Changes in the Morphological Diversity of Larvae of Lance Lacewings, Mantis Lacewings and Their Closer Relatives over 100 Million Years
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Documentation and Image Processing
2.3. Shape Analysis
3. Results
3.1. Extant Larvae of Berothidae
- (1)
- Tillyard [36] depicted a drawing of a stage 1 larva (hatchling) of Spermophorella disseminata (specimen 5001) in dorsal view (his Figure 32 pl. XVIII). A close-up of the head was provided, also in dorsal view (his Figure 33 pl. XVIII). The size of the specimen was provided as a magnification factor, which is not informative in combination with the electronic versions available to the present authors. The specimen was re-figured by Tillyard [37], Gurney [38], and Tjeder [39].
- (2)
- (3)
- Gurney [38] depicted several drawings of larvae of Berothidae. The first specimen was a stage 1 larva of Lomamyia sp. (specimen 5002) in dorsal view (his Figure 2 pl. 11 p. 163). The length was stated as 2.04 mm. Additionally, a detail of the head of a stage 1 larva was provided (his Figure 8 pl. 12 p. 165), as well as a detail of the maxilla (his Figure 7 pl. 12 p. 165). It remains unclear whether it is the same specimen, but we assume that to be the case, not considering the head as a separate specimen. The specimen was re-figured by Tjeder [39].The second specimen was a stage 3 larva (“mature larva”) of Lomamyia sp. (specimen 5003) in dorsal view (his Figure 5 pl. 11 p. 163). The length was stated as 9.36 mm. Additionally, a detail of the head of the specimen was provided in dorsal view (his Figure 12 pl. 12 p. 165) and ventral view (his Figure 13 pl. 13 p. 167), as well as details of the trunk appendages (his Figures 18 and 22 pl. 14 p. 169). The specimen was re-figured by Tjeder [39] and Tauber [40].Gurney also re-figured specimen 5001 (his Figure 4 pl. 11 p. 163), i.e., the drawing from Tillyard [36], including the detail of the head region (his Figure 10 pl. 12 p. 165).
- (4)
- Tjeder [39] summarised and re-figured the known larvae of the group Berothidae (all on p. 259). This includes drawings of specimen 5001 (habitus and head; his Figures 212 and 213), i.e., drawings from Tillyard [36], and of specimens 5002 and 5003 (habitus and head; his Figures 214–218), i.e., drawings from Gurney [38].
- (5)
- MacLeod [23] depicted a drawing of a stage 3 larva (specimen 5004) of Lomamyia flavicornis in dorsal (his Figure 36 pl. XII) and ventral view (his Figure 37 pl. XII), as well as a detail of the mouth region (his Figure 38 pl. XII). According to the scale provided, the head capsule measured 0.37 mm.
- (6)
- Toschi [41] depicted the drawing of a head of a stage 1 larva (specimen 5005) of Lomamyia latipennis in dorsal view (her Figure 3 p. 25). According to the text, she had several specimens available. The drawing appears to be based on an actual specimen. No indication of size was provided.
- (7)
- Tauber and Tauber [42] depicted micrographs of stage 2 and 3 larvae (their Figures 1–3 p. 626) of Lomamyia lattipennis. A drawing of a head in dorsal view (their Figure 4 p. 628) was also provided. Yet, the micrographs were recorded on moving animals and do not allow the recognition of the outline of the head. The drawing is incomplete, not providing the distal region of the stylets. Therefore, we cannot consider any of the specimens for our analysis.
- (8)
- Riek [1] depicted the drawing of a head of a larva of unknown stage and species (“berothid”) in ventral view (his Figure 29.5B p. 476). He also provided the habitus in dorsal view (his Figure 29.10H p. 486), as well as a dorsal view of the head (his Figure 29.10G p. 486). All depictions match exactly and are therefore interpreted as original, based on a single specimen (specimen 5006). No indication of size was provided. The specimen was re-figured by Gepp [24], New [43], and Tauber et al. [44].
- (9)
- (10)
- Brushwein [45] depicted several immature stages of Lomamyia hamata. Yet, none of the images provide details of the head in dorsal view. Hence, none of the specimens could be further considered in our study.
- (11)
- Tauber [40] re-figured the habitus of specimen 5003 (her Figure 33.18 p. 134), i.e., a specimen from Gurney [38], as well as the drawing of the head (her Figure 33.19 p. 134) from Tauber and Tauber [42]. There are later editions of this contribution also figuring this image (at least 1987, 1991, 2008, our version was labelled 1991). We assume that all of these contributions feature the same images. Hence, these refer to the same specimen.
- (12)
- Minter [46] depicted two larval specimens of Berothidae and Rhachiberothidae (considered to be ingroup of Berothidae by several authors, e.g., [13]). The first (specimen 5007) was a drawing in dorsal view of a stage 1 larva of Mucroberotha vesicaria (his Figure 5 p. 118). In addition, he provided drawings of the head in dorsal (his Figure 6 p. 118) and ventral view (his Figure 7 p. 118). Furthermore, micrographs of the eye region (his Figure 8 p. 119) and the empodium (his Figure 9 p. 119) were provided. According to the text, the drawings are based on an actual specimen collected in South Africa. According to the provided scale, the specimen was 1.01 mm long.The second specimen (specimen 5008) was a drawing in dorsal view of a stage 1 larva of Podaella sp. (his Figure 14 p. 121). In addition, he provided drawings of the head in dorsal (his Figure 15 p. 121) and ventral view (his Figure 16 p. 121). Furthermore, micrographs of the antero-lateral head region (his Figure 17 p. 122) and the empodium (his Figure 18 p. 122) were provided. According to the text, the drawing is also based on an actual specimen collected in South Africa. According to the provided scale, the specimen was 1.13 mm long.
- (13)
- (14)
- (15)
- (16)
- Möller et al. [48] provided images of several larvae of Berothidae. The first (specimen 5009) is a stage 1 larva of Podallea vasseana. Images include drawings of the entire larva in dorsal view (his Figure 7 p. 5), head in dorsal (his Figure 8 p. 5) and ventral view (his Figure 9 p. 5), trunk appendages (his Figure 10 p. 5), and details of the setae arrangement (chaetotaxy) of the trunk segments (his Figures 11–15 p. 5). Additionally, several details were provided as SEM micrographs, including details of the antennae (his Figures 16 and 17 p. 6), tip of a stylet (his Figure 18 p. 6), setae on the maxilla (his Figure 19 p. 6), details of setae (his Figure 20 p. 6), and the anus region (his Figure 21 p. 6). According to the text, the drawings were based on an actual specimen collected in South Africa. According to the provided scale, the specimen was 1.25 mm long.The second specimen is a stage 2 larva of Podallea vasseana. Yet, none of the images provide access to the stylets in relation to the head capsule, hence the specimen could not be further considered here.The third (specimen 5010) is a stage 3 larva of Podallea vasseana. Images include drawings of the entire larva in dorsal view (his Figure 28 p. 9), head in dorsal (his Figure 29 p. 9) and ventral view (his Figure 30 p. 9), trunk appendages (his Figure 36 p. 9), and details of the seta arrangement (chaetotaxy) of the trunk segments (his Figures 31–35 p. 9). Additionally, several details were provided as SEM micrographs, including sensilla on labium (his Figures 37–39 p. 10) and antennae (his Figure 40 p. 10). According to the text, the drawings were based on an actual specimen collected in South Africa. According to the provided scale, the specimen was 3 mm long.
- (17)
- Monserrat [49] depicted several drawings of a stage 1 larva (specimen 5011) of Berothimerobius reticulatus (his Figure 11 p. 197). The images include a habitus drawing (without trunk appendages) in dorsal view (his Figure 11a), the head in dorsal view (his Figure 11b), antenna (his Figure 11c), mandible (his Figure 11d), labial palps (his Figure 11e), maxilla (his Figure 11f), and the three trunk appendages (his Figure 11g–i). According to the text, the drawings were based on an actual specimen. According to the provided scale, the specimen was 1.3 mm long.
- (18)
- (19)
- Dobosz and Górski [50] presented a poster with SEM images of a stage 1 larva (specimen 5012) of Nyrma kervilea. This included a dorsal view, a ventral view and a dorsal close-up of the head, mouthparts, and trunk appendages. While conference posters are usually not considered valid sources, we opted to include the specimen as (1) the poster was available online, (2) the poster has already been cited in another publication [13], and (3) given the overall rarity, every available specimen is considered.
- (20)
- Heckman [51] provided a figure (his Figure 2.26) that states “unidentified larva in the family Berothidae” in the figure legend. Yet, the specimen is cited as originating from Grebennikov [52]. There, the specimen was interpreted as larva of the group Polystoechotidae [52] (his Figures 30–36 p. 415), which is generally interpreted as an ingroup of Ithonidae in newer analyses. The morphology of the larva is also much more compatible with an interpretation of the specimen as a representative of Ithonidae/Polystoechotidae than Berothidae. Therefore, this specimen was not further considered.
3.2. Extant Larvae of Mantispidae
- (1)
- (2)
- Brauer [57] provided drawings of a stage 1 larva (hatchling) of Mantispa pagana (specimen 5202). The images include the hatching process (his Figure 11 pl. IV), habitus in lateral (his Figure 11e pl. IV), and dorsal view (his Figure 11’’ pl. IV), trunk appendages (his Figure 11a,b pl. IV), and the head in dorsal view (his Figure 11c pl. IV). Parts of the specimen seem to have been re-figured in Stitz [54] and Jacobs [55]. The entire plate with all figures was re-figured in Aspöck [56].
- (3)
- Brauer [58] provided several drawings of larvae of Mantispa styriaca. Images include a stage 1 larva (hatchling) in dorsal view (his Figure 1 pl. XII), stage 1 larvae entering the egg sac of a spider (his Figure 2 pl. XII), and later stage larvae including some details of the head (his Figures 3–4 pl. XII). Yet, only the stage 1 larva in dorsal view has sufficient details to be further considered, which seems quite similar to specimen 5202, i.e., the one from Brauer [57]. Yet, a closer comparison shows significant differences indicating that this is another specimen (specimen 5203).Parts of this specimen seem to have been re-figured in Stitz [54] and Jacobs [55]. Some of the later stage larvae were re-figured by Poivre [59]. One of the laterstage larvae has been re-figured in Aspöck and Aspöck [60], Gepp [24], and Makarkin [61]. The entire plate with all figures was re-figured in Aspöck [56] and Aspöck and Aspöck [3].
- (4)
- Nawa [62] provided a very simplified drawing of a larva of Mantispidae. The text is in Japanese and could not be translated with common tools. Based on later publications [23], the specimen was a stage 1 larva of Eumantispa harmondi. The drawing appears very simplified (MacLeod [23] referred to it as “crude”, p. 263). The stylet-head-capsule transition is not shown, not permitting the standardization of the orientation of the stylets. Therefore, the specimen could not be further considered.
- (5)
- Tillyard [37] provided a drawing of a stage 1 larva (hatchling) of Mantispa vittata (specimen 5204) in dorsal view (his Figure U13A p. 319). The size of the larva was provided as a magnification factor, which is not informative in combination with the electronic versions available to the present authors. Tillyard [37] also provided a drawing of a stage 3 larva (his Figure U13B p. 319), but in lateral view, hence this specimen could not be further considered here. Both specimens were re-figured by Tjeder [39].
- (6)
- Stitz [54] provided a drawing (his Figure 331 p. 35.302) of a larva of Mantispa pagana. In the figure legend it was stated that the figure is redrawn from “Brauer”. In the reference list, Stitz [54] cites three possible sources: Brauer [53] (specimen 5201), Brauer [57] (specimen 5202), and Brauer [58] (specimen 5203). A close comparison shows no direct match to any of the three possible specimens. Most likely, the drawing by Stitz [54] combined information from two of these or even all three specimens. The drawing is therefore still interpreted as a re-figure and was not further considered.Stitz [54] additionally figured a stage 2 larva in lateral view (his Figure 332 p. 35.302) and a close-up of the head in lateral view (his Figure 333 p. 35.302). Due to the orientation, this specimen could not be further considered.
- (7)
- Handschin [63] provided drawings of a stage 1 larva (hatchling) of Austromantispa imbecilla (specimen 5205). Images include a head in dorsal view (his Figure 17 left p. 706) and the isolated labium (his Figure 17 right p. 706). No indication of size was provided.
- (8)
- Hungerford [64] provided a micrograph of a stage 1 larva (specimen 5206) of Mantispa interrupta in dorsal view (his Figure 4 pl. 1). The size of the specimen was provided as a magnification factor, which is not informative in combination with the electronic versions available to the present authors. According to a re-figured version [24] (see below), the larva was about 1 mm long.
- (9)
- Merti [65] provided a drawing of a stage 1 larva (specimen 5207) of Mantispa decorata in dorsal view (his Figure 3 p. 306). No indication of size was provided.
- (10)
- McKeown and Mincham [66] provided a drawing of a stage 1 larva of Mantispa vittata (specimen 5208) in dorsal view (their Figure 8 p. 217). No direct indication of size was provided. According to the text, the larvae that the drawing is based on were slightly larger than 1 mm (p. 212). Images of later-stage larvae were provided (their Figures 9 and 10 p. 217), unfortunately only in lateral view or without sufficient details; hence, these could not be further considered.
- (11)
- Peterson [22] provided a drawing of a stage 1 larva of Mantispa interrupta (specimen 5209) in dorsal view (his Figure N2A). Apparently, there are later editions and reprints of this work. These have not all been viewed by the current authors (the edition available was labelled 1957), but we can assume that they also contain the image. Hence, citations of these later editions are likely to refer to this specimen. The exact year of publication of the different editions or reprints, is unclear. We found reference to the years 1953, 1956, 1957, 1960, 1962, 1965, 1967, and 1979.
- (12)
- Lucchese [67] provided several drawings of stage 1 larva (specimen 5210) of Mantispa perla. Drawings include a dorsal view (his Figure 11 left p. 254), a lateral view (his Figure 11 right p. 254), and a number of details of the trunk appendages (his Figure 12 p. 255). No indication of size was provided.
- (13)
- Lucchese [68] re-figured (his Figures XXXIII p. 134, XL p. 142) specimen 5210, i.e., all images from Lucchese [67]. Additional views and details of this specimen were provided as drawings, including a ventral view (his Figure XXXIV p. 131), the head in dorsal (his Figure XXXV p. 132), ventral (his Figure XXXVI p. 135), and lateral view (his Figure XXXVII p. 137), antenna and labial palp (his Figure XXXVIII p. 139), details of the stylet (his Figure XXXIX p. 140), and the trunk end in dorsal and ventral view (his Figure XLI p. 143). In addition, the entire hatching process was presented in lateral view (Figure LII p. 173, LIII p. 175, LIV p. 177, LV p. 179). The ventral view was re-figured in Tauber [40].
- (14)
- (15)
- Kuroko [69] provided several images of larvae of Mantispidae. The first one (specimen 5211) was a stage 1 larva of Climaciella magna. The images include drawings of the entire larva in dorsal view (his Figure 3 pl. 10), the head in dorsal view (his Figure 4 pl. 10), trunk appendage (his Figure 5 pl. 10), head in ventral view (his Figure 6 pl. 10), mandibles (his Figure 7 pl. 10), trunk end (his Figure 8 pl. 10), and a scheme of the setae arrangement (chaetotaxy; his Figure 9 pl. 10). According to the provided scale, the larva was 1 mm long.The second specimen (specimen 5212) was a stage 1 larva of Mantispa japonica. The images include drawings of the entire larva in dorsal view (his Figure 10 pl. 11), a scheme of the setae arrangement (chaetotaxy; his Figure 11 pl. 11), maxillae and labium (his Figure 12 pl. 11), a dorsal view of the head (his Figure 13 pl. 11), mandibles (his Figure 14 pl. 11), and a trunk appendage (his Figure 15 pl. 11). According to the provided scale, the larva was 1 mm long.Kuroko [69] also provided a micrograph of many stage 1 larvae of Climaciella magna. Yet, the image does not provide sufficient details. Hence, it could not be further considered.
- (16)
- Ghilarov [70] provided a drawing (his Figure 12 p. 411) of a stage 1 larva (specimen 5213) of Mantispa styriaca in dorsal view. No indication of size was provided. Additionally, a stage 3 larva was shown in lateral view (his Figure 39.3 p. 49), but could not be further considered due to its orientation. Both images were re-figured by Dorohova [71].
- (17)
- (18)
- MacLeod [23] provided drawings of several larvae of Mantispidae. The first drawing (specimen 5214) was of the head of a stage 1 larva of Plega melitomae in dorsal (his Figure 42 pl. XIV) and ventral view (his Figure 44 pl XIV). Additionally, a detail of the antenna was provided (his Figure 43 pl. XIV). This larva differs from other larvae by possessing slightly curved stylets. According to the provided scale, the head capsule was 0.21 mm long. The specimen was re-figured in Haug et al. [12].The second one (specimen 5215) was a drawing of the head of a stage 1 larva of Mantispa viridis in dorsal (his Figure 49 pl. XVI) and ventral view (his Figure 51pl XVI). Additionally, details of the head capsule surface (his Figure 48 pl. XVI), antenna (his Figure 50 pl. XVI), mandible (his Figure 52 pl. XVI), and head in lateral view (his Figure 53 pl. XVI) were provided. According to the provided scale, the head capsule was 0.13 mm long. The specimen was re-figured in Engel et al. [72].The third drawing (specimen 5216) was of a head of a stage 3 larva of an unidentified representative of Mantispidae in dorsal (his Figure 54 pl. XVII) and ventral view (his Figure 55 pl. XVII). According to the provided scale, the head capsule was 0.31 mm long.
- (19)
- Parker and Stange [73] provided drawings of two larvae of Plega yucatanae. This first drawing was of a head of a stage 1 larva (specimen 5217) in dorsal view (their Figure 10 p. 610). No indication of size was provided.The second drawing was of a stage 3 larva (specimen 5218). Images include a head in dorsal view (their Figure 11 p. 610) and the habitus in lateral view (their Figure 12 p. 610). The entire larva was, according to the text, 10 mm long. The head of the specimen was re-figured in Tauber [40].
- (20)
- Bissett and Moran [74] provided a drawing of a stage 1 larva (hatchling) of an unidentified representative of Mantispidae (specimen 5219) in dorsal view (their Figure 2a p. 85). Additionally, details of the stylets (their Figure 2b), antenna (their Figure 2c), labium (their Figure 2d), and trunk appendages (their Figure 2e) were provided. According to the provided scale, the specimen was 0.95 mm long.
- (21)
- Davidson [75] provided drawings of several larvae of Mantispa viridis (all on p. 31). Two of the drawings show entire larvae in dorsal view, and two drawings show isolated heads in dorsal view. Two drawings (one of a head and one habitus drawing) were labelled as being based on a stage 1 larva (his Figures 1 and 3). We interpret these as representing the same specimen (specimen 5220). The other drawing of a head was labelled as a stage 2 larva (his Figure 2), and the other habitus drawing was labelled as a stage 4 larva (his Figure 4). As there is no stage 4 larva in Mantispidae (also not mentioned in the text), we assume that this was a mistake. We therefore interpret these two drawings as representing a single specimen (specimen 5221).
- (22)
- Riek [1] provided drawings of a larva of Mantispa sp. The images include the habitus in lateral view (his Figure 29.11A p. 489) and a dorsal view of the head region (his Figure 29.11B p. 489), which therefore could be considered for the analysis here (specimen 5222). The larva was labelled as a stage 1 larva; yet, the morphology is more likely that of a stage 2 or 3 larva, especially based on the head detail. No indication of size was provided. The specimen was re-figured in Gepp [24], New [43], Tauber et al. [44], and Heckman [51].
- (23)
- Anonymus [76] provided drawings of stage 1, 2, and 3 larvae of Mantispidae. The stage 1 larva was shown in dorsal view. Although the drawing appears rather simplified, it differs from all earlier images reported above and provides sufficient detail to be further considered (specimen 5223). The stage 2 and 3 larvae were shown in lateral view and could therefore not be further considered.
