Simple Summary
Pestalotiopsis fungal complex is a disease that causes damages in oil palm (Elaeis guineensis), and the lace bug, Leptopharsa gibbicarina is the main insect pest that spread this disease. Application of neurotoxic insecticides has been a common method used to control L. gibbicarina for decades in Colombia and Venezuela. The effects of four benzoylphenyl ureas (BPUs) (lufenuron, novaluron, teflubenzuron, and triflumuron) were assessed against L. gibbicarina for toxicity, survival, and reproduction. Overall, the results show that novaluron, teflubenzuron, and triflumuron cause high mortality and reduce survival time, fecundity, and fertility. Thus, BPUs exhibit detrimental effects on L. gibbicarina and can be used as alternatives to other chemical insecticides.
Abstract
The lace bug, Leptopharsa gibbicarina is a vector of Pestalotiopsis fungal complex in oil palm crops in the Americas. The effects of four benzoylphenyl ureas (BPUs) (lufenuron, novaluron, teflubenzuron, and triflumuron) were evaluated against L. gibbicarina for toxicity, survival, reproduction, and mortality in semi-field conditions. Concentration-mortality bioassays demonstrated that novaluron (LC50 = 0.33 ppm), teflubenzuron (LC50 = 0.24 ppm), lufenuron (LC50 = 0.17 ppm), and triflumuron (LC50 = 0.42 ppm) are toxic to L. gibbicarina nymphs. The survival rate was 99% in control nymphs, decreasing to 50% in nymphs exposed to LC50 of triflumuron, 47% in nymphs treated with lufenuron, 43% in nymphs treated with teflubenzuron, and 43% in those treated with novaluron. Sublethal concentrations of BPUs showed detrimental effects on the adult emergence, longevity, fecundity, and fertility of this insect. The mortality of nymphs caused by these insecticides was similar in both laboratory and semi-field conditions. Our results suggest that novaluron, teflubenzuron, and triflumuron are highly effective against L. gibbicarina, and therefore, have potential applications for this oil palm pest.
1. Introduction
The lace bug, Leptopharsa gibbicarina Froeschner (Hemiptera: Tingidae) is a significant pest and main vector of the Pestalotiopsis leaf spot in oil palm (Elaeis guineensis Jacq. (Arecales: Arecaceae)) in Colombia and Venezuela. This insect damages other palm trees species, such as Aiphanes horrida (Jacquin) Burret, Bactris gasipaes (Kunth), and Elaeis oleifera (Kunth) [1]. The life cycle of L. gibbicarina is 69 days (egg = 15, nymph = 22, and adult = 32) [2]. This insect can reach high infestations in oil palms with different steps of the Pestalotiopsis fungal complex (Pestalotiopsis palmarum (Cooke) Steyaert and Pestalotiopsis glandicola (Castagne) Steyaert) evolution [3]. The severity of the Pestalotiopsis leaf spot disease seems to be due to the easy access given to the oil palm leaves by the piercing and sucking activities of L. gibbicarina [4,5].
In Colombia, chemical insecticides, such as deltamethrin, methamidophos, methyl parathion, and permethrin, are used on oil palm crops to control L. gibbicarina [6,7,8], but monocrotophos is the preferred compound, due to its reliably high efficacy [8]. Monocrotophos, a neurotoxic insecticide of the organophosphate group, is applied in oil palm trees by trunk injection or the root absorption method [9,10], and acts via ingestion by or contact with insects [9,10]. In commercial oil palm plantations, this insecticide is a hazardous compound, because residues have been found in crude oil [11]. Also, monocrotophos is banned in the European Union, United States, and several Latin American countries [12,13]. Alternatives that are more sustainable or different from monocrotophos are needed to substitute the principal insecticide used for the past 50 years against L. gibbicarina [6].
The application of chemical insecticides is an effective strategy for controlling pest populations [14,15,16], and the use of biorational insecticides is a valuable insect pest management option for oil palm plantations [17]. The current suite of biorational insecticides includes benzoylphenyl ureas (BPUs), characterized by biological activity interfering with developmental processes of insects [17]. In this context, BPUs and their effectiveness have also been reported to control oil palm pests like Coptotermes curvignathus Holmgren (Blattodea: Rhinotermitidae) in Malaysia [18], Euprosterna elaeasa Dyar (Lepidoptera: Limacodidae) in Colombia [19], and Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) in the United States [20]. The mode of action of BPUs remains elusive; however, evidence indicates that these insecticides inhibit the N-acetylglucosamine incorporation into insect chitin in vivo, altering of transport of proteins involved in chitin polymerization [21]. Especially, BPUs acts on immature insect stages (as ovicide or larvicide), with a broad spectrum against Diptera [22], Coleoptera [23], Hemiptera [24], Lepidoptera [25], and Neuroptera [26].
BPUs are chemical substance derivates of urea (H2NCONH2) [27] and are classified as inhibitors of chitin synthesis (affecting CHS1), according to the Insecticide Resistance Action Committee (IRAC, group 15) [28]. In particular, active ingredients like lufenuron, novaluron, teflubenzuron, and triflumuron are used successfully to control hemipterous disease vectors [24,29]. Non-neurotoxic insecticides like BPUs can be used against the oil palm pest, favoring an effective approach toward Integrated Resistance Management (IRM). There are a variety of insecticides that have neurotoxic properties used to control L. gibbicarina; however, the availability and use of biorational insecticides as BPUs is an alternative for pest management programs for oil palm. We hypothesized that the effects of BPUs reduce the number of nymphs and adults of L. gibbicarina, which could be due to its ability to inhibit chitin biosynthesis.
This research evaluated the insecticidal activity of four BPUs to control L. gibbicarina, explained in different experiments evaluating their (i) toxicity, (ii) survivorship, (iii) reproduction, and (iv) mortality in field conditions. Our objective was to contribute to the development of strategies for controlling L. gibbicarina, as the current main replacement for monocrotophos against this species.
