.; Carruthers, J.M.; Williams, P.H.; Drijfhout, F.P. Host specific social parasites (Psithyrus) indicate chemical recognition system in bumblebees. J. Chem. Ecol. 2010, 36, 855–863. [Google Scholar] [CrossRef]
- Butts, D.; Camann, M.; Espelie, K. Workers and queens of the European hornet Vespa crabro L. have colony-specific cuticular hydrocarbon profiles (Hymenoptera: Vespidae). Insectes Soc. 1995, 42, 45–55. [Google Scholar] [CrossRef]
- Ebrahimi, E.; Carpenter, J.M. Distribution pattern of the hornets Vespa orientalis and V. crabro in Iran: (Hymenoptera: Vespidae). Zool. Middle East 2012, 56, 63–66.47. [Google Scholar] [CrossRef]
- Balbuena, M.S.; González, A.; Farina, W.M. Characterizing honeybee cuticular hydrocarbons during foraging. Sociobiology 2019, 661, 97–106. [Google Scholar] [CrossRef]
- McAfee, A.; Chapman, A.; Iovinella, I.; Gallagher-Kurtzke, Y.; Collins, T.F.; Higo, H.; Madilao, L.L.; Pelosi, P.; Foster, L.J. A death pheromone, oleic acid, triggers hygienic behavior in honey bees (Apis mellifera L.). Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, X. Corpse management in social insects. Int. J. Biol. Sci. 2013, 9, 313. [Google Scholar] [CrossRef]
- Diez, L.; Moquet, L.; Detrain, C. Post-mortem changes in chemical profile and their influence on corpse removal in ants. J. Chem. Ecol. 2013, 39, 1424–1432. [Google Scholar] [CrossRef]
- Wilson, E.O.; Durlach, N.I.; Roth, L.M. Chemical releasers of necrophoric behavior in ants. Psyche 1958, 65, 108–114. [Google Scholar] [CrossRef]
- Sun, Q.; Haynes, K.F.; Zhou, X. Dynamic changes in death cues modulate risks and rewards of corpse management in a social insect. Funct. Ecol. 2017, 31, 697–706. [Google Scholar] [CrossRef]
- Gordon, D.M. Dependence of necrophoric response to oleic acid on social context in the ant, Pogonomyrmex badius. J. Chem. Ecol. 1983, 9, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Westoby, M.t.; Jurado, E. Convergence of elaiosomes and insect prey: Evidence from ant foraging behaviour and fatty acid composition. Funct. Ecol. 1994, 358–365. [Google Scholar] [CrossRef]
- Zimma, B.; Ayasse, M.; Tengö, J.; Ibarra, F.; Schulz, C.; Francke, W. Do social parasitic bumblebees use chemical weapons? (Hymenoptera, Apidae). J. Comp. Physiol. A 2003, 189, 769–775. [Google Scholar] [CrossRef] [PubMed]
- D’Ettorre, P.; Errard, C.; Ibarra, F.; Francke, W.; Hefetz, A. Sneak in or repel your enemy: Dufour’s gland repellent as a strategy for successful usurpation in the slave-maker Polyergus rufescens. Chemoecology 2000, 10, 135–142. [Google Scholar] [CrossRef]
- Vidari, G.; De Bernardi, M.; Pavan, M.; Ragozzino, L. Rose oxide and iridodial from Aromia moschata L. (Coleoptera: Cerambycidae). Tetrahedron Lett. 1973, 14, 4065–4068. [Google Scholar] [CrossRef]
- Dettner, K.; Fettköther, R.; Ansteeg, O.; Deml, R.; Liepert, C.; Petersen, B.; Haslinger, E.; Francke, W. Insecticidal fumigants from defensive glands of insects a fumigant test with adults of Drosophila melanogaster. J. Appl. Entomol. 1992, 113, 128–137. [Google Scholar] [CrossRef]

| Step | Thorax | Abdomen |
|---|---|---|
| 1 | 13,15-dimethyl-C29 | 2-methyl-C26 |
| 2 | 11- and 13-methyl-C29 | 13,15-dimethyl-C29 |
| 3 | 3-methyl-C29 | 11,15-dimethyl-C27 |
| 4 | 12,16-dimethyl-C28 | n-C27 |
| 5 | - | 11- and 13-methyl-C27 |
| 6 | - | 4-methyl-C26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubiner, S.; Cohen, N.; Volov, M.; Hefetz, A.; Seltzer, R.; Levin, E. The Exocrine Chemistry of the Parasitic Wasp Sphecophaga orientalis and Its Host Vespa orientalis: A Case of Chemical Deception? Insects 2021, 12, 2. https://doi.org/10.3390/insects12010002
Dubiner S, Cohen N, Volov M, Hefetz A, Seltzer R, Levin E. The Exocrine Chemistry of the Parasitic Wasp Sphecophaga orientalis and Its Host Vespa orientalis: A Case of Chemical Deception? Insects. 2021; 12(1):2. https://doi.org/10.3390/insects12010002
Chicago/Turabian StyleDubiner, Shahar, Nitzan Cohen, Mika Volov, Abraham Hefetz, Rya Seltzer, and Eran Levin. 2021. "The Exocrine Chemistry of the Parasitic Wasp Sphecophaga orientalis and Its Host Vespa orientalis: A Case of Chemical Deception?" Insects 12, no. 1: 2. https://doi.org/10.3390/insects12010002
APA StyleDubiner, S., Cohen, N., Volov, M., Hefetz, A., Seltzer, R., & Levin, E. (2021). The Exocrine Chemistry of the Parasitic Wasp Sphecophaga orientalis and Its Host Vespa orientalis: A Case of Chemical Deception? Insects, 12(1), 2. https://doi.org/10.3390/insects12010002