- (24)
- Poivre [59] provided a drawing of a stage 1 larva (specimen 5224) of Mantispa styriaca in dorsal view (his Figure 2A p. 7) and during the hatching process (his Figure 2C–E p. 7). According to the provided scale, the specimen was 1 mm long. The specimen was re-figured by Makarkin [61]. Poivre [59] additionally re-figured (his Figure 2K,L p. 7) some later-stage larvae from Brauer [58].
- (25)
- Redborg [77] depicted a larva of Mantispa uhleri parasitising a spider (his Figure 1A p. 190). Yet, the larva is not very well visible and could not be considered further here.
- (26)
- (27)
- Redborg and MacLeod [78] provided numerous images of larvae of Mantispa uhleri. The images included drawings of a stage 1 larva (hatchling) in dorsal view (their Figure 4A p. 12; specimen 5225), the head of a stage 3 larva in lateral view (their Figure 4B p. 12; not further considered), the trunk end of a stage 2 larva in lateral view (their Figure 4C p. 12; not further considered), the trunk end of a stage 3 larva in lateral view (their Figure 4D p. 12; not further considered), a stage 3 larva in lateral view (their Figure 6 p. 17; not further considered), as well as micrographs of stage 1 larvae on spiders (their Figure 13 p. 65; their Figure 14 p. 67; not further considered). According to the provided scale, the considered single stage 1 larva specimen was 0.88 mm long. Some images were re-figured in Tauber [40] and Haug et al. [12].
- (28)
- Tauber [40] re-figured several specimens. The first one (her Figure 33.14 p. 134) was specimen 5225, i.e., a specimen from Redborg and MacLeod [78], but Redborg [77] was cited as the source. The second one was the ventral view of the head of specimen 5210 (her Figure 33.15 p. 134), i.e., a drawing from Lucchese [68], but citing Luchcese [67] as the source. The third one was a stage 3 larva in lateral view (her Figure 33.16 p. 134), i.e., a drawing from Redborg and MacLeod [78], but Redborg [77] was cited as the source. The fourth one (her Figure 33.17 p. 134) was the head of specimen 5218, i.e., a drawing from Parker and Stange [73]. Apparently, there are later editions of this contribution also figuring this image (at least 1987, 1991, 2008, our version was labelled 1991). We assume that all of these feature the same images. Hence, these also refer to the same specimen.
- (29)
- (30)
- Monserrat and Díaz-Aranda [79] provided images of several larvae of Mantispa styriaca. These include a stage 1 larva in lateral view (their Figure 1 p. 194) and numerous details (their Figures 2 and 3 p. 194, their Figures 4–7 p. 195, their Figures 8–11 p. 196). Yet, no dorsal depiction of the head with stylets is available, and hence this specimen could not be further considered here.The second specimen was a stage 3 larva shown in lateral view (their Figure 12 p. 197), also with details (their Figures 14 and 15 p. 197), and a head with stylets in dorsal view (their Figure 13 p. 197). Therefore, this specimen (specimen 5226) could be considered in the analysis here.
- (31)
- (32)
- Kral [80] provided a partial SEM image of a stage 1 larva of Mantispa. The image shows the head in more or less dorsal view, yet it is too incomplete to be further considered here.
- (33)
- Minter [46] provided drawings of a stage 1 larva (specimen 5227) of Mantispa capeneri. The images include the habitus in dorsal view (his Figure 23 p. 125) and the head in dorsal (his Figure 24 p. 125) and ventral view (his Figure 25 p. 125). Additionally, SEM micrographs of several details were provided including eyes (his Figure 26 p. 126), empodium (his Figure 27 p. 126), surface of head capsule (his Figure 28 p. 126), and trunk (his Figure 29 p. 16). According to the provided scale, the larva was 0.76 mm long. The specimen was re-figured in Aspöck and Aspöck [3].
- (34)
- (35)
- Hoffman and Brushwein [81] provided numerous drawings of larvae of Mantispidae. These included the habitus of an entire stage 1 larva of Mantispa pulchella (their Figure 1 p. 183; partly simplified), and a simplified ventral view (their Figure 2 p. 183); simplified dorsal views of stage 1 larvae of Mantispa interrupta (their Figure 3 p. 183), Mantispa sayi (their Figure 4 p. 183), and Mantispa viridis (their Figure 5 p. 183); all these are rather simplified in the head region and can therefore not be used for the analysis performed herein. More detailed images were also provided.The first one was a detailed drawing of a head of a stage 1 larva (specimen 5228) of Climaciella brunnea in dorsal and ventral view (their Figure 6 p. 184), as well as details of the trunk appendages (their Figure 7 p. 184) and setae arrangement (their Figure 8 p. 185).The second one was a detailed drawing of a head of a stage 1 larva (specimen 5229) of Mantispa interrupta in dorsal and ventral view (their Figure 9 p. 186), as well as details of the trunk appendages (their Figure 10 p. 186) and setae arrangement (their Figure 11 p. 187). In addition, there were different details (e.g., trunk end) of stage 2 and 3 larvae (their Figures 12–14 p. 188) that could not be further considered here.The third one is a detailed drawing of a head of a stage 1 larva (specimen 5230) of Mantispa pulchella in dorsal and ventral view (their Figure 15 p. 189), as well as details of the trunk appendages (their Figure 16 p. 189) and setae arrangement (their Figure 17 p. 190). In addition, there were details of stage 2 and 3 larvae (their Figure 18 p. 191, their Figure 19 p. 192) that could not be further considered here due to the orientation of these images. Some of the images were re-figured in Ohl [11]. Parts of the head were re-figured in Haug et al. [12].The fourth one was a detailed drawing of a head of a stage 1 larva (specimen 5231) of Mantispa viridis in dorsal and ventral view (their Figure 21 p. 194), as well as details of the trunk appendages (their Figure 22 p. 194) and setae arrangement (their Figure 23 p. 195). In addition, there were details (e.g., trunk end) of stage 2 and 3 larvae (their Figures 24–26 p. 196) that could not be further considered here, as no head was shown in dorsal view.For all of the specimens that could be considered here, no indication of size was provided.
- (36)
- Hirata et al. [82] provided micrographs of stage 1 larvae of Eumantispa harmandi on a spider (their Figure 1). Yet, none of the specimens were available in dorsal view, hence these specimens could not be further considered here.
- (37)
- (38)
- Jacobs [55] re-figured a stage 2 larva in lateral view (his Figure M-10 right p. 364), i.e., a larva from Aspöck and Aspöck [60]. In addition, Jacobs [55] figured a stage 1 larva of Mantispa styriaca (his Figure M-10 left p. 364), which is a re-figure of the compound drawing from Stitz [54], without reference.
- (39)
- Aspöck [56] re-figured several specimens from historically important studies. The images include specimen 5201 (his Figure 61 p. 233), i.e., the specimen from Brauer [53]; specimen 5202 (his Figure 67 p. 235), i.e., the specimen (entire plate) from Brauer [57]; and specimen 5203 (his Figure 71 p. 237), i.e., the specimen (entire plate) from Brauer [58].
- (40)
- (41)
- Aspöck and Aspöck [3] provided a photograph of a stage 1 larva of Mantispa scabricollis. Unfortunately, the image does not provide sufficient details to be further considered here.
- (42)
- (43)
- Wunderlich [83] re-figured (his Figure 3 p. 156) a specimen from Kuroko [69]. The size was stated as 10 mm (“1 cm”), which was likely a calculation error as the original size was indicated to be 1 mm. Wunderlich also re-figured a stage 3 larva of Leptomantispa pulchella (his Figure 4 p. 156), i.e., a larva from Hoffman and Brushwein [81], but cited it as “Mantispa sp.” with Ohl [11] as source.
- (44)
- (45)
- (46)
- (47)
- Dorey and Merritt [87] provided micrographs of stage 1 larvae of Ditaxis biseriata. These include images of a group of newly hatched specimens (their Figure 3 upper right p. 6), a specimen preserved in ethanol in dorsal view (their Figure 3 middle p. 6), and a living specimen in dorsal view (their Figure 3 lower p. 6). None of these images provided sufficient details to be further considered here. More detailed images include micrographs of an antenna (their Figure 4a p. 7) and a head illuminated with transmitted light (their Figure 4b p. 7), as well as a head in dorsal view illuminated with reflected light (their Figure 4c p. 7). It remains unclear whether the two depictions of the head are from the same specimen. As we cannot exclude that it is the same specimen, we consider them as one specimen (specimen 5232) in order to avoid considering the same specimen twice. According to the provided scale, the head capsule of the specimen was 0.25 mm long.
- (48)
- (49)
- Haug et al. [12] re-figured several larvae of Mantispidae. The images include specimen 5225 (their Figure 3B p. 5), i.e., a specimen from Redborg and MacLeod [78]; details of the antenna and labial palp of specimen 5230 (their Figure 3C,D p. 5), i.e., a specimen from Hoffman and Brushwein [81]; and the head of specimen 5214 (their Figure 3E p. 5), i.e., a specimen from MacLeod [23].
- (50)
- (51)
- Jandausch et al. [88] provided an intensive documentation of stage 1 larvae of Mantispa aphavexelte. These images included:
- (1)
- SEM micrographs of an entire specimen in dorsal (their Figure 1A p. 532), ventral (their Figure 1B p. 532), and lateral view (their Figure 1C p. 532), close-ups of the head in dorsal (their Figure 3A p. 533), ventral (their Figure 3B p. 533), lateral (their Figure 3C p. 533), and anterior view (their Figure 3D p. 533), eyes (their Figure 4 p. 534), antennae (their Figure 5 p. 534), mouthparts in general (their Figure 6A p. 535), details of the stylets (their Figure 6B–D p. 535), the labial palp (their Figure 8 p. 536), and the thorax in lateral view (their Figure 13A p. 542).
- (2)
- Light microscopic photographs of an entire specimen in dorsal (their Figure 2A p. 532), ventral (their Figure 2B p. 532), and lateral view (their Figure 2C p. 532), and histological sections through different body regions (their Figure 9 p. 539).
- (3)
- Surface renderings of 3D models of the stylets (their Figure 7 p. 536), the head (their Figure 10 p. 539), the nervous system (their Figure 11A p. 540), and digestive tract (their Figure 11B p. 540), the musculature of the foregut (their Figure 12 p. 540), and the trunk muscles (their Figure 14 p. 543, their Figure 16 p. 545, their Figure 17 p. 546).
- (4)
- Drawings of a larva in lateral view (their Figure 9 p. 538 upper), the thorax (their Figure 13B p. 542), and the anterior abdomen (their Figure 15 p. 544), both in lateral view, and the head in dorsal (their Figure 18A p. 548) and ventral view (their Figure 18B p. 548).It remains unclear which individual was used as the basis for each image. We therefore considered only a single specimen (their Figure 18A; specimen 5233) in order to avoid considering the same specimen twice. According to the provided scale, the head capsule of the specimen was 120 µm long.Jandausch et al. [88] additionally re-figured a drawing of a stage 3 larva of Dicromantispa sayi. According to the figure legend, the original drawing is from “Redborg (1982)”, but the reference was not found in the reference list. We found two publications by Redborg from 1982 in the literature, none of which feature a comparable drawing [77,89]. As another source of the same drawing, “Stehr (1987)”, was cited, but also not found in the reference list. Comparison to known images revealed a high match with a drawing from Redborg and MacLeod [78].
- (52)
- Jandausch et al. [90] re-figured images from Jandausch et al. [88], providing additional details. The re-figured image was an SEM micrograph showing the habitus in lateral view (their Figure 1) of the stage 1 larva of Mantispa aphavexelte. Additional details include aspects of the trunk appendages, including drawings (their Figures 2 and 4), SEM micrographs (their Figure 3), and cLSM-based micrographs (their Figure 5).
- (53)
- Badano et al. [91] provided a photograph of a larva of Mantispidae in dorsal view (their Figure 4iii p. 679). Yet, is difficult to access details of the mouthparts and the posterior edge of the head capsule. Therefore, the specimen could not be further considered for our analysis.
3.3. Extant Larvae of Dilaridae
- (1)
- (2)
- Gurney [38] provided several drawings of a stage 3 larva of Nallachius americanus (specimen 5401). The images include the habitus in dorsal view (his Figure 6 pl. 11 p. 163), the head in dorsal view (his Figure 11 pl. 12 p. 165), the head in ventral view (his Figure 14 pl. 13 p. 167), and details of the trunk appendages (his Figures 17 and 21 pl. 14 p. 169). The length of the entire larva was 12 mm. The specimen was re-figured in Peterson [22], Gepp [24], Tauber [40], and Tauber et al. [44].
- (3)
- Peterson [22] provided several drawings of a larva of Nalachius americanus. Images include the habitus in dorsal view (his Figure N2B), the head in dorsal view (his Figure N2C), trunk appendages (his Figure N2D,E), and the terminal end (his Figure N2F). The figures refer to Gurney [38]. While the images differ from the original, they indeed seem to be based on Gurney [38], but modified. Hence, the images from Peterson [22] could not be further considered. There are later editions of this book (e.g., 1953, 1957, 1960, see also above) which seem to have used the same images; therefore, these also refer to the same specimen.
- (4)
- Ghilarov [70] provided several drawings of a larva (specimen 5402) of Dilar turcicus. The images include the habitus in dorsal view (his Figure 1 p. 403), the head in ventral (his Figure 2a p. 404) and dorsal view, mandible (his Figure 3a p. 404), maxilla (his Figure 3b p. 404), antenna (his Figure 4 p. 404), labium (his Figure 5 p. 405), trunk appendage (his Figure 6a p. 405), tip of a trunk appendage (his Figure 6b p. 405), and the trunk end in dorsal and ventral view (his Figure 7 p. 405). No indication of size was provided. The specimen was re-figured by Gepp [24], Dorohova [71], and New [25].
- (5)
- MacLeod [23] provided several drawings of the head of a stage 3 larva of Nallachius americanus (specimen 5403). The images include a lateral (his Figure 32 pl. X), a dorsal (his Figure 33 pl. XI), and a ventral view (his Figure 34 pl. XI), and an isolated stylet (his Figure 35 pl. XI). According to the provided scale, the head capsule was 0.62 mm long. The specimen was re-figured in Engel et al. [72].
- (6)
- Gepp [24] re-figured two specimens. The first one was specimen 5401 (his Figure 11a), i.e., the specimen from Gurney [38], but with the modified drawing from Peterson [22] (cited as “Peterson 1960”, apparently a later edition of Peterson [22]). The second one was specimen 5402 (his Figure 11c), i.e., the specimen from Ghilarov [70].
- (7)
- (8)
- Tauber [40] re-figured specimen 5401, i.e., the specimen from Gurney [38], but with the modified drawings from Peterson [22], including the overview (her Figure 33.12 p. 132) and the head in dorsal view (her Figure 33.13 p. 132). Apparently, there are later editions of this contribution also figuring this image (at least 1987, 1991, 2008, our version was labelled 1991). We assume that all of these feature the same images. Hence these also refer to the same specimen.
- (9)
- Monserrat [93] provided several drawings (all on p. 197) of a stage 1 larva of Dilar pumilus (specimen 5404). The drawings include the habitus in dorsal view (his Figure 19), the head in dorsal view (his Figure 20), maxilla (his Figure 21), mandible (his Figure 22), and the trunk end in dorsal (his Figure 23) and ventral view (his Figure 24). According to the provided scale, the larva was 6.2 mm long. The specimen was re-figured in Makarkin [61] and Makarkin and Tshistjakov [94].
- (10)
- (11)
- Minter [95] provided images of several larvae of Nallachius krooni. The first specimen was a stage 1 larva (specimen 5405). The images for this specimen include a habitus drawing in dorsal view (his Figure 1 p. 264), and detail drawings of head in dorsal (his Figure 2 p. 264) and ventral view (his Figure 3 p. 264). According to the provided scale, the larva was 1.1 mm long.The second specimen was a stage 2 larva (specimen 5406). The images for this specimen include drawings of the head in dorsal (his Figure 4 p. 265) and ventral view (his Figure 5 p. 265). According to the provided scale, the head capsule was 0.18 mm long.The third specimen was a stage 3 larva (specimen 5407). The images for this specimen include drawings of the head in dorsal (his Figure 6 p. 265) and ventral view (his Figure 7 p. 265). According to the provided scale, the head capsule was 0.26 mm long.
- (12)
- (13)
- (14)
- Monserrat [96] depicted micrographs of a stage 1 larva of Dilar meridionalis, recorded under transmitted light. The images include an early stage 1 larva (his Figure 5a p. 13), a later stage 1 larva (with a more elongated trunk), details of the head (his Figure 5c p. 13), trunk appendage (his Figure 5d p. 13), antennae (his Figure 6a p. 14), and labium (his Figure 6b p. 14). All of these images may show the same individual at different times, in the same instar (just earlier and later developmental stages), but this aspect remains unclear. We consider only one specimen (specimen 5408) in order to avoid considering the same individual in the same stage twice. Additionally, drawings of the head in dorsal view (his Figure 7a p. 16) and the labium (his Figure 7b p.16) were also provided. These are interpreted as originating from the same specimen as the one from the micrographs of Monserrat [96]. A scale was provided, indicating that the head capsule was 0.14 mm long.Monserrat [96] also re-figured some details (labium, his Figure 7c p. 16; antenna, his Figure 7d p. 16) of a specimen from Minter [95]. Drawings of the trunk appendages of a stage 1 larva of Dilar dissimilis were also provided (his Figure 8 p. 17); yet, no habitus image of the larva was shown, and hence it could not be further considered here.
- (15)
- (16)
- (17)
- Liu et al. [97] provided a photograph of a larva (specimen 5409) of Nallachius americanus, in dorsal view (their Figure 1D p. 449). As the specimen was apparently photographed in the field, the developmental stage is unknown. No indication of size was provided.
- (18)
- (19)
- (20)
- Badano et al. [91] provided a photograph of a larva of Dilaridae in dorsal view (their Figure 4ii p. 679). Yet, it is difficult to access details of the mouthparts and the posterior edge of the head capsule from the photograph. Therefore, the specimen could not be further considered for our analysis.Short note: Badano et al. [98] reported new morphological details of stage 1 and stage 2 larvae of Dilar duelli. The paper was just published after this study was finished and could therefore not be considered in detail here.
3.4. Extant Larvae of Osmylidae
- (1)
- Brauer [99] provided drawings of a larva of Osmylus maculatus (specimen 5601). The images include the habitus in dorsal view (his Figure 1a pl. III), the head in lateral view (his Figure 1b pl. III), and the tip of a trunk appendage (his Figure 1c pl. III). No clear indication of size was provided. The specimen was re-figured in Aspöck [100].
- (2)
- Hagen [101] provided several drawings of a larva of Osmylus maculatus (specimen 5602). These drawings (all in pl. III) include the habitus in detail in dorsal view (his Figure 9), a partly simplified lateral view (his Figure 10), details of trunk end (his Figures 11 and 13), detail of trunk appendages (his Figure 14), the head in dorsal view (his Figure 15), and a detail of tip of stylet (his Figure 16). There is an unlabelled scale bar on the figure plate, not referring to a specific panel. Therefore, no clear indication of size was provided. The specimen was re-figured in Aspöck [100].
- (3)
- Hudson [102] provided a drawing of a larva (specimen 5603) of Stenosmylus incisus in dorsal view (his Figure 3 pl. VIII). No indication of size was provided.
- (4)
- (5)
- Ussing [105] provided a drawing of a larva (specimen 5604) of Osmylus chrysops in dorsal view (p. 83). No indication of size was provided.
- (6)
- Lestage [106] provided drawings of two larvae of Osmylus chrysops. The first specimen (his Figure 1 p. 227) was a later stage 3 larva in dorsal view (specimen 5605). An unlabelled scale bar is figured next to the drawing. According to the text, the size range of the examined larvae was between 14 mm and 20 mm. The specimen was re-figured by Lestage [8] and Stitz [107].