2. Materials and Methods
2.1. Lace Bugs
In the field, adults of L. gibbicarina were collected from palm trees in Brisas Oil palm plantation (Puerto Wilches, State of Santander, Colombia), placed into plastic boxes (45 × 45 × 90 cm), and transported to the entomology laboratory to establish a colony in laboratory conditions. Leptopharsa gibbicarina adults (males and females, 1:1 ratio) were isolated in glass tubes (5 × 27 cm) containing E. guineensis leaflets. After female copulation, eggs oviposited on the surface of the leaflets were collected every 24 h and placed in glass tubes containing cotton wool saturated with distilled water. After hatching, nymphs were placed on a leaf of nursery oil palm tree (4 months old), isolated with an organza bag (45 × 90 cm), and maintained in a climatized room (27 ± 1 °C, 75–85% relative humidity, and light/dark 12:12 h cycle) until adult emergence. Newly third-instar nymphs and adults of L. gibbicarina were used in the laboratory and semi-field bioassays.
2.2. Concentration-Mortality Bioassay
The following commercial BPU formulations were diluted in 100 mL of deionized water to obtain six dilutions (ranging from 75 to 2400 ppm): lufenuron (Match EC, Syngenta Crop Protection S.A., Monthey, Swaziland), 50 g L−1; novaluron (Rimon EC, Makhteshim Chemical Works Ltd., Beer-Sheva, Israel), 100 g L−1; teflubenzuron (Dart SC, Dynamit Nobel GmbH, Leverkusen, Germany), 150 g L−1; and triflumuron (Alsystin SC, Bayer CropScience AG, Dormagen, Germany), 480 g L−1. Serial dilutions of each insecticide were used to assess toxicity and determine the concentration-mortality relationship and lethal concentrations (LC25, LC50, LC75, and LC90). Water alone was used as a control. Subsequently, each insecticide concentration (0.5 µL) was applied on the body of 50 third-instar L. gibbicarina nymphs using a Hamilton microsyringe (KH Hamilton Storage GmbH, Domat/Ems, Switzerland). The insects were individualized in glass tubes (1 × 12.5 cm) and maintained in a climatized room. A piece (1 × 9 cm) of E. guineensis leaf was provided daily as food before insecticide/control exposure. Three replicates of 50 nymphs were used for each concentration. The experimental design was completely randomized and the number of dead nymphs was recorded after 72 h of exposure.
2.3. Time-Mortality Bioassay
Leptopharsa gibbicarina nymphs were exposed to the lethal concentrations (LC50 and LC25) of each insecticide, as determined by the concentration-mortality bioassay. Water was used as a control. Exposure procedures and insect conditions were the same as described above (Section 2.2), with three replicates of 50 nymphs per treatment, following a completely randomized design. The number of live nymphs was counted every 6 h for 3 d.
2.4. Adult Emergence, Longevity, and Reproduction
Leptopharsa gibbicarina nymphs were exposed to lethal concentration (LC25) of each insecticide and monitored until adult emergence. In the control group, insects were exposed to water. The general maintenance of insects and plants were as described above. Newly emerged adults of L. gibbicarina were removed, sexed, and grouped into mating pairs. Each mating pair was then transferred to a single leaflet of a nursery oil palm tree of the same treatment and covered with organza fabric (5 × 50 cm) to prevent insect escape. Emergence and adult longevity was recorded every day until female/male death. Also, the number of eggs/female and number of nymphs/female hatched from these eggs were used to calculate fecundity and fertility, respectively.
2.5. Semi-Field Assays in Oil Palm Trees
The bioassay was conducted in five-year-old commercial oil palm plantations (varieties “Tenera” and “Deli Ghana”) in the county of Puerto Wilches (Santander, Colombia), with an average temperature of 27.59 °C, 76–89% relative humidity, 1490 to 2235 h of sunshine per year, and 2283 mm annual rainfall. Under these natural conditions, 50 palm trees were selected, and L. gibbicarina nymphs were used for each treatment in the controlled semi-field bioassay. For each palm tree, 50 nymphs were placed on leaf no. 17, according to the rules of phyllotaxy [30], and isolated with an organza bag (0.65 × 0.65 × 1.25 m) for 48 h to ensure natural insect distribution. Each insecticide was prepared to the LC90 level in water and used as treatments with five replications. Water was used as the control. Treatments were applied 48 h after placing the organza bag, and applications of 200 mL of each insecticide per leaf were made using a manual pump spray (Royal Condor, Soacha, Cundinamarca, Colombia; 1.8 L capacity) at 32 psi. Leaves from palm trees were cut with a Malay knife, carefully dissected, and checked for the presence of live or dead L. gibbicarina nymphs, which were then counted. For the treatment group on the cut leaf, L. gibbicarina mortality caused by insecticides was recorded every 15 d with a completely random experimental design.
2.6. Statistical Analysis
The concentration-mortality data were submitted to probit analysis to construct a concentration-mortality curve with the PROC PROBIT procedure (SAS Institute, Campus Drive Cary, NC, USA). Time-mortality bioassays were analyzed for survival analysis (Kaplan-Meier estimators, log-rank test) using the Prism 7.0 software (GraphPad Prism Software Inc., San Diego, CA, USA). Nymphs that remained alive at the end of the bioassay were censored for the analyses. Data on emergence, longevity, reproductive (fecundity and fertility) parameters, and field mortality of L. gibbicarina were subjected to one-way analysis of variance (ANOVA), with treatment as a fixed effect, and Tukey’s honest significance difference (HSD) test (p < 0.05) was used a mean separation test and analyzed with SAS 9.0 software. Data on emergence, longevity, reproduction, and field mortality test were arcsine-transformed to satisfy the premises of normality and homoscedasticity.