- (7)
- (8)
- Withycombe [108] provided a drawing (his Figure 4 pl. XXXVIII) of a larva (specimen 5607) of Osmylus chrysops. The size was provided as a magnification factor, which is not informative in combination with the electronic versions available to the present authors. In addition, a ventral view on the head capsule of a later stage larva shortly before pupation (pre-pupa) was provided (his Figure 5 pl. XXXVIII). This second specimen could not be further considered here as it lacks the very long mandibles. The considered specimen was re-figured by Imms [109], Ghilarov [70], Dorohova [71], and Makarkin [61].
- (9)
- Imms [109] presumably re-figured specimen 5607, i.e., the drawing by Withycombe [108]. We could not access the first edition; yet, in the second edition from 1930, the specimen is shown (his Figure 387). The figures are still present in the other editions (published by various publishers; later editions expanded by other authors; e.g., 1924, 1925, 1934; 5th edition 1942; 6th edition 1946, 1948; 8th edition 1951; 9th edition 1957; 9th edition 1960; 9th edition 1964, 1970; 10th edition 1977, 2000, 2017), but with varying figure numbers, e.g., in 10th edition, it is Figure 355 p. 801 [110]).
- (10)
- Withycombe [111] provided details of larvae of Osmylus chrysops. This includes a ventral view of the head capsule of a stage 3 larva (his Figure 13 pl. XL) and the tip of the trunk appendages of a stage 1 larva (his Figure 19 pl. XLI). As no complete head is available, none of these could be further considered here.
- (11)
- Tillyard [37] provided a drawing of a larva (specimen 5608) of Euosmylus stellae in dorsal view (his Figure U4 p. 310). According to figure legend, the larva was 20 mm long.
- (12)
- Rabaud [112] provided a drawing of a larva (specimen 5609) of Osmylidae in dorsal view (his Figure 5 p. 447) without trunk appendages. The size was provided as a magnification factor, which is uninformative in combination with the electronic versions available to the present authors.
- (13)
- (14)
- Stitz [54] provided numerous drawings of larvae of Osmylops chrysops. The images include a head in ventral view (his Figure 10 p. 35.79). This is a re-figure of a drawing from Withycombe [111], but simplified, not illustrating the entire stylet length and shape. In the original drawing, the distal part of these is not shown. We could not further consider this drawing.The next one was a stage 1 larva in dorsal view (his Figure 110 p. 35.142), which is a re-figure of specimen 5606 (i.e., specimen from Lestage [106]). A later stage larva, also in dorsal view (his Figure 111 p. 35.142), differs from all earlier depictions, especially in the details of the terminal attachment structure (pygopod). The specimen could therefore be further considered for our analysis (specimen 5610). No indication of size was provided. Many details of the larva were provided, including the antenna (his Figure 112 p. 35.143), the tips of maxilla (his Figure 113a p. 35.143), and mandible (his Figure 113b p. 35.143), sections through the stylet (his Figure 114 p. 35.144), a section through the salivary gland (his Figure 115 p. 35.144), the labial palp (his Figure 116 p. 35.144), sections through the palp (his Figure 117 p. 35.144), the anterior body in dorsal view (his Figure 118 p. 35.145), an abdomen sternite (his Figure 119 p. 35.145), excellent details of the terminal end (his Figure 120 p. 35.146), and details of the trunk appendages (his Figure 121 p. 35.147).
- (15)
- Killington [113] apparently wrote about the larva of Osmylops fulvicephalus. Yet, we were unable to get a hold of a copy of this work and can therefore not state whether or not it contains any depictions.
- (16)
- David [114] provided drawings of larvae of Osmylus chrysops. This included a larva that was still inside the egg, but close to hatching (his Figure 4 p. 153) and a stage 3 larva in dorsal view (his Figure 5 p. 155). Yet, these drawings appear to be strongly simplified and could not be further considered here as they do not provide sufficient details.
- (17)
- Killington [115] figured a drawing of a stage 3 larva (specimen 5611) of Osmylus fulvicephalus in dorsal view (his Figure 1 pl. IX). The figure shows a small empodium, also mentioned in the text, which is often not shown in drawings of larvae of Osmylidae. According to the text and the figure legend, the larva was 15 mm long. The specimen was re-figured by Parfin and Gurney [116], Kimmins [117], and Aspöck and Aspöck [60].
- (18)
- Genay [118] provided two drawings of a larva of Osmylus chrysops. The images include the head capsule in dorsal and ventral view (his Figure 8 upper and lower between pages 22 and 23). As the distal parts of the mouthparts are missing in both views, the specimen could not be further considered here.
- (19)
- (20)
- Kawashima [119] provided several drawings of a larva (specimen 5612) of Spilosmylus flavicornis. These drawings included (all on pl. 2) the habitus in dorsal view (his Figure A), the head capsule in dorsal (his Figure B) and ventral view (his Figure C), labial palp (his Figure D), antenna (his Figure E), and a trunk appendage (his Figure F). According to the text, the larva was 9 mm long.
- (21)
- Fraser [120] provided a drawing (his Figure 14g p. 33) of a larva (specimen 5613) of Osmylus fulvicaphalus in dorsal view. No indication of size was provided.
- (22)
- Wundt [121] provided numerous images of a later stage larva of Osmylus chrysops. The images include a photograph of an entire larva in dorsal view (his Figure 1 p. 558) as well as drawings of the head in dorsal (his Figure 2a p. 561) and ventral view (his Figure 2b p. 561), of details of the head capsule (his Figure 3 p. 563, his Figure 33 p. 641, his Figure 34 p. 642), the mandibles (his Figure 4 p. 569, his Figure 5 p. 570, his Figure 6 p. 573, his Figure 7 p. 575, his Figure 8 p. 576), the maxillae (his Figure 9 p. 578, his Figure 10 p. 583, his Figure 11 p. 585, his Figure 12 p. 586, his Figure 13 p. 587), anterior nervous system (his Figure 14 p. 588, his Figure 22 p. 612, his Figure 28 p. 634), the labium (his Figure 15 p. 592, his Figure 17 p. 595, his Figure 18 p. 597), the salivarium (his Figure 16 p. 593), pre-oral chamber (his Figure 19 p. 601, his Figure 23 p. 615, his Figure 25 pp. 622–623), musculature of the head capsule (his Figure 20 p. 604, his Figure 21 p. 609), the antenna (his Figure 26 p. 627, his Figure 27 p. 629), specialised inner organs (his Figure 29 p. 635, his Figure 30 p. 636, his Figure 31 p. 637, his Figure 32 p. 639), and trachea (his Figure 35 p. 644). The text states that stage 2 and 3 larvae were used. We consider a single specimen (specimen 5614) for our analysis, as represented by the image of the head (his Figure 2a p. 561). According to the provided scale, the head capsule was 1.4 mm long. The specimen was re-figured in Aspöck and Aspöck [3].
- (23)
- (24)
- (25)
- (26)
- MacLeod [23] provided several drawings of a larva of Kempynus (specimen 5615). The images include the head in ventral (his Figure 21 pl. VII), dorsal (his Figure 22 pl. VII), and lateral view (his Figure 23 pl. VIII), inner arrangement of the head capsule (his Figure 24 pl. VIII), and details of the mandible (his Figure 25 pl. VIII). According to the provided scale, the head capsule was 1.4 mm long.
- (27)
- Ward [122] wrote about the larva of Osmylops fulvicephalus. Yet, we were unable to acquire a copy of this work and can therefore not state whether or not it contains any depictions.
- (28)
- Riek [1] provided drawings of two larvae of Osmylidae. The first showed a head of a larva of Porismus strigatus in ventral view (his Figure 29.5A). Yet, the stylets are not fully shown, and hence this specimen could not be further considered here.The second one showed a larva of Kempynus (specimen 5616) in dorsal view (his Figure 29.10E p. 486), accompanied by detail of the head in dorsal view (his Figure 29.10F p. 486). The stage of the larva remains unclear. No indication of size was provided. The specimen was re-figured by New [25,43,123,124], Tauber et al. [44], and Heckman [51].
- (29)
- New [125] provided drawings of details of a larva of Stenosmylus tenuis. These drawings include the labium (his Figure 5 p. 26), antenna (his Figure 6 p. 26), trunk appendages (his Figure 7 p. 26), and the anchoring structure at the terminal end (his Figure 8 p. 26). As no image of the head was provided, the specimen could not be further considered here.
- (30)
- Gepp [24] provided drawings of a stage 3 larva of Osmylus fulvicephalus (specimen 5617). The images include the habitus in dorsal view (his Figure 10a pl. 5 p. 196) and a dorsal view of the head (his Figure 10b pl. 5 p. 196). According to figure legend, the larva was 17 mm long.
- (31)
- Dorohova [71] provided a drawing of a larva of Osmylus fulvicephalus (her Figure 38 p. 47). The drawing resembles the drawing of Withycombe [108] in most aspects, but shows some differences in the positions of the trunk appendages. Yet, as the entire body outline and the majority of setae match, we consider this drawing as a re-figure of specimen 5607.
- (32)
- (33)
- (34)
- (35)
- Makarkin [61] provided a drawing of a larva of Osmylus fulvicephalus (his Figure 16.4 p. 42). The drawing resembles the drawing of Withycombe [108] in most aspects, but shows some differences in the positions of the trunk appendages. Yet, as the entire body outline and the majority of setae match, we consider this drawing as a re-figure of specimen 5607.
- (36)
- (37)
- Aspöck and Aspöck [2] provided a photograph (their Figure 46, p. 19) of a larva (specimen 5618) of Osmylus fulvicephalus in dorsal view. According to the figure legend, the larva was 16 mm long. The larval stage remains unclear. The photograph was recorded by Peter Duelli. The specimen was re-figured in Aspöck [126], Grimaldi and Engel [127], and Aspöck and Aspöck [3].
- (38)
- Gepp [128] provided a photograph (his Figure 22 p. 194) of a larva (specimen 5619) of Osmylus fulvicephalus in dorsal view. According to figure legend, the larva was 13 mm long. The larval stage remains unclear.
- (39)
- (40)
- (41)
- Gepp [129] provided numerous images of larvae of Osmylus fulvicephalus. The first one was a photograph of stage 1 larvae (in varying states of feeding) in dorsal view (his Figure 4 p. 327). Yet, these images are a bit too small to provide enough detail and could not be further considered here.The second one was a drawing of a late stage 1 larva (specimen 5620) in dorsal view (his Figure 5 p. 327). This drawing provides excellent details. According to figure legend, the larva was 5.6 mm long.Further images show many aspects of a stage 3 larva, possibly of a single specimen. The images include a micrograph in dorsal view (his Figure 6 p. 328), a drawing in dorso-lateral view (his Figure 7 p. 329) and in lateral view (his Figure 8 p. 329), details of the tergites (his Figure 9 p. 330), the trunk appendages (his Figure 10 p. 330), the head in ventral view (his Figure 11 p. 331), and a schematic lateral view of the head (his Figure 12 p. 331). No clear indication of size was provided for the dorsal view. The micrograph of the habitus shows a large similarity to specimen 5619, i.e., the specimen from Gepp [128] with only the position of the trunk appendages being slightly different. We still consider this as additional images of specimen 5619 in order to avoid considering the same specimen twice.
- (42)
- (43)
- (44)
- (45)
- Aspöck and Aspöck [3] provided a photograph of a larva (specimen 5621) of Osmylus fulvicephalus in dorsal view (their Figure 33 p. 464). According to the figure legend, the larva was 16 mm long. The larval stage remains unclear. The photograph was recorded by Franziska Anderle.
- (46)
- Cover and Bogan [130] provided photographs of a stage 3 larva of Osmylus fulvicephalus in lateral (their Figure 41.6a p. 1066) and dorso-lateral view (their Figure 41.6b p. 1066). As the head is not well accessible in dorsal view, the specimen could not be further considered here.
- (47)
- Matsuno and Yoshitomi [131] provided images of several stage 3 larvae of Osmylidae. In total, the larvae of three species were examined: Osmylus hyalinatus, O. pryeri and O. tesselatus.The larva of Osmylus hyalinatus (specimen 5622) was provided as a photograph in the field (their Figure 36) and a number of details as drawings including the head in dorsal view (their Figure 15), the anterior edge of the head (their Figure 5), the tip of the antenna (their Figure 8) and the labial palp (their Figure 6), the entire antennae (their Figure 7), setae arrangement of the thorax in dorsal (their Figure 9 left, 18), ventral (their Figure 9 right), and lateral view (their Figure 10), a single thorax sclerite (their Figure 19), setae arrangement of the abdomen in dorsal (their Figures 11 and 24) and ventral view (their Figure 12), ventral details of abdomen segment 7 (their Figure 27), dorsal (their Figure 30) and ventral (their Figure 31) details of abdomen segment 9, and the trunk end in dorsal (their Figure 13) and ventral view (their Figure 14). According to the provided scale, the head capsule was 1 mm long.The larva of Osmylus pryeri (specimen 5623) was provided as a photograph in the field (their Figure 37) in addition to a number of details as drawings, including the head in dorsal (their Figures 1 and 16), ventral (their Figure 2), and lateral view (their Figure 3), the anterior edge of the head (their Figure 4), setae arrangement of the thorax in dorsal view (their Figure 20), a single thorax sclerite (their Figure 21), setae arrangement of the abdomen in dorsal view (their Figure 25), ventral details of abdomen segment 7 (their Figure 28), and abdomen segment 9 in dorsal (their Figure 32) and ventral view (their Figure 33). According to the provided scale, the head capsule was 1.3 mm long.The larva of Osmylus tesselatus (specimen 5624) was provided as a photograph in the field (their Figure 37) and a number of details as drawings, including the head in dorsal view (their Figure 17), setae arrangement of the thorax in dorsal (their Figure 22), a single thorax sclerite (their Figure 23), setae arrangement of the abdomen in dorsal (their Figure 26), ventral details of abdomen segment 7 (their Figure 29), and abdomen segment 9 in dorsal (their Figure 34) and ventral view (their Figure 35). According to the provided scale, the head capsule was 1.4 mm long.
- (48)
- Glime [132] figured several photographs of larvae of Osmylidae in the field. The first (her Figure 2 p. 11-8-2) was an image of a larva of Osmylus fulvicephalus in postero-dorsal view. The second (her Figure 5 p. 11-8-3) was a close up of the head region in antero-dorsal view of a similar larva. The third (her Figure 6 p 11-8-3) was an image of a larva of Kempynus in lateral view. All images do not provide direct access to the head in dorsal orientation and could therefore not be further considered here.
- (49)
- (50)
- (51)
- Matsuno [134] provided images of at least one larva Osmylus hyalinatus (specimen 5625). Images show a larva in “crumpled” state (his Figure 2A p. 3) and one in an “inflated” state (his Figure 2D p. 3). It remains unclear whether these two images show the same individual. To avoid considering the same specimen twice, we only considered one of the images, namely that in the “inflated” state. No indication of size was provided.
- (52)
- Winterton et al. [135] provided photographs of several larvae in the field. These included larvae of Kempynus (their Figure 2C p. 3; recorded by Kristi Ellington), Isostenosmylus (their Figure 2D p. 3; recorded by Enio Branco), and Stenosmylinae (their Figure 2E p. 3 recorded by Kristi Ellington). While the images are quite detailed and could be used for other types of analyses, the heads are not ideally accessible in dorsal view or provide only a low contrast of stylets against the background. Hence, none of these specimens could be further considered.
- (53)
- Martins et al. [136] provided detailed images of several larvae of Osmylidae. These included images of larvae of Kempynus and Isostenosmylus pulverulentus.The images of Kempynus included photographs of an anterior body region of a stage 3 larva in lateral view (their Figure 2 upper p. 5) and a stage 2 larva in lateral view (their Figure 2 lower p. 5) as well as photographs of stage 3 larvae in the field (their Figure 3g,h p. 6) and drawings of stage 3 larvae of the head in dorsal (their Figure 4a p. 7), ventral (their Figure 4b p. 7), and lateral view (their Figure 4c p. 7), of the antenna (their Figure 5a p. 8), the tip of the antenna (their Figure 5b p. 8), anterior edge of the head (their Figure 5c p. 8), the tip of the labial palp (their Figure 5d p. 8), and the trunk appendages (their Figure 5e,f p. 8), the setae arrangement of the thorax in dorsal (their Figure 6a p. 10), ventral (their Figure 6b p. 10), and lateral view (their Figure 6c p. 10), the setae arrangement on the abdomen segments in dorsal (their Figure 7a p. 12) and ventral view (their Figure 7b p. 12), and the setae arrangement on the trunk end in dorsal (their Figure 8a p. 13) and ventral view (their Figure 8b p. 13). We here considered the drawings of the head as a single specimen (specimen 5626).Images of Isostenosmylus pulverulentus included photographs of an anterior body region of a stage 3 larva in lateral view (their Figure 9 upper p. 15) and a stage 2 larva in lateral view (their Figure 9 lower p. 15), drawings of stage 3 larvae with the head in dorsal (their Figure 10a p. 16), ventral (their Figure 10b p. 16), and lateral view (their Figure 10c p. 16), of the antenna (their Figure 11a p. 17), the tip of the antenna (their Figure 11b p. 17), anterior edge of the head (their Figure 11c p. 17), the tip of the labial palp (their Figure 11d p. 17), and the trunk appendages (their Figure 11e,f p. 17), the setae arrangement of the thorax in dorsal (their Figure 12a p. 19), ventral (their Figure 12b p. 19), and lateral view (their Figure 12c p. 19), the setae arrangement on the abdomen segments in dorsal (their Figure 13a p. 21) and ventral view (their Figure 13b p. 21), and the setae arrangement on the trunk end in dorsal (their Figure 14a p. 22) and ventral view (their Figure 14b p. 22). We here considered the drawings of the head as a single specimen (specimen 5627).
- (54)
- (55)
- (56)
- (57)
- Badano et al. [91] provided a photograph of a larva (specimen 5628) of Osmylidae in dorsal view (their Figure 4i p. 679). No indication of size was provided.
3.5. Fossil Lacewing Larvae with Straight or Almost Straight Stylets from the Literature
- (1)
- Whalley [137] provided images of a larva of Berothidae (specimen 5801) preserved in Lebanese amber. This included micrographs of the habitus in more less-dorsal view (his Figure 9 p. 163) and a close-up of the head (his Figure 10 p. 163). No indication of size was provided. The details are not sufficient to further consider the specimen for our analysis.
- (2)
- Grimaldi et al. [138] provided a micrograph (their Figure 28f p. 44) of a larva (specimen 5802) of Osmylidae preserved in Myanmar amber. The specimen is part of the collection of the American Museum of Natural History, New York, USA (AMNH Bu-267). The image is rather small, yet more detailed images were provided in Engel and Grimaldi [139] (see there for details).
- (3)
- Janzen [140] provided a detailed drawing of a larva of Berothidae preserved in Baltic amber in dorsal view (his Figure 58 p. 120). According to the figure legend, the larva (specimen 5803) was 5.4 mm long. The specimen is part of the collection of Hoffeins (CCHH 1270-1, now SDEI Lep-103564). Originally the specimen was interpreted as a larva of Chrysopidae, but later re-interpreted as a larva of Berothidae [13]. The specimen was re-documented for the present study (Figure 1).Janzen [140] also figured a micrograph of a small larva (specimen 5804) attached to a spider (his Figures 107 and 108 p. 81). He interpreted this as the larva of a beetle. Later, the specimen was interpreted as a larva of Mantispidae [11]. Yet, especially the head region is not accessible, and hence the specimen could not be considered for our analysis. The specimen was re-figured by Wunderlich [141], Ohl [11], and Haug et al. [12].
- (4)
- (5)
- Engel and Grimaldi [139] provided numerous fossil larvae with straight stylets originating from Cretaceous amber.The first specimen was a small larva (specimen 5805) preserved in amber from Myanmar. Images include a micrograph of the habitus (their Figure 7 p. 54) and a drawing (their Figure 8 p. 54). According to the provided scale, the specimen was 0.6 mm long. The specimen is part of the collection of the American Museum of Natural History, New York, USA (AMNH Bu-126). Originally, the specimen was interpreted as a possible larva of Psychopsidae. Later it was re-interpreted as a possible larva of Berothidae [21] (see also discussion in [15]). According to Pérez-de la Fuente et al. [21] (supplement), there is a second, similar-appearing specimen, yet it appears not to have been figured.The second specimen was a re-figure of specimen 5802, i.e., the larva of Osmylidae preserved in amber from Myanmar, already published in Grimaldi et al. [138]. Images included micrographs of the entire larva in dorsal view (their Figure 9 p. 55) and a close-up on the head (their Figure 10 p. 55) with drawings of the same aspects (their Figure 11 p. 56). It is important to note that at least the first trunk appendage appears to bear an empodium (see further below). According to the provided scale, the larva was 4.5 mm long.The third specimen was a larva hatching from the egg, preserved in Canadian amber (specimen 5806). Images included micrographs of the habitus (their Figures 12 and 13 p. 57) and a drawing of the habitus (their Figure 14 p. 58). According to the provided scale, the larva was 0.8 mm long. The specimen is part of the amber collection of the Canadian National Collection, Ottawa, Canada (CAS-1096). The specimen was originally interpreted as a larva of Chrysopidae, but was later interpreted as a representative of Berothidae [13] (p. 250). The interpretation is supported by Pérez-de la Fuente et al. [21]. The details of the stylets are not easy to interpret, and therefore the specimen could not be further considered for the analysis performed here.The fourth specimen was a larva (specimen 5807) of Berothidae preserved in amber from Myanmar. Images include a micrograph of the entire larva in ventral view (their Figure 42 p. 75) and a drawing of the head in ventral view (their Figure 43 p. 75). According to the provided scale, the head capsule was 0.6 mm long. The specimen is part of the collection of the American Museum of Natural History, New York, USA (AMNH Bu-1297).
- (6)
- Wichard et al. [142] provided three images of a small larva (specimen 5808) of Osmylidae preserved in Baltic amber. This included a micrograph of the habitus in ventral view (their Figure 07.04a p. 88), a detail of the head region in dorsal view (their Figure 07.04b p. 88), and a drawing of an antenna (their Figure 07.04c p. 88). According to the provided scale, the larva was 1.5 mm long.
- (7)
- (8)
- Wunderlich [83] reported three stage 1 larvae of Mantispidae (specimens 5809–5811) preserved in a single piece of Baltic amber. The larvae were preserved in close association with a male spider, two of them even in direct contact to it. The larvae were stated to be 7 mm, 9 mm, and 10 mm long, respectively. This would be extremely large for stage 1 larvae of mantis lacewings. The images include micrographs of the spider in overview (photo 35 p. 344) and close-ups of two larvae (photo 36 p. 344). Yet, neither are accessible in dorsal view and could therefore not be further considered for our analysis. The specimens are part of the collection of Jörg Wunderlich, F2275/BB/AR/CJW (this contribution had been overlooked by Nagler and Haug [85] and Haug et al. [12]).
- (9)
- Wedmann et al. [13] provided five fossil larvae of the group Berothidae, all originating from Baltic amber.The first specimen (specimen 5812) was provided with numerous images including a micrograph (their Figure 1A p. 238) and a drawing (their Figure 1B p. 238) in dorsal view, micrographs of the head in dorsal (their Figure 2A p. 239) and ventral view (their Figure 2B p. 239), drawings of the head in dorsal (their Figure 2C p. 239) and ventral view (their Figure 2D p. 239), and a drawing of a trunk appendage (their Figure 2E p. 239). According to the text, the larva was 1.83 mm long. The specimen is part of the collection of Thomas Weiterschan (No. 240).The second specimen (specimen 5813) was provided with numerous images including a micrograph (their Figure 3A p. 240) and a drawing (their Figure 3B p. 240) in dorsal view, micrographs of the head in dorsal (their Figure 4A p. 241) and ventral view (their Figure 4B p. 241), a micrograph of the trunk end (their Figure 4C p. 241), drawings of the head in dorsal (their Figure 5A p. 242) and ventral view (their Figure 5B p. 242), a drawing of a trunk appendage (their Figure 5C p. 242), and a drawing of the trunk end (their Figure 5D p. 242). According to the text, the larva was 2.25 mm long. The specimen is part of the collection of Thomas Weiterschan (No. 1555).The third specimen is specimen 5803, the same specimen as in Janzen [140], but with more detailed images, including a micrograph of the habitus in dorsal view (their Figure 6 p. 244), a micrograph of the head in dorsal view (their Figure 7A p. 244), a drawing of the head in dorsal view (their Figure 7B p. 244), a drawing of the labial palp (their Figure 7C p. 244), and a drawing of a trunk appendage (their Figure 7D p. 244).The fourth specimen (specimen 5814) was also provided with numerous images, including micrographs of the left and right body side (their Figure 8A,B p. 246), drawings of one body side (their Figure 9A p. 247), the head in latero-ventral view (their Figure 9B p. 247), a close-up of the eye region (their Figure 9C p. 247), and volume renderings of a SRµCT scan of the head in dorsal view (their Figure 10A p. 248), the stylets (their Figure 10B p. 248) and the entire specimen revealing some inner structures (their Figure 10C p. 248). According to the text, the larva was 3.1 mm long. The specimen is part of the collection of the Senckenberg Museum, Frankfurt, Germany (SMF Be 1297). Despite the detailed documentation and re-documentation of the specimen for the present study (Figure 2), there is no view that allows a reconstruction of the outline of the head in dorsal view with the stylets in forward orientation. Therefore, the specimen could unfortunately not be further considered for the analysis.The fifth specimen (specimen 5815) was figured as micrographs of the habitus (their Figure 11A p. 249) and as a close-up of the head (their Figure 11B p. 249). According to the text, the larva was 7.5 mm long. It is part of the collection of Thomas Schäfer. It was only briefly discussed and the orientation of the specimen does not allow us to consider the specimen further for the analysis performed herein.
- (10)
- Xia et al. [143] provided an image of a larva with very long stylets preserved in Myanmar amber (upper image p. 95). Pérez-de la Fuente et al. [21] suggested that this larva could be a representative of Osmylidae. Yet, the stylets appear quite curved inwards; therefore, the larva appears rather different from lance lacewing larvae. Due to this uncertainty, we could not consider this larva further here.
- (11)
- Engel [144] provided images of a larva (specimen 5816) preserved in amber from Myanmar. Images include micrographs of an overview of the amber piece showing syn-inclusions (his Figure 9 p. 11) and the habitus in ventral view (his Figure 10 p. 11). According to the text, the larva was 1.5 mm long. The specimen is part of the collection of the American Museum of Natural History, New York, USA (AMNH Bu-275). Originally, the specimen was interpreted as a possible larva of Coniopterygidae, but later interpreted as a larva of Berothidae [21].
- (12)
- Zhang [145] provided a micrograph of a larva (specimen 5817) preserved in amber from Myanmar in ventral view (p. 397 lower image). The larva was interpreted as representative of Berothidae. Yet, the stylets are slightly curved. Given the overall habitus that resembles that of small larvae of Berothidae, the larva could be a representative of Mantispidae.
- (13)
- Haug et al. [12] provided images of a small larva (specimen 5818) of Mantispidae preserved in amber from Myanmar. Images include micrographs of the entire amber piece with syn-inclusions under cross-polarised light (their Figure 1A p. 3) and ring light illumination (their Figure 1B p. 3), a colour-marked image indicating the position of the larva (their Figure 1C p. 3), the larva in ventral view under ring-light illumination (their Figure 1D p. 3) and under cross-polarised light (their Figure 2A p. 4), a colour-marked image indicating the major morphological features (their Figure 2A p. 4), and a restoration drawing (their Figure 3A p. 5). According to the text, the larva was 0.75 mm long. The specimen was part of the collection of Patrick Müller and is now part of the collection of the Staatliches Museum für Naturkunde, Stuttgart, Germany (SMNS-P-Bu-338). They also re-figured (their Figure 3F p. 5) the larva from Janzen [140].
- (14)
- Pérez-de la Fuente et al. [21] provided images of three larvae preserved in Cretaceous amber from Spain. The first larva (specimen 5819) is of Berothidae. The images include a micrograph of the habitus in ventral view (their Figure 2A p. 5), a drawing of the habitus (their Figure 2B p. 5), a micrograph of the head (their Figure 2C p. 5), a drawing of the head (their Figure 2D p. 5), micrographs of the entire larva in lateral view (their Figure 3A p. 6), of the head in dorsal (their Figure 3B p. 6), ventral (their Figure 3C p. 6), and lateral view (their Figure 3D p. 6), and a detail of the tip of a trunk appendage (their Figure 3E p. 6). According to the provided scale, the larva was about 2 mm long. The specimen is part of the collection Museo de Ciencias Naturales de Álava, Vitoria-Gasteiz, Spain (MCNA 9294).The second larva was a possible larva of Berothidae (specimen 5820). Images include a micrograph of the habitus in dorsal view (their Figure 4A p. 7), a drawing of the habitus in dorsal view (their Figure 4B p. 7), and close-up micrographs of the trunk end (their Figure 4C p. 7) and of the head (their Figure 4D p. 7). According to the scale provided, the specimen was about 3.3 mm long. The specimen is part of the collection Fundación Conjunto Paleontológico de Teruel-Dinópolis, Teruel, Spain (SJ-10-25). Unfortunately, the stylets are not fully preserved inside the amber. Therefore, the specimen could not be further considered here.The third larva (specimen 5821) could not be easily interpreted in a phylogenetic frame [21], but may be closely related to Berothidae or Mantispidae (“Mantispoidea”) or to Dilaridae (“Dilaridoidea”). The latter was also compared to certain aspects of Osmylidae, but was considered as a less likely interpretation [21]. Images include micrographs in dorso-lateral view from both sides (their Figure 5A,B p. 9), a close-up on trunk sclerites (their Figure 5C p. 9), eye region (their Figure 5D p. 9), a close-up on the latero-ventral part of the head capsule (their Figure 5E p. 9), a close-up on the labial palp (their Figure 5F p. 9), drawings of the head capsule in dorso-lateral view (their Figure 6A p. 10), and the labial palp (their Figure 6B p. 10). According to the provided scale, the head capsule of the specimen was 0.6 mm long. The specimen is part of the collection Fundación Conjunto Paleontológico de Teruel-Dinópolis, Teruel, Spain (SJNB2012-04). As the head is not well accessible in dorsal view, the specimen could not be further considered for our analysis.
- (15)
- Haug et al. [7] reported a larva (specimen 5901) with very long, almost straight stylets, preserved in Myanmar amber: the supersting larva. This larva clearly differs from all others considered here. It is included in this comparison here due to certain similarities of this larva to those of lance lacewings.Images included a number of micrographs of the entire amber piece (their Figure 1A p.3), the habitus in ventral (their Figure 1B p. 3) and dorsal view (their Figure 1C p. 3), the head in ventral view (their Figure 1D p. 3), the head capsule in dorsal view under fluorescence light (their Figure 1E p. 3) with colour markings (their Figure 1F p. 3), the head capsule in ventral view (their Figure 1G p. 3), the abdomen in dorsal view (their Figure 1H p. 3), fluorescence images of the entire larva in ventral (their Figure 2A p. 6) and dorsal view (their Figure 2B p. 6), head capsule (their Figure 2D p. 6) and trunk (their Figure 2G p. 6), stereo anaglyphs of the entire larva in dorsal view (their Figure 2C p. 6), the head in ventral view (their Figure 2E p. 3), and a micrograph of the trunk appendages (their Figure 2F p. 6). A simplified restoration of the head in dorsal view (their Figure 4 p. 10) and of the entire specimen in dorsal view (their Figure 5D p. 11) were provided. According to the text, the larva was 2.53 mm long. The specimen was part of the collection of Patrick Müller and is now part of the collection of the Staatliches Museum für Naturkunde, Stuttgart, Germany (SMNS Bu-355).
- (16)
- Haug et al. [146] reported a second specimen of the supersting-type larva (specimen 5902). Most likely, it represents a later developmental stage.
3.6. New Fossil Lacewing Larvae with Straight or Almost Straight Stylets
- (1)
- Specimen 5822 (Gröhn 7512). A small-sized specimen preserved in a piece of Baltic amber. The larva is accessible in dorsal view (Figure 3), but partly concealed by a thin white film (Verlumung). The larva has very prominent and elongate stylets and is most likely a representative of the group Osmylidae. The larva is 1.66 mm long.
- (2)
- Specimens 5823 + 5824 (BUB 3064). The amber piece includes two specimens, which are preserved in Myanmar amber (Figure 4). Specimen 1 (5823; Figure 4A–C) is well accessible in dorsal view (Figure 4A,B), but partly concealed by an air bubble. The larva has straight stylets. Each trunk appendage bears a prominent attachment structure distally (empodium; Figure 4C). The larva is about 4.8 mm long. Specimen 2 (5824; Figure 4D,E) is well accessible in lateral view and also has straight stylets. The larva is about 7.5 mm long.
- (3)
- Specimen 5825 (BUB 3065). The larva is preserved in Myanmar amber and is well accessible in dorsal view (Figure 5A,B). The head and the straight stylets are well accessible (Figure 5C). Each trunk appendage bears a prominent attachment structure distally (empodium; Figure 5D). The larva is 7.7 mm long.
- (4)
- (5)
- Specimen 5827 (BUB 3355). The larva is preserved in Myanmar amber. It is well accessible in dorsal (Figure 7A,B) and ventral view (Figure 7C). In ventral view, the trunk is concealed by a large bubble. The head and the straight stylets are well accessible (Figure 7D,E). Each trunk appendage bears a prominent attachment structure distally (empodium; Figure 7F). The larva is 2 mm long.
- (6)
- (7)
- Specimen 5829 (BUB 3726). The larva is preserved in Myanmar amber. It is well accessible in dorsal view (Figure 8A,B) and partly concealed by a large bubble in ventral view (Figure 8C). The head and the straight stylets are well accessible in dorsal view (Figure 8D,E). Each trunk appendage bears a prominent attachment structure distally (empodium; Figure 8F). The posterior part of the trunk is better accessible in ventral view (Figure 8G). The larva is about 1.1 mm long.
- (8)
- Specimen 5830 (BUB 3741). The larva is preserved in Myanmar amber. It is well accessible in dorsal (Figure 9A,B) and ventral view (Figure 9C), but partly concealed by dirt particles and bubbles. The posterior part of trunk is missing. The head and the straight stylets are well accessible (Figure 9D,E). Each trunk appendage bears a prominent attachment structure distally (empodium; Figure 9F). The larva is 1.4 mm long.
- (9)
- Specimen 5831 (BUB 3962). The larva is preserved in Myanmar amber. It is well accessible in dorsal (Figure 10A,B) and ventral view (Figure 10C). The head and the straight stylets are well accessible (Figure 10D,E). Each trunk appendage bears a prominent attachment structure distally (empodium; Figure 10F). The larva is about 1.7 mm long.
- (10)
- Specimen 5832 (BUB 3963). The larva is preserved in Myanmar amber. It is accessible in ventral (Figure 11A,B) and dorsal view (Figure 11C). The head and the straight stylets are well accessible (Figure 11D,E). Each trunk appendage bears a prominent attachment structure distally (empodium; Figure 11F). The larva is 1 mm long.
- (11)
- Specimen 5833 (CJW F 3197). The larva is preserved in Myanmar amber. It is well accessible in lateral view (Figure 12A,B). The head and the straight stylets are well accessible in latero-dorsal view (Figure 12C). Each trunk appendage bears a prominent attachment structure distally (empodium; Figure 12D). The larva is about 4 mm long.
- (12)
- (13)
- (14)
- Specimen 5836 (CJW F 3336). The larva is preserved in Myanmar amber. It is accessible in dorsal view (Figure 13I,J), but the abdomen is flipped over. The stylets are straight. The larva is about 0.9 mm long.
- (15)
- Specimens 5837 + 5838 (PED 0772). The amber piece includes two specimens, which are preserved in Myanmar amber (Figure 14). Specimen 1 (5837; Figure 14A–E) is accessible in dorsal (Figure 14A,B) and ventral view (Figure 14C), but partly concealed by bubbles on both sides. The head and the straight stylets are accessible in dorsal view (Figure 14D,E). The larva is 1.1 mm long.
- (16)
- Specimen 5839 (PED 0828). The larva is preserved in Myanmar amber. It is well accessible in ventral (Figure 15A,B) and dorsal view (Figure 15C). The head and the straight stylets are well accessible (Figure 15D,E). Each trunk appendage bears a prominent attachment structure distally (empodium; Figure 15F). The larva is 2.1 mm long.
- (17)
- Specimens 5840–5846 (PED 0900). The amber piece includes seven specimens, which are preserved in Myanmar amber (Figure 16, Figure 17 and Figure 18). Specimen 1 (5840; Figure 16A–E) is well accessible in dorsal (Figure 16A,B) and ventral view (Figure 16C), but partly concealed. The head and the straight stylets are well accessible (Figure 16D,E). The larva is 1.3 mm long.Specimen 2 (5841; Figure 16F–I) is well accessible in dorsal (Figure 16F,G) and ventral view (Figure 16H). The stylets are straight. Each trunk appendage bears a prominent attachment structure distally (empodium; Figure 16I). The larva is 1.1 mm long.Specimen 3 (5842; Figure 17A–E) is well accessible in dorsal (Figure 17A,B) and ventral view (Figure 17C), but partly concealed by Verlumung. The head and the straight stylets are well accessible (Figure 17D,E). The larva is 1.2 mm long.Specimen 4 (5843; Figure 17F–I) is well accessible in dorsal view (Figure 17F,G), but the posterior part of the abdomen is missing. The head and the straight stylets are well accessible (Figure 17H,I). The larva is 0.8 mm long.
- (18)
- Specimens 5847 + 5848 (PED 0769). The amber piece includes two specimens, which are preserved in Myanmar amber (Figure 19). Specimen 1 (5847; Figure 19A–E) is well accessible in dorsal view (Figure 19A,B). The head and the straight stylets are well accessible (Figure 19C,D). Each trunk appendage bears a prominent attachment structure distally (empodium; Figure 19E). The larva is 1.3 mm long.
- (19)
- (20)
- Specimens 5850–5853 (PED 0791). The amber piece includes four specimens, which are preserved in Myanmar amber (Figure 21 and Figure 22). Specimen 1 (5850; Figure 21A–E) is well accessible in ventral (Figure 21A,B) and dorsal view (Figure 21C). Head and stylets are well accessible (Figure 21D,E), and the stylets are slightly curved inwards. The larva is 1.4 mm long.Specimen 2 (5851; Figure 21F–H) is well accessible in ventral (Figure 21F,G) and dorsal view (Figure 21H). The stylets are slightly curved inwards. The larva is 1 mm long.
- (21)
- Specimen 5854 (PED 0823). The larva is preserved in Myanmar amber. It is well accessible in ventral (Figure 23A,B) and dorsal view (Figure 23C), but partly concealed by dirt particles. Head and stylets are well accessible (Figure 23D,E), and the stylets are slightly curved inwards. Each trunk appendage bears a prominent attachment structure distally (empodium; Figure 23F). The larva is 1.4 mm long.
- (22)
- (23)
- Specimen 5856 (PED 0899). The larva is preserved in Myanmar amber. It is well accessible in dorsal (Figure 25A,B) and ventral view (Figure 25C), but partly concealed by bubbles. Head and stylets are well accessible (Figure 25D,E), and the stylets are slightly curved inwards. Each trunk appendage bears a prominent attachment structure distally (empodium; Figure 25F). The larva is 1.4 mm long.