3. Results
3.1. Concentration-Mortality Bioassay
The concentration-response model used was suitable (p > 0.05), which confirmed the toxicity of each insecticide to L. gibbicarina and provided the estimates of the desired toxicological endpoints for subsequent use (Table 1). For the estimated LC50 value, testing indicated that novaluron with LC50 = 0.55 (0.36–0.74) ppm was the most effective BPU insecticide for L. gibbicarina, followed by teflubenzuron with LC50 = 1.71 (1.44–1.89) ppm, lufenuron with LC50 = 2.05 (1.78–2.33) ppm, and triflumuron with LC50 = 2.38 (2.07–2.71) ppm. In the control, mortality remained at <1%.

Table 1.
Lethal concentration of four benzoylphenyl ureas (BPUs) against Leptopharsa gibbicarina nymphs after 72 h exposure, obtained from probit analysis (df = 5). The chi-square (χ2) value refers to the goodness of fit test at p > 0.05.
3.2. Time-Mortality Bioassay
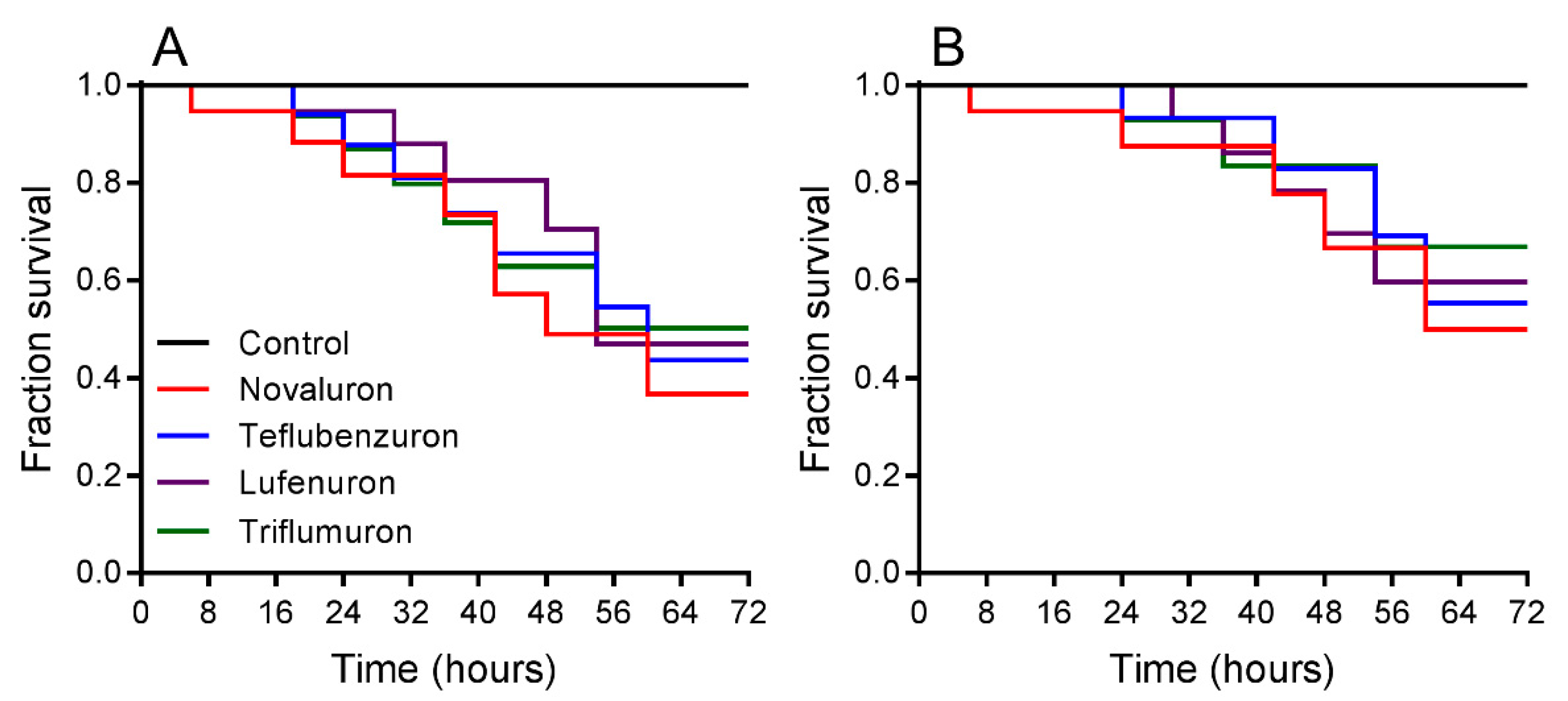
Survival rates were registered when L. gibbicarina nymphs were exposed for 3 d to BPUs and indicated differences at LC50 (log-rank test; χ2 = 15.53, df = 4, p < 0.0001) (Figure 1A). For the treatments, L. gibbicarina survival decreased from 99.9% in the control to 50.3% with triflumuron, 47.1% with lufenuron, 43.2% with teflubenzuron, and 36.7% with novaluron. Survival rates differed between treatments at LC25 (log-rank test; χ2 = 8.94, df = 4, and p < 0.0012). Leptopharsa gibbicarina survival decreased from 99.9% in the control to 66.9% with triflumuron, 59.7% with lufenuron, 55.3% with teflubenzuron, and 50.01% with novaluron (Figure 1B).
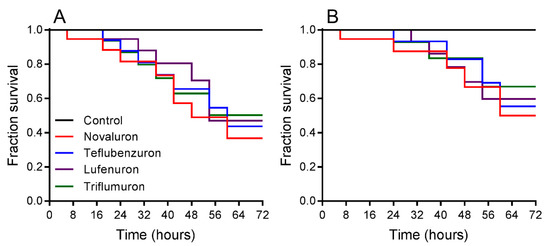
Figure 1.