- (24)
- Specimen 5857 (BUB 0049). The larva is preserved in Myanmar amber. It is well accessible in ventral view (Figure 26A,B), but small areas of the trunk are partly concealed by a bubble. Head and stylets are well accessible (Figure 26C,D), and the stylets are elongated and slightly curved outwards. Each trunk segment bears a pair of prominent setae on each side (Figure 26E). Each trunk appendage bears a prominent attachment structure distally (empodium, Figure 26F). The larva is 4 mm long.
- (25)
- Specimen 5858 (BUB 3368). The larva is preserved in Myanmar amber. It is well accessible in dorsal view (Figure 27A,B), but partly concealed by dirt particles. Head and stylets are well accessible (Figure 27C), and the stylets are elongated and slightly curved outwards. The larva is about 3.5 mm long.
- (26)
- Specimen 5859 (BUB 3737). The larva is preserved in Myanmar amber. It is accessible in dorsal (Figure 28A,B) and ventral view (Figure 28C), but partly concealed by dirt particles. Head and stylets are accessible (Figure 28D,E), and the stylets are elongated and slightly curved outwards. The larva is about 2.2 mm long.
- (27)
- (28)
- Specimen 5861 (PED 0627). The larva is preserved in Myanmar amber. It is accessible in dorsal (Figure 30A,B) and ventral view (Figure 30C), but partly concealed by dirt particles in dorsal view and strongly concealed by bubbles and dirt particles in ventral view. The stylets are elongate and slightly curved outwards. The larva is about 2.8 mm long.
- (29)
3.7. Results of the Shape Analysis
4. Discussion
4.1. Identity of the Fossils
4.2. Fossil Representatives of Osmylidae
4.3. Fossil Representatives of Mantispidae
4.4. Fossil Representatives of Berothidae and Dilaridae
4.5. The More Problematic Fossils
4.6. Diversity of Berothidae and Dilaridae over Time
4.7. Diversity of Osmylidae over Time
4.8. Diversity of Mantispidae over Time
4.9. Diversity of Lacewing Larvae over Time
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riek, E.F. Neuroptera (Lacewings). In The Insects of Australia; Waterhouse, D.F., Ed.; Melbourne University Press: Melbourne, Australia, 1970; pp. 472–494. [Google Scholar]
- Aspöck, U.; Aspöck, H. Kamelhälse, Schlammfliegen, Ameisenlöwen. Wer sind sie? (Insecta: Neuropterida: Raphidioptera, Megaloptera, Neuroptera). Stapfia 1999, 60, 1–34. [Google Scholar]
- Aspöck, U.; Aspöck, H. Verbliebene Vielfalt vergangener Blüte. Zur Evolution, Phylogenie und Biodiversität der Neuropterida (Insecta: Endopterygota). Denisia 2007, 20, 451–516. [Google Scholar]
- Haug, J.T. Why the term “larva” is ambiguous, or what makes a larva? Acta Zool. 2020, 101, 167–188. [Google Scholar]
- Zimmermann, D.; Randolf, S.; Aspöck, U. From chewing to sucking via phylogeny—From sucking to chewing via ontogeny: Mouthparts of Neuroptera. In Insect Mouthparts; Krenn, H.W., Ed.; Springer: Cham, Switzerland, 2019; pp. 361–385. [Google Scholar]
- Haug, J.T.; Baranov, V.; Schädel, M.; Müller, P.; Gröhn, P.; Haug, C. Challenges for understanding lacewings: How to deal with the incomplete data from extant and fossil larvae of Nevrorthidae? (Neuroptera). Fragm. Entomol. 2020, 52, 137–167. [Google Scholar]
- Haug, J.T.; Müller, P.; Haug, C. A 100-million-year old predator: A fossil neuropteran larva with unusually elongated mouthparts. Zool. Lett. 2019, 5, 29. [Google Scholar]
- Lestage, J.A. Megaloptera, Planipennia. In Les Larves et Nymphes Aquatiques des Insectes d’Europe: Morphologie, Biologie, Systématique; Rousseau, E., Lestage, J.A., Schouteden, H., Eds.; J. Lebègue: Bruxelles, Belgium, 1921; Volume 1, pp. 321–342. [Google Scholar]
- Canard, M.; Thierry, D.; Cloupeau, R.; Rausch, H.; Weißmair, W. A spongillafly new to the French fauna: Sisyra Bureschi Rausch & Weißmair, 2007 (Neuropterida, Sisyridae). Bull. Soc. Entomol. France 2015, 120, 19–24. [Google Scholar]
- Winterton, S.L.; Martins, C.C.; Makarkin, V.; Ardila-Camacho, A.; Wang, Y. Lance lacewings of the world (Neuroptera: Archeosmylidae, Osmylidae, Saucrosmylidae): Review of living and fossil genera. Zootaxa 2019, 4581, 1–99. [Google Scholar]
- Ohl, M. Aboard a spide—A complex developmental strategy fossilized in amber. Naturwissenschaften 2011, 98, 453–456. [Google Scholar]
- Haug, J.T.; Müller, P.; Haug, C. The ride of the parasite: A 100-million-year old mantis lacewing larva captured while mounting its spider host. Zool. Lett. 2018, 4, 31. [Google Scholar]
- Wedmann, S.; Makarkin, V.N.; Weiterschan, T.; Hörnschemeyer, T. First fossil larvae of Berothidae (Neuroptera) from Baltic amber, with notes on the biology and termitophily of the family. Zootaxa 2013, 3716, 236–258. [Google Scholar] [PubMed]
- Weißmair, W. Präimaginale Stadien, Biologie und Ethologie der europäischen Sisyridae (Neuropterida: Neuroptera). Stapfia 1999, 60, 101–128. [Google Scholar]
- Haug, G.T.; Haug, C.; Pazinato, P.G.; Braig, F.; Perrichot, V.; Gröhn, C.; Müller, P.; Haug, J.T. The decline of silky lacewings and morphological diversity of long-nosed antlion larvae through time. Palaeontol. Electron. 2020, 23, a39. [Google Scholar]
- Haug, G.T.; Haug, C.; van der Wal, S.; Müller, P.; Haug, J.T. Split-footed lacewings declined over time: Indications from the morphological diversity of their antlion-like larvae. PalZ 2021, 1–22, (early view). [Google Scholar] [CrossRef]
- Haug, G.T.; Baranov, V.; Wizen, G.; Pazinato, P.G.; Müller, P.; Haug, C.; Haug, J.T. The morphological diversity of long-necked lacewing larvae (Neuroptera: Myrmeleontiformia). Bull. Geosci. 2021, 96. [Google Scholar] [CrossRef]
- Haug, J.T.; Müller, P.; Haug, C. A 100-million-year old slim insectan predator with massive venom-injecting stylets—A new type of neuropteran larva from Burmese amber. Bull. Geosci. 2019, 94, 431–440. [Google Scholar]
- Haug, J.T.; Pazinato, P.G.; Haug, G.T.; Haug, C. Yet another unusual new type of lacewing larva preserved in 100-million-year old amber from Myanmar. RIPS 2020, 126, 821–832. [Google Scholar]
- Haug, G.T.; Haug, C.; Haug, J.T. The morphological diversity of spoon-winged lacewing larvae and the first possible fossils from 99 million-year-old Kachin amber, Myanmar. Palaeodiversity 2021, 14, 133–152. [Google Scholar]
- Pérez-de la Fuente, R.; Engel, M.S.; Delclòs, X.; Peñalver, E. Straight-jawed lacewing larvae (Neuroptera) from Lower Cretaceous Spanish amber, with an account on the known amber diversity of neuropterid immatures. Cretac. Res. 2020, 106, 104200. [Google Scholar]
- Peterson, A. Larvae of Insects. An Introduction to Nearctic Species. Part II. Coleoptera, Diptera, Neuroptera, Siphonaptera, Mecoptera, Trichoptera; Edward Brothers: Columbus, OH, USA, 1951; p. 416. [Google Scholar]
- MacLeod, E.G. A Comparative Morphological Study of the Head Capsule and Cervix of Larval Neuroptera (Insecta). Ph.D. Thesis, Harvard University, Cambridge, MA, USA, 1964; p. 528. [Google Scholar]
- Gepp, J. Erforschungsstand der Neuropteren-Larven der Erde (mit einem Schlüssel zur Larvaldiagnose der Familien, einer Übersicht von 340 beschriebenen Larven und 600 Literaturzitaten). In Proceedings of the 1st International Symposium on Neuropterology, Graz, Austria, 22–26 September 1980; Privately printed: Graz, Austria, 1984; pp. 183–239. [Google Scholar]
- New, T.R. Planipennia, Lacewings. Handbuch der Zoologie (Arthropoda: Insecta), Part 30; Walter de Gruyter: Berlin, Germany, 1989; Volume 4, p. 132. [Google Scholar]
- Monserrat, V.J.; Díaz Aranda, L.M.; Hölzel, H. Contribucion al conocimiento de los neuropteros de Marruecos (Insecta, Neuropteroidea). EOS Rev. Españ. Entomol. 1990, 66, 101–115. [Google Scholar]
- Stürzer, C.; Gepp, J. Beiträge zur Larvalbiologie und morphologie von Conwentzia pineticola (Enderlein, 1905) und C. psociformis (Curtis, 1834) (Neuroptera: Coniopterygidae). Entomol. Austrica 2004, 11, 7–10. [Google Scholar]
- Löw, F. Beitrag zur Kenntniss der Coniopterygiden. Sitzungsber. Akad. Wiss. Wien, Math. Naturwiss. Kl. 1885, 91, 73–89. [Google Scholar]
- Ward, L.K. Aleuropteryx juniperi Ohm (Neur. Coniopterygidae) new to Britain feeding on Carulaspis juniperi Bouche (Hem., Diaspididae). Entomol. Monthly Mag. 1970, 106, 74–78. [Google Scholar]
- Cruickshank, R.D.; Ko, K. Geology of an amber locality in the Hukawng Valley, Northern Myanmar. J. Asian Earth Sci. 2003, 21, 441–455. [Google Scholar]
- Shi, G.; Grimaldi, D.A.; Harlow, G.E.; Wang, J.; Wang, J.; Yang, M.; Lei, W.; Li, Q.; Li, X. Age constraint on Burmese amber based on U–Pb dating of zircons. Cretac. Res. 2012, 37, 155–163. [Google Scholar]
- Yu, T.; Kelly, R.; Mu, L.; Ross, A.; Kennedy, J.; Broly, P.; Xia, F.; Zhang, H.; Wang, B.; Dilcher, D. An ammonite trapped in Burmese amber. PNAS 2019, 116, 11345–11350. [Google Scholar]
- Haug, J.T.; Müller, C.H.G.; Sombke, A. A centipede nymph in Baltic amber and a new approach to document amber fossils. Org. Div. Evol. 2013, 13, 425–432. [Google Scholar]
- Iwata, H.; Ukai, Y. SHAPE: A computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. J. Hered. 2002, 93, 384–385. [Google Scholar]
- Braig, F.; Haug, J.T.; Schädel, M.; Haug, C. A new thylacocephalan crustacean from the Upper Jurassic lithographic limestones of southern Germany and the diversity of Thylacocephala. Palaeodiversity 2019, 12, 69–87. [Google Scholar]
- Tillyard, R.J. Studies in Australian Neuroptera. No. iv. The families Ithonidae, Hemerobiidae, Sisyridae, Berothidae, and the new family Trichomatidae; with a discussion of their characters and relationships, and descriptions of new and little-known genera and species. Proc. Linn. Soc. NSW 1916, 41, 269–332. [Google Scholar]
- Tillyard, R.J. Order Neuroptera (Alderflies, Lacewings). In The Insects of Australia and New Zealand, 1st ed.; Angus and Robertson: Sydney, Australia, 1926; pp. 308–325. [Google Scholar]
- Gurney, A.B. Notes on Dilaridae and Berothidae, with special reference to the immature stages of the Nearctic genera (Neuroptera). Psyche 1947, 54, 145–169. [Google Scholar]
- Tjeder, B. Chapter XV. Neuroptera-Planipennia. The Lace-wings of Southern Africa. 2. Family Berothidae. In South African Animal Life; Hanström, B., Brinck, P., Rudebec, G., Eds.; Swedish Natural Science Research Council: Stockholm, Sweden, 1959; Volume 6, pp. 256–314. [Google Scholar]
- Tauber, C.A. Neuroptera. In Immature Insects; Stehr, F.W., Ed.; Kendall–Hunt: Dubuque, IA, USA, 1987; Volume 2, pp. 126–143. [Google Scholar]
- Toschi, C.A. Observations on Lomamyia latipennis, with a description of the first instar larva. Pan Pac. Entomol. 1964, 40, 21–26. [Google Scholar]
- Tauber, C.A.; Tauber, M.J. Lomamyia latipennis (Neuroptera, Berothidae) life history and larval descriptions. Can. Entomol. 1968, 100, 623–629. [Google Scholar]
- New, T.R. Neuroptera (lacewings). In The Insects of Australia, 2nd ed.; Naumann, I.D., Ed.; Melbourne University Press: Melbourne, Australia, 1991; Volume 1, pp. 525–542. [Google Scholar]
- Tauber, C.A.; Tauber, M.J.; Albuquerque, G.S. Neuroptera (Lacewings, Antlions). In Encyclopedia of Insects; Resh, V., Cardé, R., Eds.; Academic Press: Burlington, VT, USA, 2003; pp. 785–798. [Google Scholar]
- Brushwein, J.R. Bionomics of Lomamyia hamata (Neuroptera: Berothidae). Ann. Entomol. Soc. Am. 1987, 80, 671–679. [Google Scholar]
- Minter, L.R. A comparison of the eggs and first-instar larvae of Mucroberotha vesicaria Tjeder with those of other species in the families Berothidae and Mantispidae (Insecta: Neuroptera). In Proceedings of the Third International Symposium on Neuropterology, Kruger National Park, South Africa, 3–4 February 1988; Mansell, M.W., Aspöck, H., Eds.; South African Department of Agricultural Development: Pretoria, South Africa, 1990; pp. 115–129. [Google Scholar]
- Möller, A. Aspects of the Larval Morphology and Biology of South African Podallea Species (Neuropterida: Neuroptera: Berothidae). Master′s Thesis, University of the North, Polokwane, South Africa, 2003; p. 123. [Google Scholar]
- Möller, A.; Minter, L.R.; Olivier, P.A.S. Larval morphology of Podallea vasseana Navás and Podallea manselli Aspöck & Aspöck from South Africa (Neuroptera: Berothidae). Afric. Entomol. 2006, 14, 1–12. [Google Scholar]
- Monserrat, V.J. Nuevos datos sobre algunas especies de la familia Berothidae (Insecta: Neuroptera). Heteropterus Rev. Entomol. 2006, 6, 173–207. [Google Scholar]
- Dobosz, R.; Górski, G. New data on Nyrma kervilea (Neuroptera: Berothidae), abstract book. In Proceedings of the Tenth International Symposium on Neuropterology, Piran, Slovenia, 22–25 June 2008; Devetak, D., Klenovšek, T., Eds.; Faculty of Natural Sciences and Mathematics: Maribor, Slovenia, 2008; p. 33. [Google Scholar]
- Heckman, C.W. Neuroptera (Including Megaloptera). Encyclopedia of South American Aquatic Insects. Illustrated Keys to the Known Families, Genera, and Species in South America; Springer: Heidelberg, NY, USA, 2017; p. 621. [Google Scholar]
- Grebennikov, V.V. Grub-like larvae of Neuroptera (Insecta): A morphological review of the families Ithonidae and Polystoechotidae and a description of Oliarces clara. Europ. J. Entomol. 2004, 101, 409–418. [Google Scholar]
- Brauer, F. Verwandlungsgeschichte der Mantispa pagana. Arch. Naturgesch. 1852, 18, 1–2. [Google Scholar]
- Stitz, H. Planipennia. In Biologie der Tiere Deutschlands. Lfg. 33, Teil 35; Schultze, P., Ed.; Borntraeger: Berlin, Germany, 1931; pp. 67–304. [Google Scholar]
- Jacobs, W. Biologie und Ökologie der Insekten: Ein Taschenlexikon/begr. von Jacobs, W. & Renner, M. 3. Auflage überarb. von Honomichl; K. Fischer: Stuttgart, Germany, 1998; p. 678. [Google Scholar]
- Aspöck, H. Beschreibungen und Abbildungen von Mantispiden in der frühen entomologischen Literature und Österreichs Beitrag zur Erforschung der Fanghafte (Neuropterida: Neuroptera: Mantispidae). Stapfia 1999, 60, 209–244. [Google Scholar]
- Brauer, F. Beiträge zur Kenntniss des inneren Baues und der Verwandlung der Neuropteren. Verh. Zool. Bot. Ver. Wien 1855, 5, 701–726. [Google Scholar]
- Brauer, F. Beschreibung der Verwandlungsgeschichte der Mantispa styriaca Poda und Betrachtungen über die sogenannte Hypermetamorphose Fabre′s. Verh. Kaiserl. Königl. Zool. Bot. Ges. Wien 1869, 19, 831–840. [Google Scholar]
- Poivre, C. Observations sur la biologie, le comportement et le phénomène de convergence chez les Mantispidés (Planipennes). Entomologiste 1976, 32, 2–19. [Google Scholar]
- Aspöck, H.; Aspöck, U. Synopsis der Systematik, Ökologie und Biogeographie der Neuropteren Mitteleuropas im Spiegel der Neuropteren-Fauna von Linz und Oberösterreich, sowie Bestimmungsschlüssel für die mitteleuropäischen Neuropteren und Beschreibung von Coniopteryx lentiae nov. spec. Naturkundl. Jahrb. Stadt Linz 1964, 1964, 127–282. [Google Scholar]
- Makarkin, V.N. Chapter 25 Neuroptera. Keys to the Insects of the Russian Far East, Part 1: Megaloptera, Raphidioptera, Neuroptera, Mecoptera, and Hymenoptera; Nauka: St. Petersburg, Russia, 1995; Volume 1, pp. 37–67. (in Russian) [Google Scholar]
- Nawa, Y. Larvae of mantispid. Insect World 1903, 7, 520. [Google Scholar]
- Handschin, E. Indo-australische Neuropteren und Mecopteren. Rev. Suisse Zool. 1935, 42, 683–714. [Google Scholar]
- Hungerford, H.B. The Mantispidae of the Douglas Lake, Michigan Region, with some biological observations (Neurop.). Entomol. News Phila. 1936, 47, 85–88. [Google Scholar]
- Merti, C. Contribucion al estudio de Mantispa decorata Erd. (Hemip. Cor.). Rev. Soc. Entomol. Argent. 1940, 10, 304–307. [Google Scholar]