Survival curves of Leptopharsa gibbicarina nymphs exposed to four BPUs, estimated using the Kaplan-Meier log-rank test. Lethal concentrations: (A) LC50 (χ2 = 15.53, p < 0.0001) and (B) LC25 (χ2 = 8.94, p < 0.0012).
3.3. Adult Emergence, Longevity, and Reproduction
The effects caused by four BPUs on L. gibbicarina adults, such as emergence, survival, fecundity, and fertility, were determined (Table 2). The emergence of L. gibbicarina adults was different between the BPUs tested, with concentrations estimated for the LC25 values to females (F4,19 = 46.25, p < 0.0001) and males (F4,19 = 28.23, p < 0.0001). The longevity of adult L. gibbicarina decreased significantly when the insects were exposed to BPUs in females (F4,19 = 59.44, p < 0.0001) and males (F4,19 = 33.16, p < 0.0001). Similarly, the reproduction of this insect differed between the insecticides tested for fecundity (F4,19 = 11.68, p < 0.0001) and fertility (F4,19 = 21.62, p < 0.0001).

Table 2.
Effects on the emergence, longevity, and reproduction of Leptopharsa gibbicarina caused by sublethal concentration (LC25) of the four BPUs. In the table, values followed with the same letter in the row do not differ significantly, according to the Tukey’s honest significance difference (HSD) test (p < 0.05).
3.4. Semi-Field Assays in Palm Trees
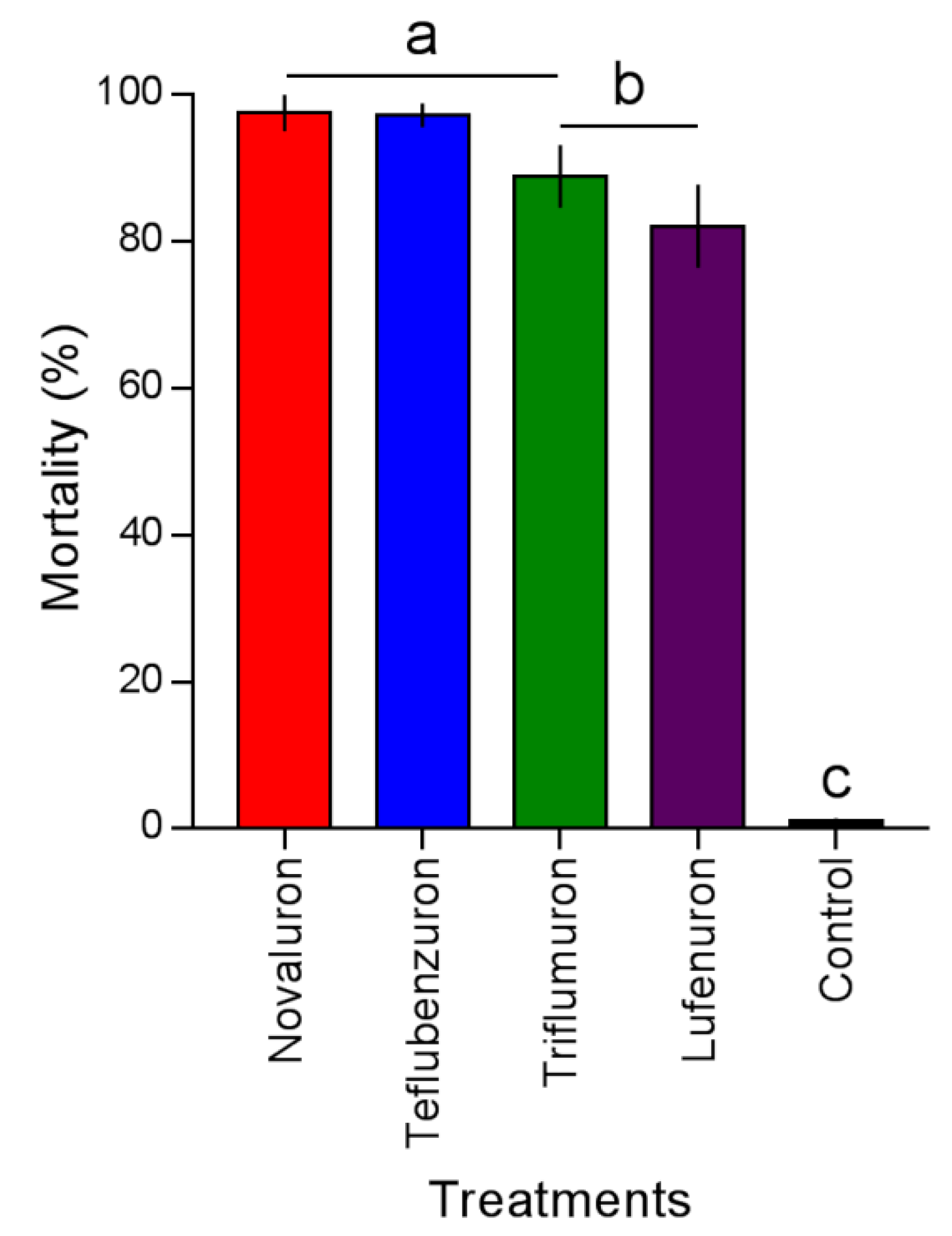
The mortality caused by the BPUs on L. gibbicarina was different (F4,9 = 39.02; p < 0.0001) (Figure 2). Novaluron and teflubenzuron caused mortality of 97.5% ± 2.5% and 97.2% ± 1.6%, followed by triflumuron and lufenuron with 88.9% ± 2.7% and 82.1% ± 5.6%, respectively. Mortality did not exceed 1.04% ± 0.4% in the control.
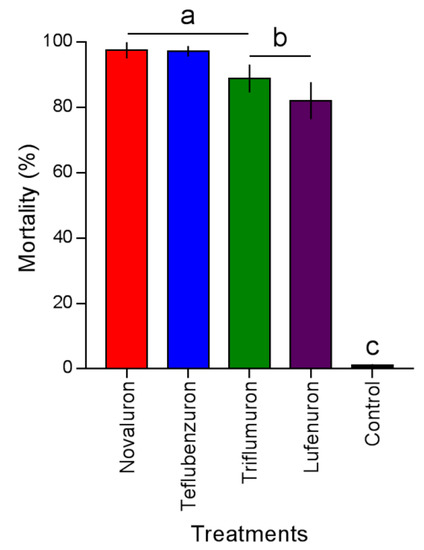
Figure 2.
Mortality of Leptopharsa gibbicarina third-instar nymphs by four BPUs to level LC90 application on oil palm leaves. Treatment means (percent mortality ± SEM) with different letters show significant differences by Tukey’s HSD test at the p < 0.05 level.
4. Discussion
The use of various BPUs was effective in causing mortality, compromising survivorship, and affecting the reproduction of L. gibbicarina under laboratory and semi-field conditions. Novaluron, teflubenzuron, lufenuron, and triflumuron were toxic to L. gibbicarina nymphs and exerted a strong effect through contact exposure. BPUs caused mortality in L. gibbicarina in a concentration-dependent manner, as demonstrated in other insect vectors [24,29]. Leptopharsa gibbicarina nymphs exposed to high concentrations of BPUs displayed immobilization, cuticle malformation, and consequently, abortive molting. In this sense, symptoms in L. gibbicarina nymphs were consistent with the known effects of inhibitors of chitin synthesis. A set of results point to the effects on the cuticle of hemipteran pests, such as Adelphocoris lineolatus Goeze (Miridae) [30], Aleurodicus rigioperculatus (Aleyrodidae) [31], and Stephanitys pyriodes Scott (Tingidae) [32], after the topical application of BPUs. In general, BPUs exhibited toxicity against L. gibbicarina nymphs at different concentrations and reinforced their use as an alternative to neurotoxic insecticides on this species.
High variability in L. gibbicarina survival is mediated by the interaction of BPUs attaching to the external body surface and penetrating through the insect cuticle, leading to the suppression of ecdysis. The time taken for these BPUs to induce mortality in L. gibbicarina nymphs, from 48 to 72 h, presents quick action on this insect. In this study, the compared effects of BPUs on L. gibbicarina occur at various periods. These time differences occur due to BPUs’ ability to penetrate the integument cuticle layers [33], by changes in the proliferation of epidermal imaginal discs [34], and by altering the intracellular exocytosis process during chitin biosynthesis [35]. BPUs have been reported to induce nymph malformation, affect egg hatching, and interrupt the insect’s life cycle [32,36,37]. Low L. gibbicarina survival suggests that the insecticidal activity of novaluron, teflubenzuron, lufenuron, and triflumuron causes detrimental effects on nymphs, with an appreciable population reduction. Thus, they may represent a valuable alternative to monocrotophos and other pesticides to protect oil palm leaves.
The sublethal effect caused by the LC25 of each insecticide on the emergence, longevity, and adult reproduction of L. gibbicarina was observed. Exposure to novaluron, teflubenzuron, lufenuron, and triflumuron affects adult emergence, with a significant reduction in longevity. In this case, a low number of adults emerged as a result of the disruption of the reproductive cycle of this insect. Our results indicate that BPUs have the potential to suppress the development of L. gibbicarina populations, as observed in other studies [38,39]. With regard to reproduction, a smaller egg and nymph quantity was observed in the females of L. gibbicarina after novaluron, teflubenzuron, lufenuron, and triflumuron exposure. There are demonstrated ovicidal and nymphacidal activities in various hemipteran pests, such as Agonoscena targionii Lichtenstein (Aphalaridae) exposed to teflubenzuron [40], Bagrada hilaris Burmeister (Pentatomidae) exposed to novaluron [41], Ceroplastes destructor Newstead (Coccidae) exposed to triflumuron [42], and Oxycarenus hyalinipennis Costa (Lygaeidae) exposed to lufenuron [43]. The effects caused by BPUs on the fecundity and fertility of L. gibbicarina can be attributed to different changes during the embryonic developmental phase, compromising immature survival for various insects. Preliminary studies show that BPUs induce transovarial effects to produce a low number of eggs/nymphs when the insects are exposed during the adult stage [32] or before the adult emergence [44]. In this context, BPUs cause degeneration in the follicular epithelial cells of ovaries, reduction of vitellogenin deposits, distorted oocytes, and abnormal egg hatching [45]. The results suggest that BPUs have a high impact on the emergence, longevity, and reproduction of L. gibbicarina, affecting the fecundity, fertility, and offspring of this insect.
Novaluron, teflubenzuron, lufenuron, and triflumuron showed lethal effects against L. gibbicarina in palm trees in the field, and results were consistent with those observed in the laboratory. However, the mortality level at the nymphal stage was lower than those obtained under laboratory conditions. It is possible that the efficacy of BPUs in field conditions may be due to physical environmental factors [46], systemic or non-systemic action [47], chemical degradation [48], and limited persistence of insecticides in foliage [49]. However, while it is difficult to accurately determine the amount of insecticide penetrating to each insect, the mortality caused by these BPUs on L. gibbicarina was similar to trends observed for the application of insecticidal concentration. The lethality of BPUs and their effectiveness has also been studied with other oil palm pests under field conditions, proving them to be potent chemical agents against Euprosterna elaeasa Dyar (Lepidoptera: Limacodidae) exposed to teflubenzuron and triflumuron [50], as well as R. ferrugineus exposed to lufenuron and novaluron [51,52]. This was similar to findings that treating immature stages of Drosophila suzukii Matsumura (Diptera: Drosophilidae) with lufenuron in the United States [53], Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae) with novaluron in Canada [54], Schistocerca gregaria Forskal (Orthoptera: Acrididae) with teflubenzuron in Egypt [55], and Spodoptera litura Fabricius (Lepidoptera: Noctuidae) with triflumuron in Pakistan [56] reduced the population level of these pests. Our results show that BPUs have a specific physiological effect on insect growth that affects a high number of L. gibbicarina nymphs. In particular, novaluron, teflubenzuron, and triflumuron exhibit excellent insecticidal activity on this insect in the field, and the maximum efficiency from insecticides should be used during the nymph stage. Testing with these BPUs suggests that applications on oil palm leaves can drastically decrease L. gibbicarina infestation.