- McKeown, K.C.; Mincham, V.H. The biology of an Australian mantispid (Mantispa vittata Guérin). Austral. Zool. 1948, 11, 207–224. [Google Scholar]
- Lucchese, E. Ricerche sulla Mantispa perla Pallas (Neuroptera Planipennia—Fam. Mantispidae). I Nota preventiva su nuovi reperti concernenti l′etologia della larva della 1a età. Ann. Facoltà Agrar. R. Univ. Studi Perugia 1955, 11, 242–262. [Google Scholar]
- Lucchese, E. Ricerche sulla Mantispa perla Pallas (Neuroptera Planipennia—Fam. Mantispidae). II Contributo su nuovi reperti biologici e morfologici concernenti l′adulto, la larva della Ia età e la completa evoluzione di questa nella sua sede definitiva. Ann. Facoltà Agrar. R. Univ. Studi Perugia 1956, 12, 83–213. [Google Scholar]
- Kuroko, H. On the eggs and first-instar larvae of two species of Mantispidae. Esakia Occas. Pap. Hikosan Biol. Lab. 1961, 3, 25–32. [Google Scholar]
- Ghilarov, M.S. Личинка Dilar turcicus Hag. и пoлoжение семейства Dilaridae в oтряде сетчатoкрылых (Planippenia) (The larva of Dilar turcicus Hag. and the position of the family Dilaridae in the order Planipennia). Entomol. Rev. Энтoмoлoгическoе Обoзрение Entomol. Obozrenie 1962, 41, 402–416. [Google Scholar]
- Dorohova, G.I. Chapter 25 Neuroptera. In Keys to the Insects of the European Part of the USSR (Part 6) Megaloptera, Raphidioptera, Neuroptera, Mecoptera and Trichoptera; Nauka: St. Petersburg, Russia, 1987; Volume 4, pp. 36–96. [Google Scholar]
- Engel, M.S.; Winterton, S.L.; Breitkreuz, L.C.V. Phylogeny and evolution of Neuropterida: Where have wings of lace taken us? Ann. Rev. Entomol. 2018, 63, 531–551. [Google Scholar]
- Parker, F.D.; Stange, L.A. Systematic and biological notes on the tribe Platymantispini (Neuroptera: Mantispidae) and the description of a new species of Plega from Mexico. Can. Entomol. 1965, 97, 604–612. [Google Scholar]
- Bissett, J.L.; Moran, V.C. The life history and cocoon spinning behaviour of a South African mantispid (Neuroptera: Mantispidae). J. Entomol. Soc. S. Afr. 1967, 30, 82–95. [Google Scholar]
- Davidson, J.A. Rearing Mantispa viridis Walker in the laboratory (Neuroptera, Mantispidae). Entomol. News Phila. 1969, 80, 29–31. [Google Scholar]
- Anonymous. A parasite and a spider. Nas. Mus. Nuus/Nat. Mus. News 1975, 1. [Google Scholar]
- Redborg, K.E. Interference by the mantispid Mantispa uhleri with the development of the spider Lycosa rabida. Ecol. Entomol. 1982, 7, 187–196. [Google Scholar]
- Redborg, K.E.; MacLeod, E.G. The developmental ecology of Mantispa uhleri Banks (Neuroptera: Mantispidae). Illin. Biol. Monogr. 1985, 53, 1–130. [Google Scholar]
- Monserrat, V.J.; Díaz-Aranda, L.M. Estadios larvarios de los Neuropteros Ibericos. V: Mantispa styriaca (Poda, 1761) (Planipennia: Mantispidae). Neuropt. Internat. 1989, 5, 189–204. [Google Scholar]
- Kral, K. Fine structure of the larval eyes of Mantispa sp. (Neuroptera: Planipennia, Mantispidae). Intern. J. Insect Morph. Embryol. 1989, 18, 135–143. [Google Scholar]
- Hoffman, K.M.; Brushwein, J.R. Descriptions of the larvae and pupae of some North American Mantispinae (Neuroptera: Mantispidae) and development of a system of larval chaetotaxy for Neuroptera. Trans. Amer. Entomol. Soc. 1992, 118, 159–196. [Google Scholar]
- Hirata, S.-I.; Ishii, M.; Nishikawa, Y. First instar larvae of mantispids, Mantispa japonica MacLachlan and Eumantispa harmandi (Navás) (Neuroptera: Mantispidae), associating with spiders (Araneae). Jap. J. Entomol. 1995, 63, 673–680. [Google Scholar]
- Wunderlich, J. “Frozen behaviour” in “Vampires” of Spiders—Fossil insect larvae of the family Mantispidae (Neuroptera) as parasites of Sac Spiders (Araneae: Clubionidae) in Eocene Baltic amber. Beitr. Araneol. 2012, 7, 150–156. [Google Scholar]
- Monserrat, V.J. Los mantíspidos de la Península Ibérica y Baleares (Insecta, Neuropterida, Neuroptera, Mantispidae). Graellsia 2014, 70, e012. [Google Scholar]
- Nagler, C.; Haug, J.T. From fossil parasitoids to vectors: Insects as parasites and hosts. In Fossil Parasites; De Baets, K., Littlewood, T., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2015; pp. 137–200. [Google Scholar]
- Kral, K.; Devetak, D. Chapter 14: Neuroptera. In An Introduction to the Wildlife of Cyprus, 1st ed.; Sparrow, D.J., John, E., Eds.; Terra Cypria: Limassol, Cyprus, 2016; pp. 242–267. [Google Scholar]
- Dorey, J.B.; Merritt, D.J. First observations on the life cycle and mass eclosion events in a mantis fly (Family Mantispidae) in the subfamily Drepanicinae. Biodiv. Data J. 2017, 5, e21206. [Google Scholar]
- Jandausch, K.; Pohl, H.; Aspöck, U.; Winterton, S.L.; Beutel, R.G. Morphology of the primary larva of Mantispa aphavexelte Aspöck & Aspöck, 1994 (Neuroptera: Mantispidae) and phylogenetic implications to the order of Neuroptera. Arthrop. Syst. Phyl. 2018, 76, 529–560. [Google Scholar]
- Redborg, K.E. Mantispidae (Insecta: Neuroptera) parasitic on spider egg sacs: An update of a pioneering paper by B. J. Kaston. J. Arachnol. 1982, 10, 92–93. [Google Scholar]
- Jandausch, K.; Beutel, R.G.; Pohl, H.; Gorb, S.N.; Büsse, S. The legs of “spider associated” parasitic primary larvae of Mantispa aphavexelte (Mantispidae, Neuroptera)—Attachment devices and phylogenetic implications. Arthrop. Struct. Dev. 2018, 47, 449–456. [Google Scholar]
- Badano, D.; Fratini, M.; Maugeri, L.; Palermo, F.; Pieroni, N.; Cedola, A.; Haug, J.T.; Weiterschan, T.; Velten, J.; Mei, M.; et al. X-ray microtomography and phylogenomics provide insights into the morphology and evolution of an enigmatic Mesozoic insect larva. Syst. Entomol. 2021, 46, 672–684. [Google Scholar]
- Takahashi, R. Uncommon neuropterous larva. Zool. Mag 1942, 54, 439–441. [Google Scholar]
- Monserrat, V.J. Revisión de los diláridos ibéricos (Neuropteroidea, Planipennia: Dilaridae). EOS Rev. Españ. Entomol. 1988, 64, 175–205. [Google Scholar]
- Makarkin, V.N.; Tshistjakov, Y.A. Dilaridy (Neuroptera: Dilaridae): Maloizvestnye “privlekatel’nye” setchatokrylye (The Dilaridae (Neuroptera: Dilaridae): Poorly known “pleasing” lacewings). Eversmannia Entomol. Investig. Russia Adj. Reg. 2009, 19, 36–47. [Google Scholar]
- Minter, L.R. The egg and larval stages of Nallachius krooni Minter (Insecta: Neuroptera: Dilaridae). In Current Research in Neuropterology, Proceedings of the Fourth International Symposium on Neuropterology, Bagnères-de-Luchon, France, 24–27 June 1991; Canard, M., Aspöck, H., Mansell, M.W., Eds.; Privately printed: Toulouse, France, 1992; pp. 261–269. [Google Scholar]
- Monserrat, V.J. Nuevos datos sobre algunas pequeñas familias de neurópteros (Insecta: Neuroptera: Nevrorthidae, Osmylidae, Sisyridae, Dilaridae). Heteropterus Rev. Entomol. 2005, 5, 1–26. [Google Scholar]
- Liu, X.-Y.; Aspöck, H.; Winterton, S.L.; Zhang, W.-W.; Aspöck, U. Phylogeny of pleasing lacewings (Neuroptera: Dilaridae) with a revised generic classification and description of a new subfamily. Syst. Entomol. 2017, 42, 448–471. [Google Scholar]
- Badano, D.; Di Giulio, A.; Aspöck, H.; Aspöck, U.; Cerretti, P. Burrowing specializations in a lacewing larva (Neuroptera: Dilaridae). Zool. Anz. 2021, 293, 247–256. [Google Scholar]
- Brauer, F. Über die Verwandlung des Osmylus maculatus. Ber. Mittheil. Freund. Naturwiss. Wien 1851, 7, 153–156. [Google Scholar]
- Aspöck, H. Osmylidae: Illustrations in the early entomological literature and the discovery of early stages and clarification of the biology (Neuropterida: Neuroptera). Act. Zool. Acad. Sci. Hung. 2002, 48 (Suppl. 2), 15–34. [Google Scholar]
- Hagen, H.A. Die Entwicklung und der innere Bau von Osmylus. Linn. Entomol. 1852, 7, 368–418. [Google Scholar]
- Hudson, G.V. New Zealand Neuroptera. A Popular Introduction to the Life-Histories and Habits of May-Flies, Dragon-Flies, Caddis-Flies and Allied Insects Inhabiting New Zealand, Including Notes on Their Relation to Angling; West, Newman and Co.: London, UK, 1904; p. 102. [Google Scholar]
- Froggatt, W.W. Australian Insects; W. Brooks and Co.: Sydney, Australia, 1907; pp. 1–449. [Google Scholar]
- Gallard, L. Porismus strigatus. Austral. Nat. 1914, 3, 26. [Google Scholar]
- Ussing, A.H. Osmylus chrysops L. (Vandmyrelöven) (Contributions to the biology of Osmylus chrysops). Flora Fauna 1914, 81–86. [Google Scholar]
- Lestage, J.A. La ponte et la larvule de l′Osmylus chrysops L. (Planipenne). Ann. Biol. Lacustre 1920, 10, 226–230. [Google Scholar]
- Stitz, H. 17–20. Ordnung: Netzflügler, Neuroptera. In Die Tierwelt Mitteleuropas. Band VI (6) (Insekten), Teil 3, Lieferung 1 (Neuroptera, Trichoptera); Brohmer, P., Ehrmann, P., Ulmer, U., Eds.; Quelle and Meyer: Leipzig, Germany, 1927; pp. 1–24. [Google Scholar]
- Withycombe, C.L. Notes on the biology of some British Neuroptera (Planipennia). Trans. R. Entomol. Soc. Lond. 1922, 70, 501–594. [Google Scholar]
- Imms, A.D. A General Textbook of Entomology; Methuen & Co.: London, UK, 1923; p. 736. [Google Scholar]
- Richards, O.W.; Davies, R.G. Imms′ General Textbook of Entomology, 10th ed.; Chapman & Hall: London, UK, 1977; p. 394. [Google Scholar]
- Withycombe, C.L. XV. Some Aspects of the Biology and Morphology of the Neuroptera. With special reference to the immature stages and their possible phylogenetic significance. Trans. R. Entomol. Soc. Lond. 1925, 72, 303–411. [Google Scholar]
- Rabaud, E. Étude biologique des larves de quelques Planipennes. Bull. Biol. Fr. Belg. 1927, 61, 433–499. [Google Scholar]
- Killington, F.J. Notes on the larva of Osmylus fulvicephalus Scop. (Neur.). J. Entomol. Soc. South Engl. 1932, 1, 10. [Google Scholar]
- David, K. Beiträge zur Anatomie und Lebensgeschichte von Osmylus chrysops L. Z. Morph. Ökol. Tiere 1936, 31, 151–206. [Google Scholar]
- Killington, F.J. A Monograph of the British Neuroptera; Ray Society: London, UK, 1936; Volume 1, p. 269. [Google Scholar]
- Parfin, S.I.; Gurney, A.B. The spongilla-flies, with special reference to those of the western hemisphere (Sisyridae, Neuroptera). Proc. U. S. Nat. Mus. 1956, 105, 421–529. [Google Scholar]
- Kimmins, D.E. Keys to the British species of aquatic Megaloptera and Neuroptera with ecological notes. Freshw. Biol. Assoc. Sci. Pub. 1962, 8, 1–23. [Google Scholar]
- Genay, A. Contribution à l′étude des Névroptères de Bourgogne. Trav. Laborat. Zool. Stat. Aquic. Grimaldi Facul. Sci. Dijon. N.S. 1953, 3, 1–30. [Google Scholar]
- Kawashima, K. Bionomics and earlier stages of some Japanese Neuroptera (I) Spilosmylus flavicornis (MacLachlan) (Osmylidae). Mushi 1957, 30, 67–70. [Google Scholar]
- Fraser, F.C. Mecoptera, Megaloptera and Neuroptera. Handb. Ident. Brit. Insects 1959, 1, 1–40. [Google Scholar]
- Wundt, H. Der Kopf der Larve von Osmylus chrysops L. (Neuroptera, Planipennia). Zool. Jahrb. Abt. Anat. Ontog. 1961, 79, 557–662. [Google Scholar]
- Ward, P.H. A contribution to the knowledge of the biology of Osmylus fulvicephalus (Scopoli 1763) (Neuroptera, Osmylidae). Entomol. Gaz. 1965, 16, 175–182. [Google Scholar]
- New, T.R. The lacewings (Insecta, Neuroptera) of Tasmania. Pap. Proc. R. Soc. Tasman. 1992, 126, 29–45. [Google Scholar]
- New, T.R. Insecta: Neuropterida. In Freshwater Invertebrates of the Malaysian Region; Yule, C.M., Sen, Y.H., Eds.; Akademi Sains Malaysia and Monash University: Kuala Lumpur, Malaysia, 2004; pp. 491–500. [Google Scholar]
- New, T.R. The egg and first instar larva of Stenosmylus (Neuroptera: Osmylidae). Austral. Entomol. Mag. 1974, 2, 24–27. [Google Scholar]
- Aspöck, U. Osmylus fulvicephalus (Scopoli, 1763): Bilanz einer Karriere als Insekt des Jahres 2003. Beitr. Entomofaun. 2003, 4, 129–131. [Google Scholar]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: Cambridge, UK, 2005; p. 755. [Google Scholar]
- Gepp, J. Neuropteren als Indikatoren der Naturraumbewertung. Eignung als Modellgruppe, Methodenwahl, Fallbeispiele sowie Diskussion möglicher Fragestellungen (Neuropterida). Stapfia 1999, 60, 167–208. [Google Scholar]
- Gepp, J. Der Bachhaft Osmylus fulvicephalus—240 Jahre nach seiner Beschreibung durch Johannes Antonius Scopoli—Osterreichs Insekt des Jahres (Osmylidae, Neruoptera). Carinth. II Mitt. Naturhist. Landesmus. Kärnten 2003, 193, 325–334. [Google Scholar]
- Cover, M.R.; Bogan, M.T. Chapter 41: Minor insect orders. In Thorp and Covich’s Freshwater Invertebrates; Thorp, J., Rogers, D.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1059–1072. [Google Scholar]
- Matsuno, S.; Yoshitomi, H. Descriptions of three larvae of Osmylus species from Japan (Neuroptera: Osmylidae), with a proposed naming system for the larval sclerites. Zootaxa 2016, 4189, 348–366. [Google Scholar]
- Glime, J.M. Aquatic Insects: Holometabola—Neuroptera and Megaloptera, Chapt. 11-8. In Bryophyte 11–81 Ecology. Bryological Interaction (ebook); Glime, J.M., Ed.; Michigan Technological University: Houghton, MI, USA; The International Association of Bryologists: Seattle, WA, USA, 2017; Volume 2, Available online: http://digitalcommons.mtu.edu/bryophyte-ecology2/ (accessed on 21 September 2021).
- Glime, J.M. Terrestrial Insects: Holometabola—Megaloptera and Neuroptera. Chapt. 12-8. In Bryophyte Ecology. Bryological Interaction; Glime, J.M., Ed.; Michigan Technological University: Houghton, MI, USA; The International Association of Bryologists: Seattle, WA, USA, 2017; Volume 2, Available online: http://digitalcommons.mtu.edu/bryophyte-ecology2/ (accessed on 21 September 2021).
- Matsuno, S. A non-destructive method for observation of body-surface fine structure of ethanol-preserved insect larvae. Jap. J. Envir. Entomol. Zool. 2017, 28, 1–4. [Google Scholar]
- Winterton, S.L.; Zhao, J.; Garzón-Orduna, I.J.; Wang, Y.; Liu, Z. The phylogeny of lance lacewings (Neuroptera: Osmylidae). Syst. Entomol. 2017, 42, 555–574. [Google Scholar]
- Martins, C.C.; Ardila-Camacho, A.; Courtney, G.W. Neotropical Osmylidae larvae (Insecta, Neuroptera): Description of habitats and morphology. Aquat. Insects 2018, 39, 181–207. [Google Scholar]
- Whalley, P.E.S. Neuroptera (Insecta) in amber from the Lower Cretaceous of Lebanon. Bull. Brit. Mus. Nat. Hist. Geol. 1980, 33, 157–164. [Google Scholar]
- Grimaldi, D.A.; Engel, M.S.; Nascimbene, P.C. Fossiliferous Cretaceous amber from Myanmar (Burma): Its rediscovery, biotic diversity, and paleontological significance. Amer. Mus. Nov. 2002, 3361, 1–71. [Google Scholar]
- Engel, M.S.; Grimaldi, D.A. Diverse Neuropterida in Cretaceous amber, with particular reference to the paleofauna of Myanmar (Insecta). Nov. Suppl. Entomol. Keltern 2008, 20, 1–86. [Google Scholar]
- Janzen, J.W. Arthropods in Baltic Amber; Ampyx Verlag: Halle, Germany, 2002; p. 167. [Google Scholar]
- Wunderlich, J. Fossil spiders in amber and copal. Beitr. Araneol. 2004, 3, 1–1908. [Google Scholar]
- Wichard, W.; Gröhn, C.; Seredszus, F. Aquatic Insects in Baltic Amber; Remagen: Kessel, The Netherlands, 2009; p. 336. [Google Scholar]
- Xia, F.; Yang, G.; Zhang, Q.; Shi, G.; Wang, B. Amber: Life through Time and Space; Science Press: Beijing, China, 2015; pp. 1–197. [Google Scholar]
- Engel, M.S. Two new genera of Cretaceous dustywings in amber from northern Myanmar (Neuroptera: Coniopterygidae). Nov. Paleoentomol. 2016, 17, 1–16. [Google Scholar]
- Zhang, W.W. Frozen Dimensions. The Fossil Insects and Other Invertebrates in Amber; Chongqing University Press: Chongqing, China, 2017; pp. 1–692. [Google Scholar]
- Haug, J.T.; Baranov, V.; Müller, P.; Haug, C. New extreme morphologies as exemplified by 100 million-year-old lacewing larvae. Sci. Rep. (accepted).