5. Conclusions
The side effects caused by four BPUs on the survival and reproduction of L. gibbicarina were investigated. Novaluron, teflubenzuron, lufenuron, and triflumuron inhibit the polymerization of chitin, cause mortality, and affect the reproduction of this insect, with the potential to control its field populations. The toxic effects of these insecticides may efficiently manage L. gibbicarina and reduce the insect’s damage and Pestalotiopsis fungal infection to oil palm leaves. In the field, L. gibbicarina was highly susceptible to novaluron, teflubenzuron, and triflumuron, and can be an alternative to monocrotophos in oil palm plantations.
Author Contributions
Conceptualization, A.P.-R., J.E.S., and L.C.M.; methodology, A.P.-R., J.E.S., and L.C.M.; formal analysis, A.P.-R., J.E.S., and L.C.M.; investigation, A.P.-R., J.E.S., and L.C.M.; resources, A.P.-R., J.E.S., and L.C.M.; writing, A.P.-R., J.E.S., and L.C.M.; supervision, A.P.-R., J.E.S., and L.C.M.; project administration, A.P.-R., J.E.S., and L.C.M.; funding acquisition, A.P.-R., J.E.S., and L.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Brazilian research agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant number 305165/2013-5), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (grant number 2815/11), and Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG) (grant number APQ-01079-13).
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable for studies not involving humans.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
We thank Oliverio de Jesús Agudelo for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Froeschner, R.C. Description of a new species of lace bug attacking the oil palm in Colombia (Hemiptera: Tingidae). Proc. Entomol. Soc. Wash. 1976, 78, 104–107. [Google Scholar]
- Genty, P.; Desmier de Chenon, R.; Morin, J.P. Les ravageurs du palmier a huile en Amerique latine. Oléagineux 1978, 33, 325–419. [Google Scholar]
- Escalante, M.; Damas, D.; Márquez, D.; Gelvez, W.; Chacón, H.; Díaz, A.; Moreno, B. Diagnosis and evaluation of Pestalotiopsis, and insect vectors, in an oil palm plantation at the South of Maracaibo Lake, Venezuela. Bioagro 2010, 22, 211–216. [Google Scholar]
- Martínez, L.C.; Plata-Rueda, A. Lepidoptera vectors of Pestalotiopsis fungal disease: First records in oil palm plantations from Colombia. Int. J. Trop. Insect Sci. 2013, 33, 239–246. [Google Scholar] [CrossRef]
- Gitau, C.W.; Gurr, G.M.; Dewhurst, C.F.; Fletcher, M.J.; Mitchell, A. Insect pests and insect-vectored diseases of palms. Austral Entomol. 2009, 48, 328–342. [Google Scholar] [CrossRef]
- Martínez, O.L.; Plata-Rueda, A.; Martínez, L.C. Oil palm plantations as an agroecosystem: Impact on integrated pest management and pesticide use. Outlooks Pest Manag. 2013, 24, 225–229. [Google Scholar] [CrossRef]
- Genty, P.; Garzon, M.; García, R. Damage and control of the Leptopharsa-Pestalotiopsis complex in oil palm. Oléagineux 1983, 38, 291–299. [Google Scholar]
- Mariau, D. Control methods against the bug-Pestalotiopsis complex on oil palm in Latin America. Oléagineux 1994, 49, 189–195. [Google Scholar]
- Reyes, A.R.; Cruz, M.A.; Genty, P. The root absorption technique for controlling oil-palm pests. Oléagineux 1988, 43, 363–370. [Google Scholar]
- Martínez, L.C.; Plata-Rueda, A.; Agudelo, O. Efficacy of insecticides on Leptopharsa gibbicarina Froeschner (Hemiptera: Tingidae) applied by root absorption technique in oil palm. Persian Gulf Crop Prot. 2013, 2, 10–17. [Google Scholar]
- Yeoh, C.B.; Chong, C.L. Acephate, methamidophos and monocrotophos residues in a laboratory-scale oil refining process. Eur. J. Lipid Sci. Technol. 2009, 111, 593–598. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Rotterdam Convention on the Prior Informed Consent (PIC) Procedure for Certain Hazardous Chemicals and Pesticides in International Trade; UNEP: Geneva, Switzerland, 2008. [Google Scholar]
- Environmental Protection Agency, EPA’s Pesticides Industry Sales and Usage, 2006 and 2007 Market Estimates. 2011. Available online: http://www.epa.gov/oppbead1/pestsales/ (accessed on 1 February 2011).
- Martínez, L.C.; Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E. Comparative toxicity of six insecticides on the rhinoceros beetle (Coleoptera: Scarabaeidae). Fla. Entomol. 2014, 97, 1056–1062. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Rodríguez-Dimaté, F.A.; Campos, J.M.; Santos Júnior, V.C.; Rolim, G.D.S.; Fernandes, F.L.; Silva, W.M.; Wilcken, C.F.; Zanuncio, J.C.; et al. Exposure to insecticides reduces populations of Rhynchophorus palmarum in oil palm plantations with Bud Rot disease. Insects 2019, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Plata-Rueda, A.; Quintero, H.A.; Serrão, J.E.; Martínez, L.C. Insecticidal activity of Bacillus thuringiensis strains on the nettle caterpillar, Euprosterna elaeasa (Lepidoptera: Limacodidae). Insects 2020, 11, 310. [Google Scholar] [CrossRef]
- Catchot, B.; Anderson, C.J.H.; Gore, J.; Jackson, R.; Rakshit, K.; Musser, F.; Krishnan, N. Novaluron prevents oogenesis and oviposition by inducing ultrastructural changes in ovarian tissue of young adult Lygus lineolaris. Pest Manag. Sci. 2020, 76, 4057–4063. [Google Scholar] [CrossRef]
- Sajap, A.S.; Amit, S.; Welker, J. Evaluation of hexaflumuron for controlling the subterranean termite Coptotermes curvignathus (Isoptera: Rhinotermitidae) in Malaysia. J. Econ. Entomol. 2000, 93, 429–433. [Google Scholar] [CrossRef]
- Reyes, A. Primeros resultados en el control de Euprosterna elaeasa Dyar defoliador de palma africana Elaeis guineensis Jacq. con triflumuron y teflubenzuron inhibidores de síntesis de quitina. Oléagineux 1991, 46, 139–144. [Google Scholar]
- Milosavljević, I.; El-Shafie, H.A.F.; Faleiro, J.R.; Hoddle, C.D.; Lewis, M.; Mark, S.; Hoddle, M.S. Palmageddon: The wasting of ornamental palms by invasive palm weevils, Rhynchophorus spp. J. Pest Sci. 2019, 92, 143–156. [Google Scholar] [CrossRef]
- Matsumura, F. Studies on the action mechanism of benzoylurea insecticides to inhibit the process of chitin synthesis in insects: A review on the status of research activities in the past, the present and the future prospects. Pestic. Biochem. Physiol. 2010, 97, 133–139. [Google Scholar] [CrossRef]
- Meyer, F.; Flotenmeyer, M.; Moussian, B. The sulfonylurea receptor Sur is dispensable for chitin synthesis in Drosophila melanogaster embryos. Pest Manag. Sci. 2013, 69, 1136–1140. [Google Scholar] [CrossRef]
- Karimzadeh, R.; Hejazi, M.J.; Khoei, F.R.; Moghaddam, M. Laboratory evaluation of five chitin synthesis inhibitors against the Colorado potato beetle Leptinotarsa decemlineata. J. Insect Sci. 2007, 7, 1–6. [Google Scholar] [CrossRef]
- Joseph, S. Ingestion of novaluron elicits transovarial activity in Stephanitis pyrioides (Hemiptera: Tingidae). Insects 2020, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Perveen, F.; Miyata, T. Effects of sublethal dose of chlorfluazuron on ovarian development and oogenesis in the common cutworm Spodoptera litura (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 2000, 93, 1131–1137. [Google Scholar] [CrossRef]
- Liu, T.X.; Chen, T.Y. Effects of the chitin synthesis inhibitor buprofezin on survival and development of immatures of Chrysoperla rufilabris (Neuroptera: Chrysopidae). J. Econ. Entomol. 2000, 93, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liu, C.; Zhang, H.; Wang, Q. Benzoylurea synthesis inhitors. J. Agric. Food Chem. 2015, 63, 6847–6865. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E. Leucothyreus femoratus (Coleoptera: Scarabaeidae): Feeding and behavioral activities as an oil palm defoliator. Fla. Entomol. 2013, 96, 55–63. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Liu, B.; Ali, A.; Luo, S.P.; Lu, Y.H.; Liang, G.M. Insecticide toxicity to Adelphocoris lineolatus (Hemiptera: Miridae) and its nymphal parasitoid Peristenus spretus (Hymenoptera: Braconidae). J. Econ. Entomol. 2015, 108, 1779–1785. [Google Scholar] [CrossRef]
- Kumar, V.; Francis, A.; Avery, P.B.; McKenzie, C.L.; Osborne, L.S. Assessing compatibility of Isaria fumosorosea and buprofezin for mitigation of Aleurodicus rugioperculatus (Hemiptera: Aleyrodidae): An invasive pest in the Florida landscape. J. Econ. Entomol. 2018, 111, 1069–1079. [Google Scholar] [CrossRef]
- Joseph, S.V. Influence of insect growth regulators on Stephanitis pyrioides (Hemiptera: Tingidae) eggs and nymphs. Insects 2019, 10, 189. [Google Scholar] [CrossRef]
- Farnesi, L.C.; Brito, J.M.; Linss, J.G.; Pelajo-Machado, M.; Valle, D.; Rezende, G.L. Physiological and morphological aspects of Aedes aegypti developing larvae: Effects of the chitin synthesis inhibitor novaluron. PLoS ONE 2012, 7, e30363. [Google Scholar] [CrossRef] [PubMed]
- Meola, S.M.; Mayer, R.T. Inhibition of cellular proliferation of imaginal epidermal cells by diflubenzuron in pupae of the stable fly. Science 1980, 207, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Gangishetti, U.; Breitenbach, S.; Zander, M.; Saheb, S.K.; Muller, U.; Schwarz, H.; Moussian, B. Effects of benzoylphenylurea on chitin synthesis and orientation in the cuticle of the Drosophila larva. Eur. J. Cell Biol. 2009, 88, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-Y.; Meng, Q.-W.; Shi, J.-F.; Deng, P.; Guo, W.-C.; Li, G.-Q. Novaluron ingestion causes larval lethality and inhibits chitin content in Leptinotarsa decemlineata fourth-instar larvae. Pestic. Biochem. Physiol. 2017, 143, 173–180. [Google Scholar] [CrossRef]