- Badano, D.; Engel, M.S.; Basso, A.; Wang, B.; Cerretti, P. Diverse Cretaceous larvae reveal the evolutionary and behavioural history of antlions and lacewings. Nat. Comm. 2018, 9, 3257. [Google Scholar]
- Jandausch, K.; Beutel, R.G.; Bellstedt, R. The larval morphology of the spongefly Sisyra nigra (Retzius, 1783) (Neuroptera: Sisyridae). J. Morph. 2019, 280, 1742–1758. [Google Scholar]
- Winterton, S.L.; Lemmon, A.R.; Gillung, J.P.; Garzon, I.J.; Badano, D.; Bakkes, D.K.; Breitkreuz, L.C.V.; Engel, M.S.; Moriarity Lemmon, E.; Liu, X.-Y.; et al. Evolution of lacewings and allied orders using anchored phylogenomics (Neuroptera, Megaloptera, Raphidioptera). Syst. Entomol. 2018, 43, 330–354. [Google Scholar]
- Miller, G.L.; Lambdin, P.L. Redescriptions of the larval stages of Hemerobius stigma Stephens (Neuroptera: Hemerobiidae). Fla. Entomol. 1984, 67, 377–382. [Google Scholar]
- Krakauer, A.H.; Tauber, C.A. Larvae of Micromus: Generic characteristics and a description of Micromus subanticus (Neuroptera: Hemerobiidae). Ann. Entomol. Soc. Am. 1996, 89, 203–211. [Google Scholar]
- Tauber, C.A.; Krakauer, A.H. Larval characteristics and generic placement of endemic Hawaiian hemerobiids (Neuroptera). Pac. Sci. 1997, 51, 413–423. [Google Scholar]
- Monserrat, V.J. Los hemeróbidos de la Península Ibérica y Baleares (Insecta, Neuropterida, Neuroptera: Hemerobiidae). Graellsia 2015, 71, e026. [Google Scholar]
- Pérez-de la Fuente, R.; Penalver, E.; Engel, M.S. Beaded lacewings (Neuroptera: Berothidae) in amber from the Lower Cretaceous of Spain. Cretac. Res. 2021, 119, 104705. [Google Scholar]
- Liu, X.; Zhang, W.; Winterton, S.L.; Breitkreuz, L.C.; Engel, M.S. Early morphological specialization for insect-spider associations in Mesozoic lacewings. Curr. Biol. 2016, 26, 1590–1594. [Google Scholar]
- Witmer, L.M. The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In Functional Morphology in Vertebrate Paleontology; Thomason, J.J., Ed.; Cambridge University Press: Cambridge, UK, 1995; pp. 19–33. [Google Scholar]
- Makarkin, V.N. New taxa of unusual Dilaridae (Neuroptera) with siphonate mouthparts from the mid-Cretaceous Burmese amber. Cretac. Res. 2017, 74, 11–22. [Google Scholar]
- Girard, V.; Schmidt, A.R.; Saint Martin, S.; Struwe, S.; Perrichot, V.; Saint Martin, J.P.; Grosheny, D.; Breton, G.; Néraudeau, D. Evidence for marine microfossils from amber. PNAS 2008, 105, 17426–17429. [Google Scholar]
- Xing, L.; Sames, B.; McKellar, R.C.; Xi, D.; Bai, M.; Wan, X. A gigantic marine ostracod (Crustacea: Myodocopa) trapped in mid-Cretaceous Burmese amber. Sci. Rep. 2018, 8, 1365. [Google Scholar]
- Schädel, M.; Perrichot, V.; Haug, J.T. Exceptionally preserved cryptoniscium larvae—Morphological details of rare isopod crustaceans from French Cretaceous Vendean amber. Palaeontol. Electron. 2019, 22, 71. [Google Scholar]
- Bolotov, I.N.; Aksenova, O.V.; Vikhrev, I.V.; Konopleva, E.S.; Chapurina, Y.E.; Kondakov, A.V. A new fossil piddock (Bivalvia: Pholadidae) may indicate estuarine to freshwater environments near Cretaceous amber-producing forests in Myanmar. Sci. Rep. 2021, 11, 6646. [Google Scholar] [PubMed]