- Amarasekare, K.G.; Shearer, P.W. Laboratory bioassays to estimate the lethal and sublethal effects of various insecticides and fungicides on Deraeocoris brevis (Hemiptera: Miridae). J. Econ. Entomol. 2013, 106, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Alyokhin, A.; Guillemette, R.; Choban, R. Stimulatory and suppressive effects of novaluron on the Colorado potato beetle reproduction. J. Econ. Entomol. 2009, 102, 2078–2083. [Google Scholar] [CrossRef]
- Parys, K.A.; Snodgrass, G.L.; Luttrell, R.G.; Allen, K.C.; Little, N.S. Baseline susceptibility of Lygus lineolaris (Hemiptera: Miridae) to novaluron. J. Econ. Entomol. 2016, 109, 339–344. [Google Scholar] [CrossRef]
- Saour, G. Efficacy of kaolin particle film and selected synthetic insecticides against pistachio psyllid Agonoscena targionii (Homoptera: Psyllidae) infestation. Crop Prot. 2005, 24, 711–717. [Google Scholar] [CrossRef]
- Joseph, S.V. Effects of insect growth regulators on Bagrada hilaris (Hemiptera: Pentatomidae). J. Econ. Entomol. 2017, 110, 2471–2477. [Google Scholar] [CrossRef]
- Wakgari, W.; Giliomee, J. Effects of some conventional insecticides and insect growth regulators on different phenological stages of the white wax scale, Ceroplastes destructor Newstead (Hemiptera: Coccidae), and its primary parasitoid, Aprostocetus ceroplastae (Girault) (Hymenoptera: Eulophidae). Int. J. Pest Manag. 2001, 47, 179–184. [Google Scholar]
- Atta, B.; Gogi, M.D.; Arif, M.J.; Mustafa, F.; Raza, M.F.; Hussain, M.J.; Farooq, M.A.; Nisar, M.J.; Iqbal, M. Toxicity of some insect growth regulators (IGRs) against different life stages of Dusky cotton bugs Oxycarenus hyalinipennis Costa (Hemiptera: Lygaeidae: Oxycareninae). Bulg. J. Agric. Sci. 2015, 21, 367–371. [Google Scholar]
- Mansur, J.F.; Figeira-Mansur, J.; Santos, A.S.; Santos-Junior, H.; Ramos, I.B.; de Medeiros, M.N.; Machado, E.A.; Kaiser, C.R.; Muthukrishnan, S.; Masuda, H.; et al. The effect of lufenuron, a chitin synthesis inhibitor, on oogenesis of Rhodnius prolixus. Pestic. Biochem. Physiol. 2010, 98, 59–67. [Google Scholar] [CrossRef]
- Joseph, S.V. Transovarial effects of insect growth regulators on Stephanitis pyrioides (Hemiptera: Tingidae). Pest Manag. Sci. 2019, 75, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Guillebeau, L.P.; All, J.N.; Javid, A.M. Influence of weather on efficacy of pyrethroid insecticides for boll weevil (Coleoptera: Curculionidae) and bollworm (Lepidoptera: Noctuidae) in cotton. J. Econ. Entomol. 1989, 82, 291–296. [Google Scholar] [CrossRef]
- Hayasaka, D.; Korenaga, T.; Sánchez-Bayo, F.; Goka, K. Differences in ecological impacts of systemic insecticides with different physicochemical properties on biocenosis of experimental paddy fields. Ecotoxicology 2012, 21, 191–201. [Google Scholar] [CrossRef]
- Kitsiou, V.; Filippidis, N.; Mantzavinos, D.; Poulios, I. Heterogeneous and homogeneous photocatalytic degradation of the insecticide imidacloprid in aqueous solutions. Appl. Catal. B 2009, 86, 27–35. [Google Scholar] [CrossRef]
- Cutler, G.C.; Scott-Dupree, C.D.; Tolman, J.H.; Harris, C.R. Field efficacy of novaluron for control of Colorado potato beetle (Coleoptera: Chrysomelidae) on potato. Crop Prot. 2007, 26, 760–767. [Google Scholar] [CrossRef]
- Cruz, M.A.; Reyes, Y.A. Initial results in controlling Euprosterna elaesa Dyar a leaf-eating pest on oil palm (Elaeis guineensis jacq) using triflumuron and teflubenzuron, chitin synthesis inhibitors. Oléagineux 1991, 46, 139–145. [Google Scholar]
- Ghoneim, K.S.; Al-Dali, A.G.; Abdel-Ghaffar, A.A. Effectiveness of lufenuron (CGA-184699) and diofenolan (CGA-59205) on the general body metabolism of the red palm weevil, Rhynchophorus ferrugineus (Curculionidae: Coleoptera). Pak. J. Biol. Sci. 2003. [Google Scholar]
- Hussain, A.; AlJabr, A.M.; Al-Ayedh, H. Development-disrupting chitin synthesis inhibitor, novaluron, reprogramming the chitin degradation mechanism of red palm weevils. Molecules 2019, 24, 4304. [Google Scholar] [CrossRef]
- Sampson, B.J.; Marshall, D.A.; Smith, B.J.; Stringer, S.J.; Werle, C.T.; Magee, D.J.; Adamczyk, J.J. Erythritol and Lufenuron detrimentally alter age structure of wild Drosophila suzukii (Diptera: Drosophilidae) populations in blueberry and blackberry. J. Econ. Entomol. 2017, 110, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.G.; Scott-Dupree, C.D.; Tolman, J.H.; Harris, R.C. Acute and sublethal toxicity of novaluron, a novel chitin synthesis inhibitor, to Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Pest Manag. Sci. 2005, 61, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Al-Mokhlef, A.A.; Mariy, F.M.; Emam, A.K.; Ali, G.M. Effect of teflubenzuron on ultrastructure and components of the integument in Schistocerca gregaria (Forskal) 5th instar nymphs. Ann. Agric. Sci. 2012, 57, 1–6. [Google Scholar] [CrossRef][Green Version]
- Ahmad, M.; Gull, S. Susceptibility of armyworm Spodoptera litura (Lepidoptera: Noctuidae) to novel insecticides in Pakistan. Can. Entomol. 2017, 149, 649–661. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).