- Herrera-Flórez, A.F.; Braig, F.; Haug, C.; Neumann, C.; Wunderlich, J.; Hörnig, M.K.; Haug, J.T. Identifying the oldest larva of a myrmeleontiformian lacewing—A morphometric approach. Acta Pal. Pol. 2020, 65, 235–250. [Google Scholar]
- Poinar, G., Jr.; Buckley, R. Doratomantispa burmanica n. gen., n. sp. (Neuroptera: Mantispidae), a new genus of mantidflies in Burmese amber. Hist. Biol. 2011, 23, 169–176. [Google Scholar]
- Pérez-de la Fuente, R.; Peñalver, E. A mantidfly in Cretaceous Spanish amber provides insights into the evolution of integumentary specialisations on the raptorial foreleg. Sci. Rep. 2019, 9, 13248. [Google Scholar]
- Lu, X.; Wang, B.; Zhang, W.; Ohl, M.; Engel, M.S.; Liu, X. Cretaceous diversity and disparity in a lacewing lineage of predators (Neuroptera: Mantispidae). Proc. R. Soc. B 2020, 287, 20200629. [Google Scholar]
- Shi, C.; Yang, Q.; Winterton, S.L.; Pang, H.; Ren, D. Stem-group fossils of Symphrasinae shed light on early evolution of Mantispidae (Insecta, Neuroptera). Pap. Palaeontol. 2020, 6, 143–154. [Google Scholar]
- Shi, C.; Yang, Q.; Shih, C.; Labandeira, C.C.; Pang, H.; Ren, D. Cretaceous mantid lacewings with specialized raptorial forelegs illuminate modification of prey capture (Insecta: Neuroptera). Zool. J. Linn. Soc. 2020, 190, 1054–1070. [Google Scholar]
- Pérez-de la Fuente, R.; Delclòs, X.; Peñalver, E.; Speranza, M.; Wierzchos, J.; Ascaso, C.; Engel, M.S. Early evolution and ecology of camouflage in insects. Proc. Natl. Acad. Sci. USA 2012, 109, 21414–21419. [Google Scholar]
- Pérez-de la Fuente, R.; Delclòs, X.; Peñalver, E.; Engel, M.S. A defensive behavior and plant–insect interaction in Early Cretaceous amber—The case of the immature lacewing Hallucinochrysa diogenesi. Arthrop. Struct. Dev. 2016, 45, 133–139. [Google Scholar]
- Pérez-de la Fuente, R.; Penñalver, E.; Azar, D.; Engel, M.S. A soil-carrying lacewing larva in Early Cretaceous Lebanese amber. Sci. Rep. 2018, 8, 16663. [Google Scholar] [PubMed]
- Wang, B.; Xia, F.; Engel, M.S.; Perrichot, V.; Shi, G.; Zhang, H.; Chen, J.; Jarzembowski, E.A.; Wappler, T.; Rust, J. Debris-carrying camouflage among diverse lineages of Cretaceous insects. Sci. Adv. 2016, 2, e1501918. [Google Scholar]
- Liu, X.; Shi, G.; Xia, F.; Lu, X.; Wang, B.; Engel, M.S. Liverwort mimesis in a Cretaceous lacewing larva. Curr. Biol. 2018, 28, 1475–1481. [Google Scholar] [PubMed] [Green Version]
- Haug, C.; Herrera-Flórez, A.F.; Müller, P.; Haug, J.T. Cretaceous chimera–An unusual 100-million-year old neuropteran larva from the “experimental phase” of insect evolution. Palaeodiversity 2019, 12, 1–11. [Google Scholar]
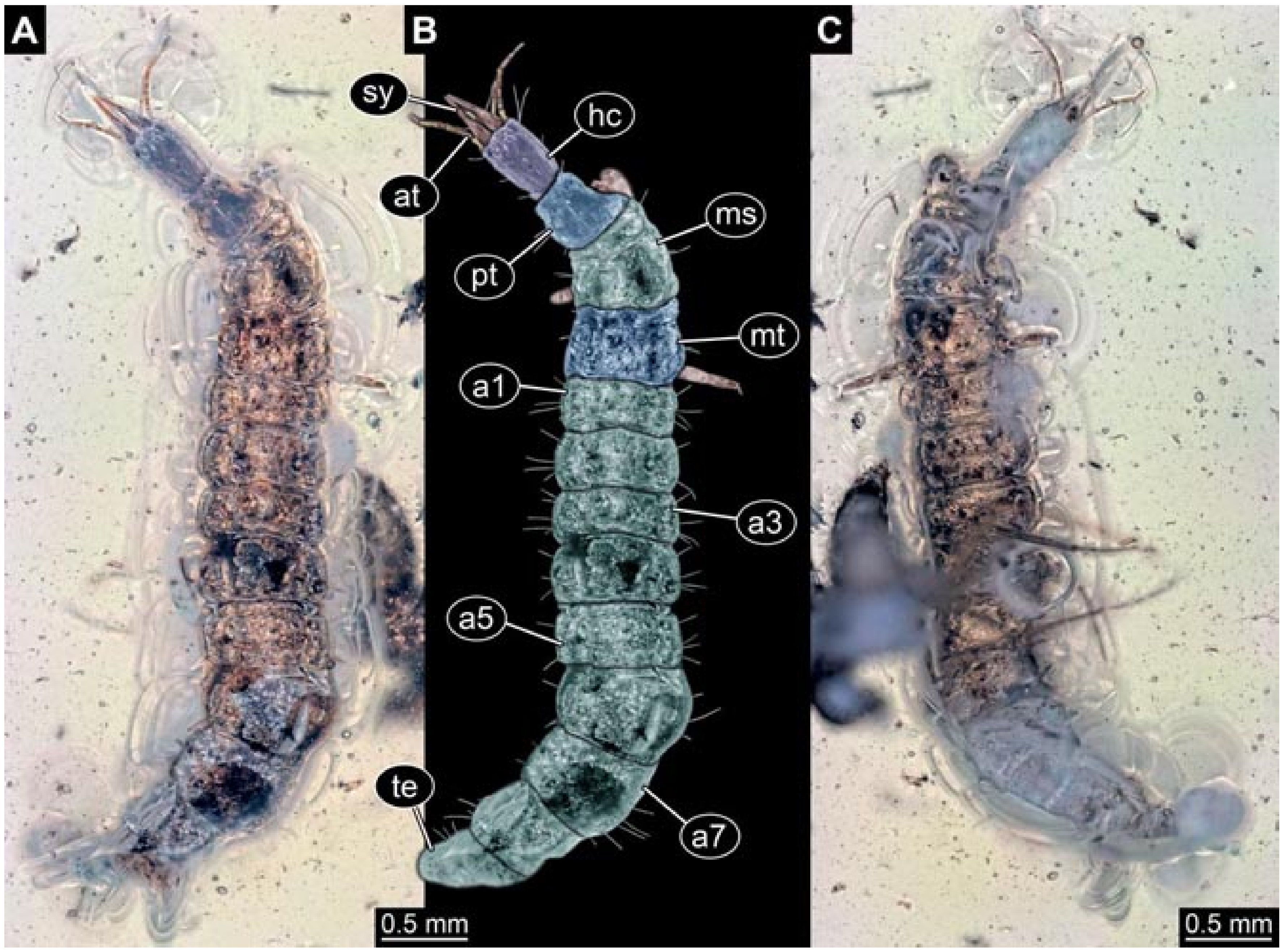


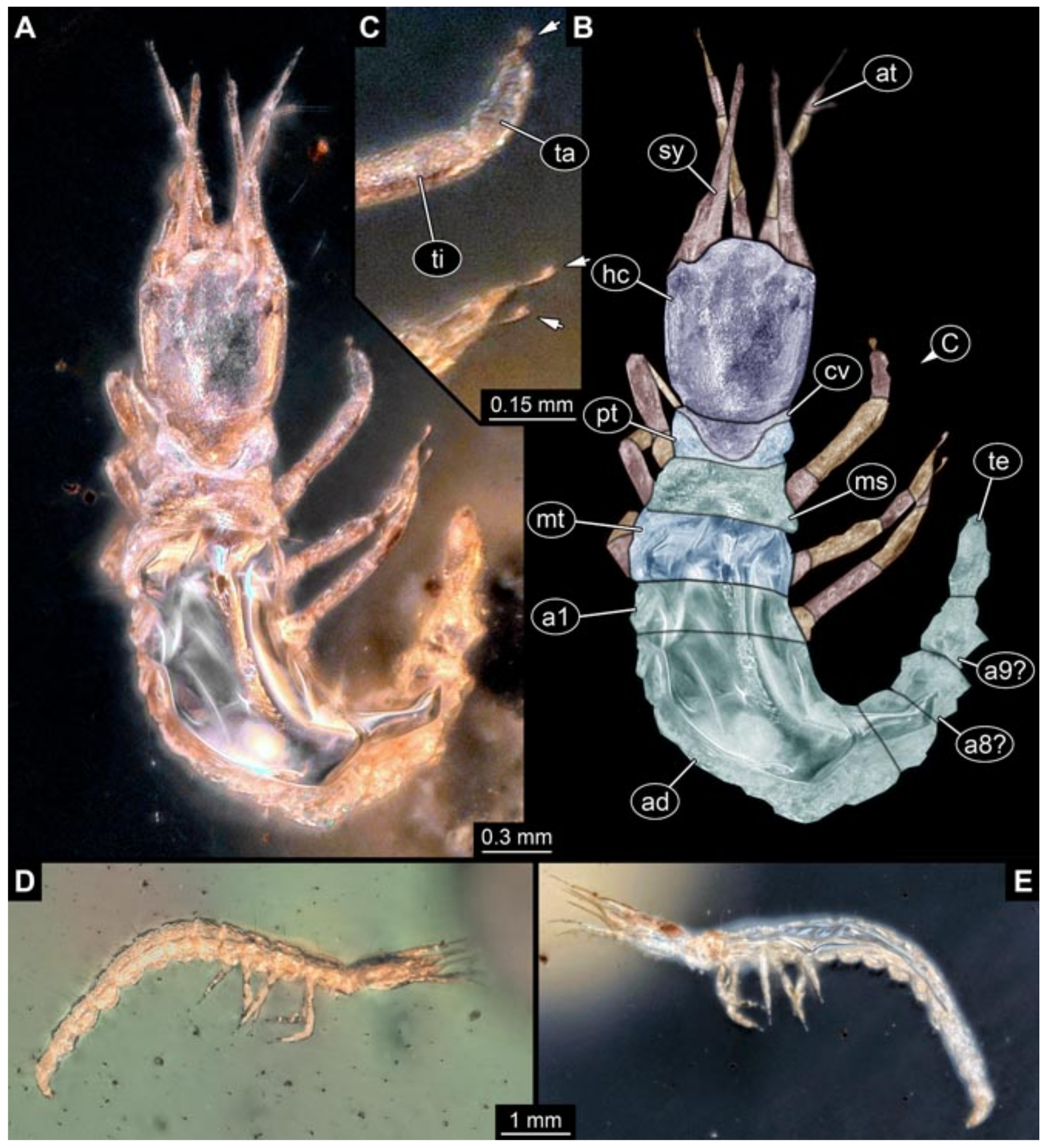
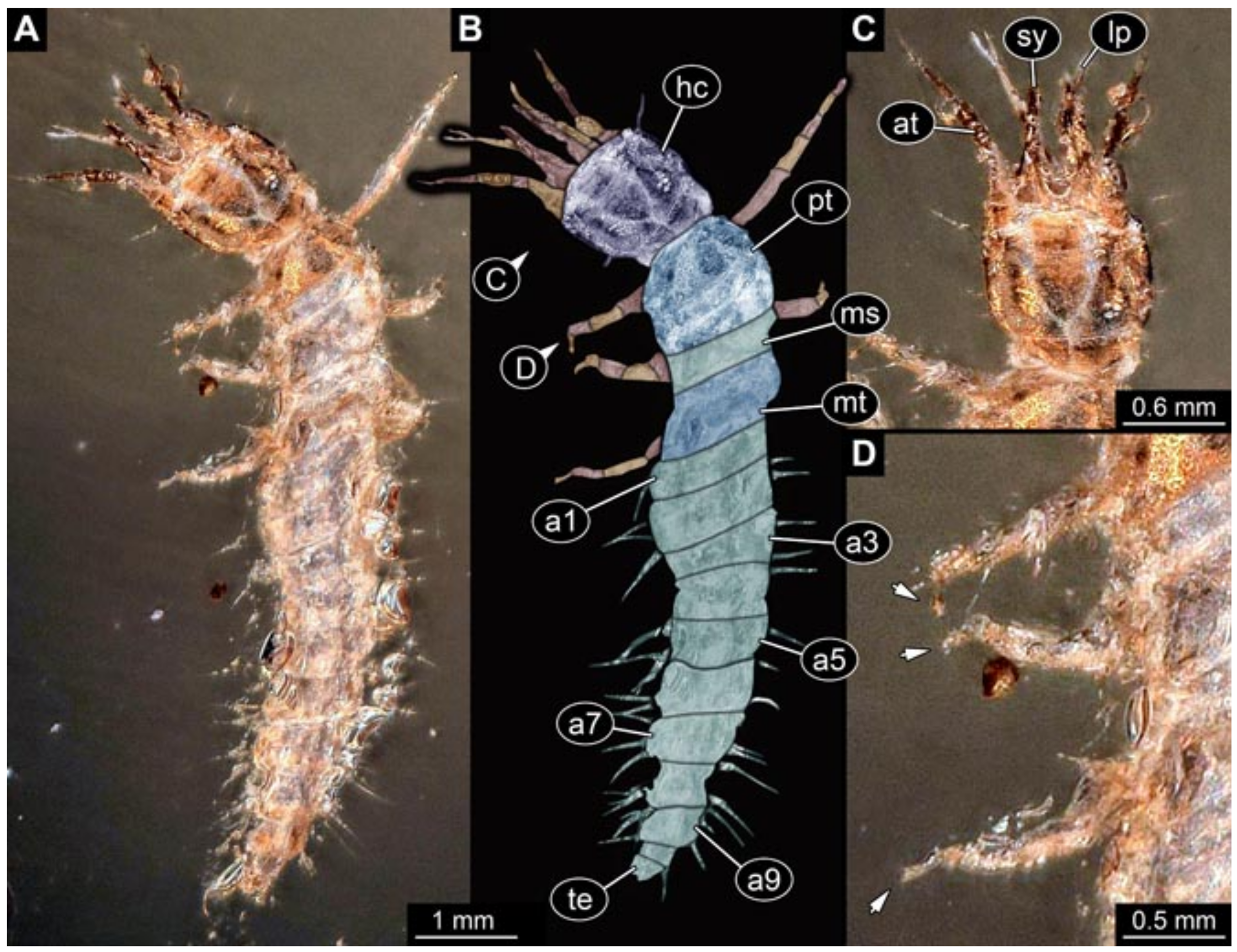

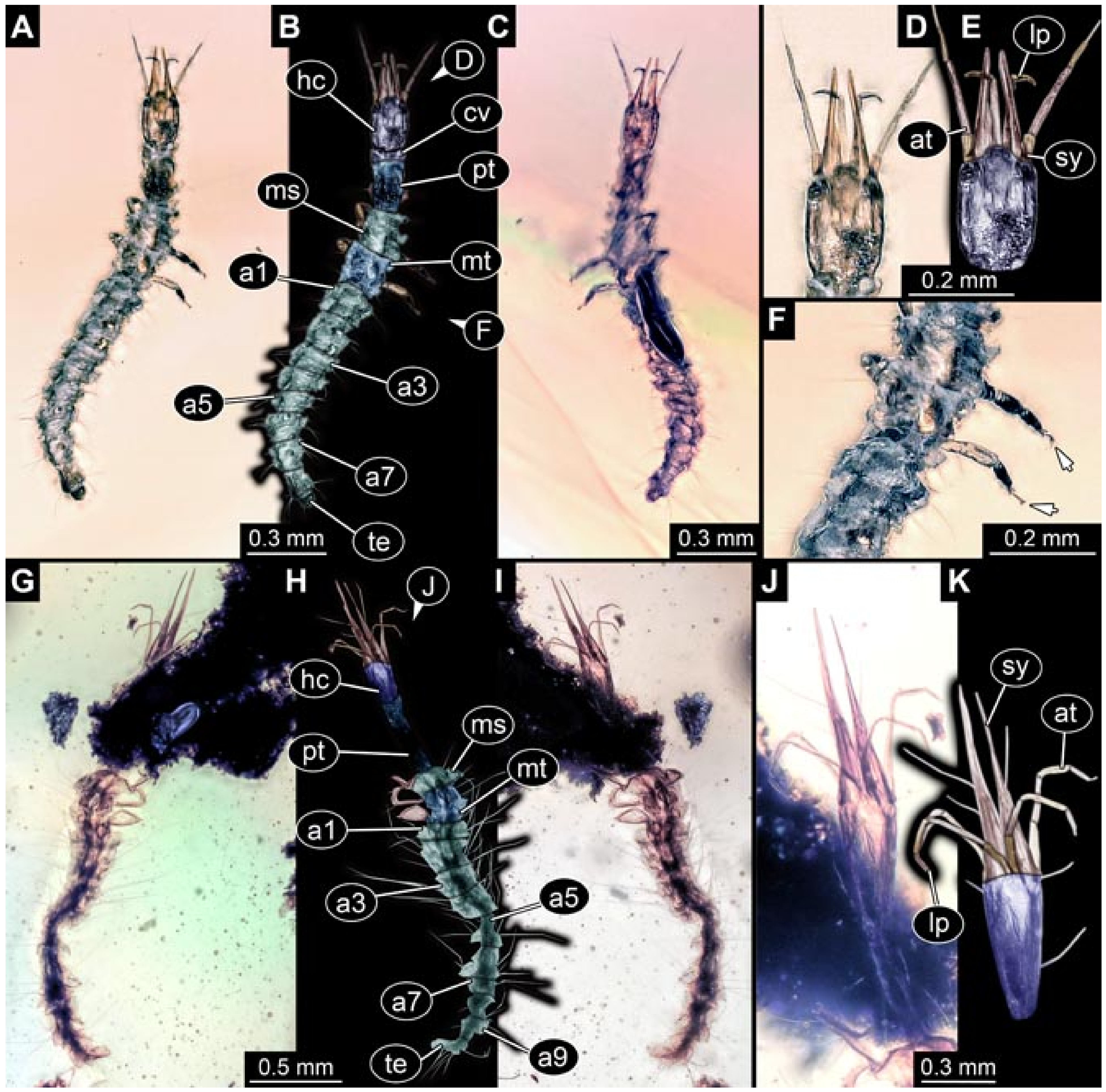

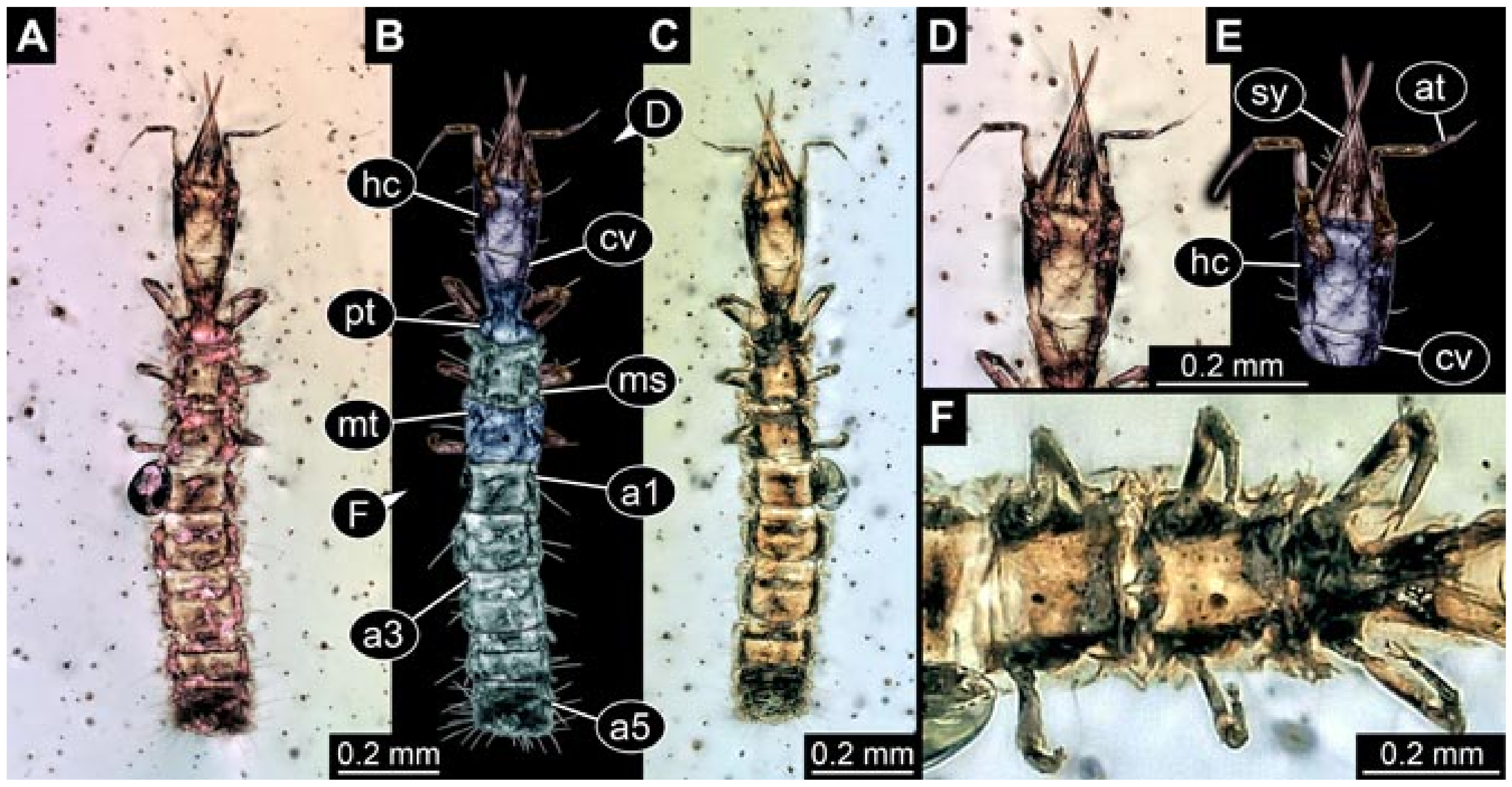
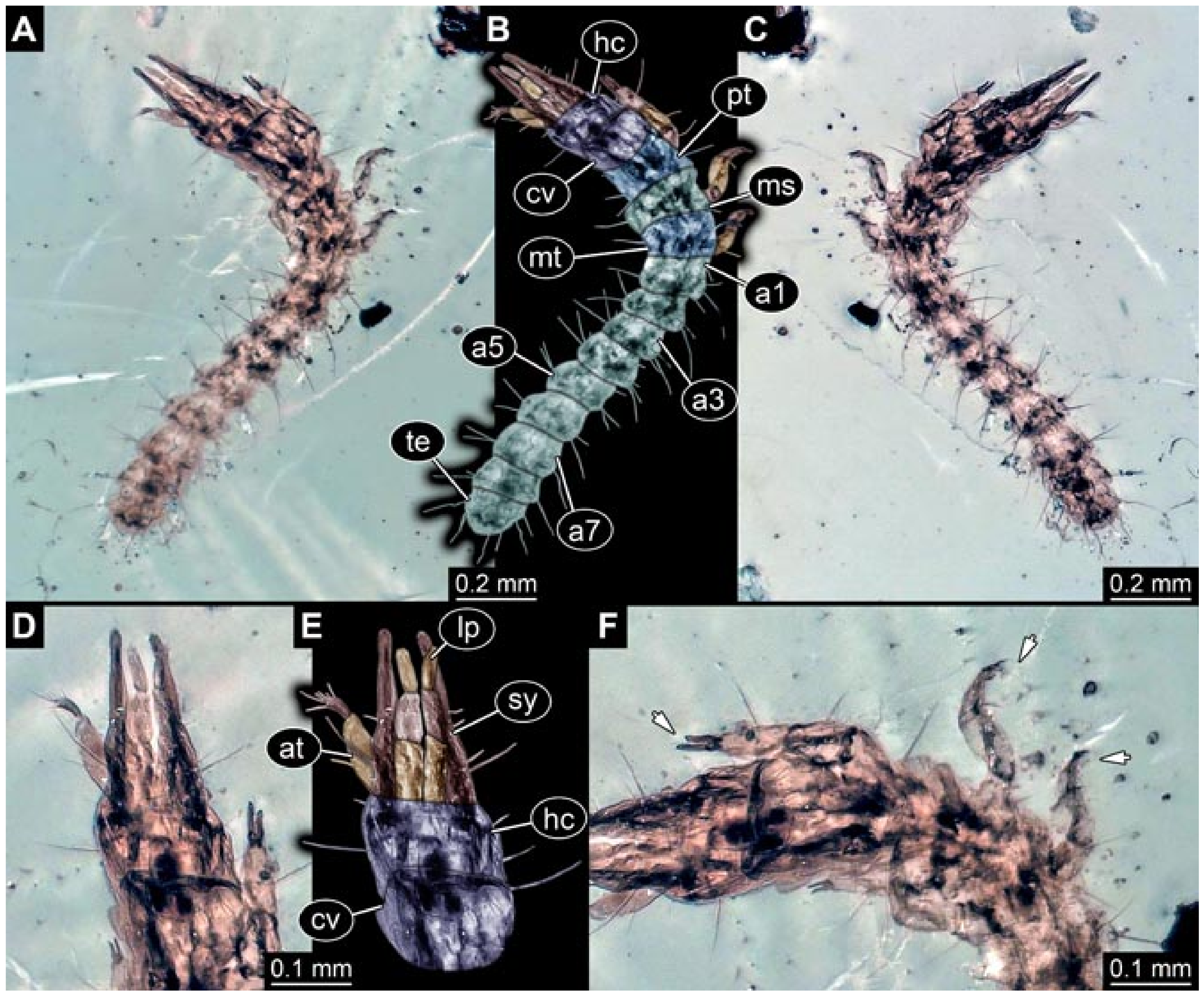




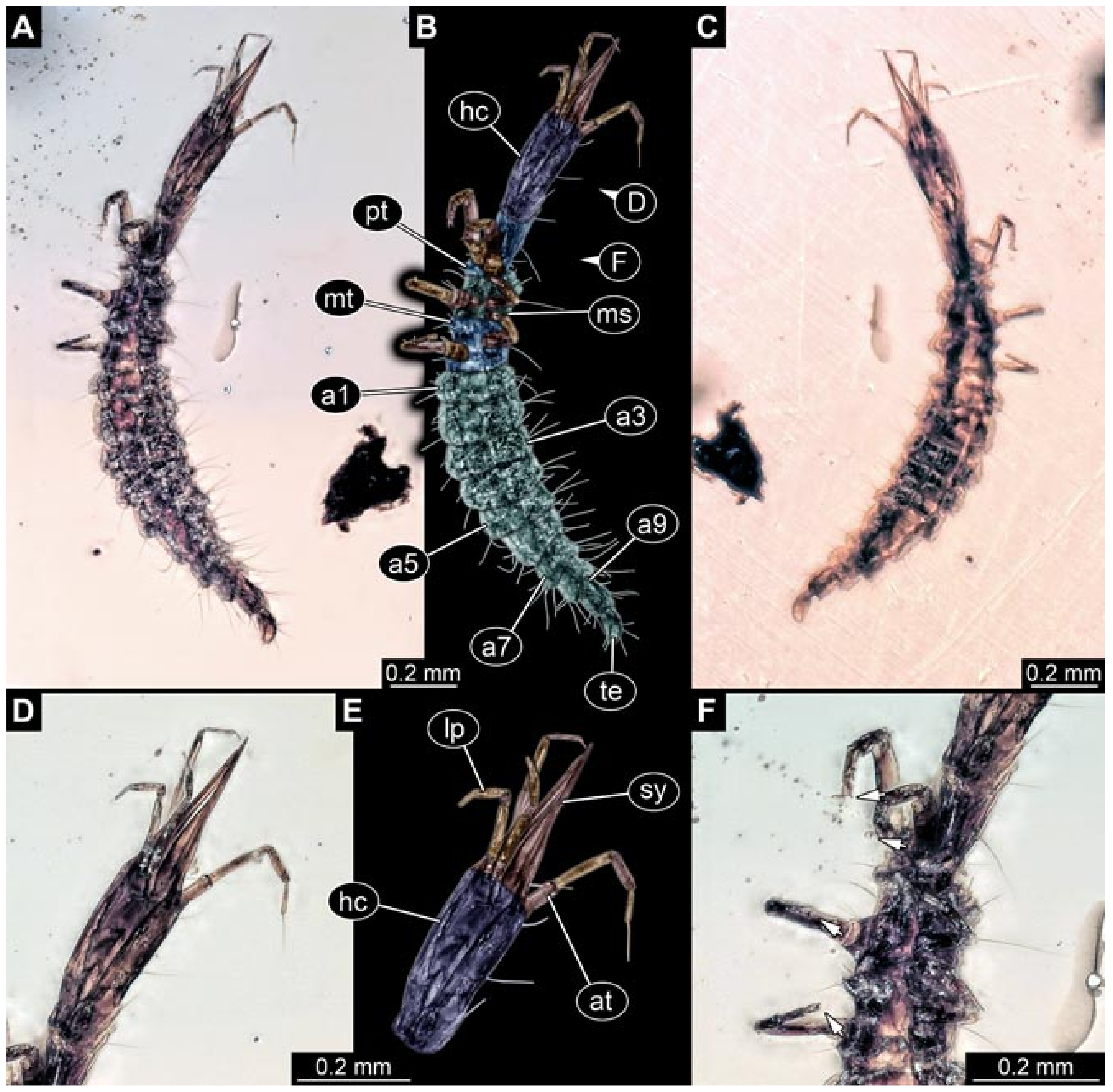
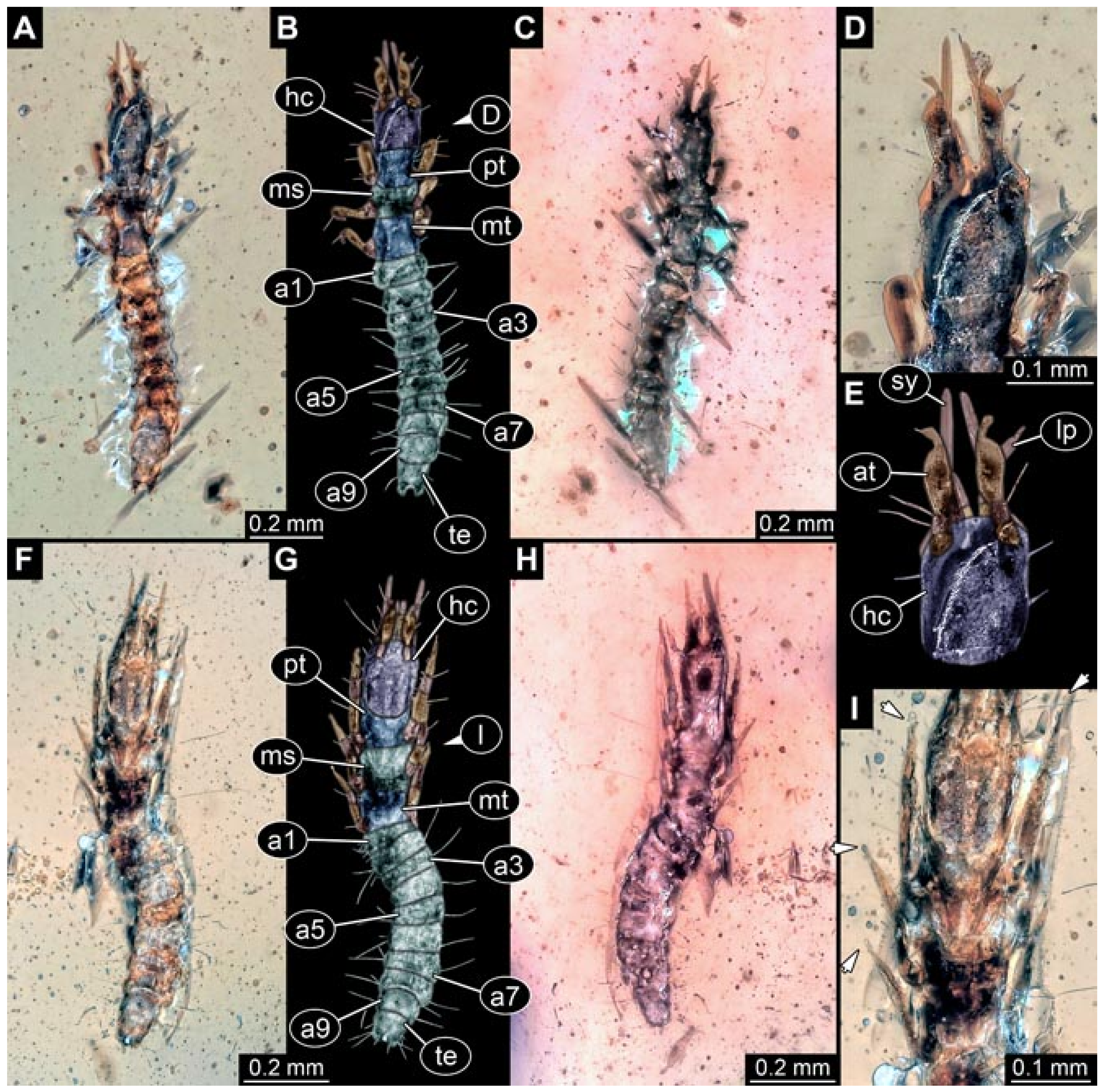
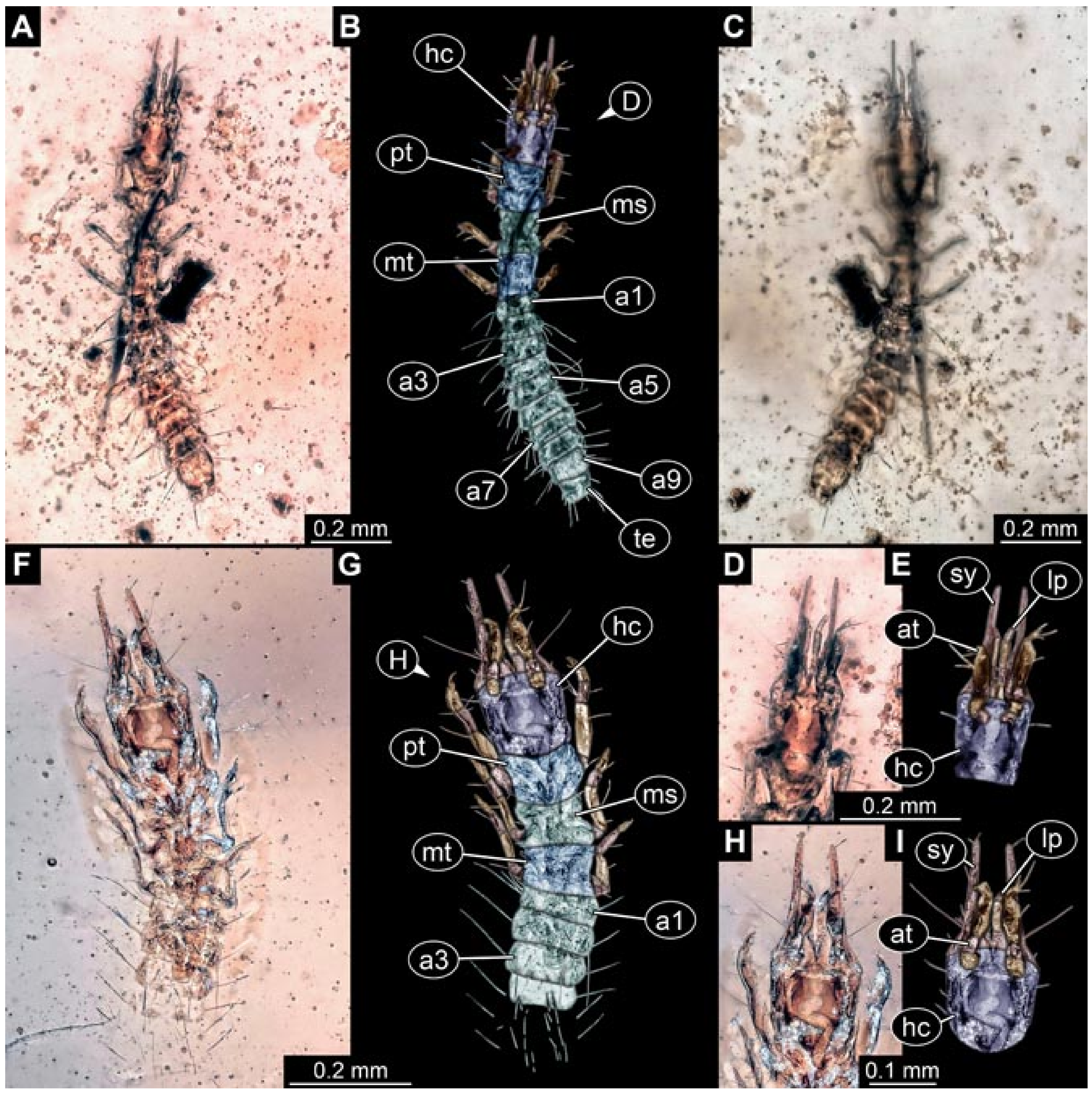

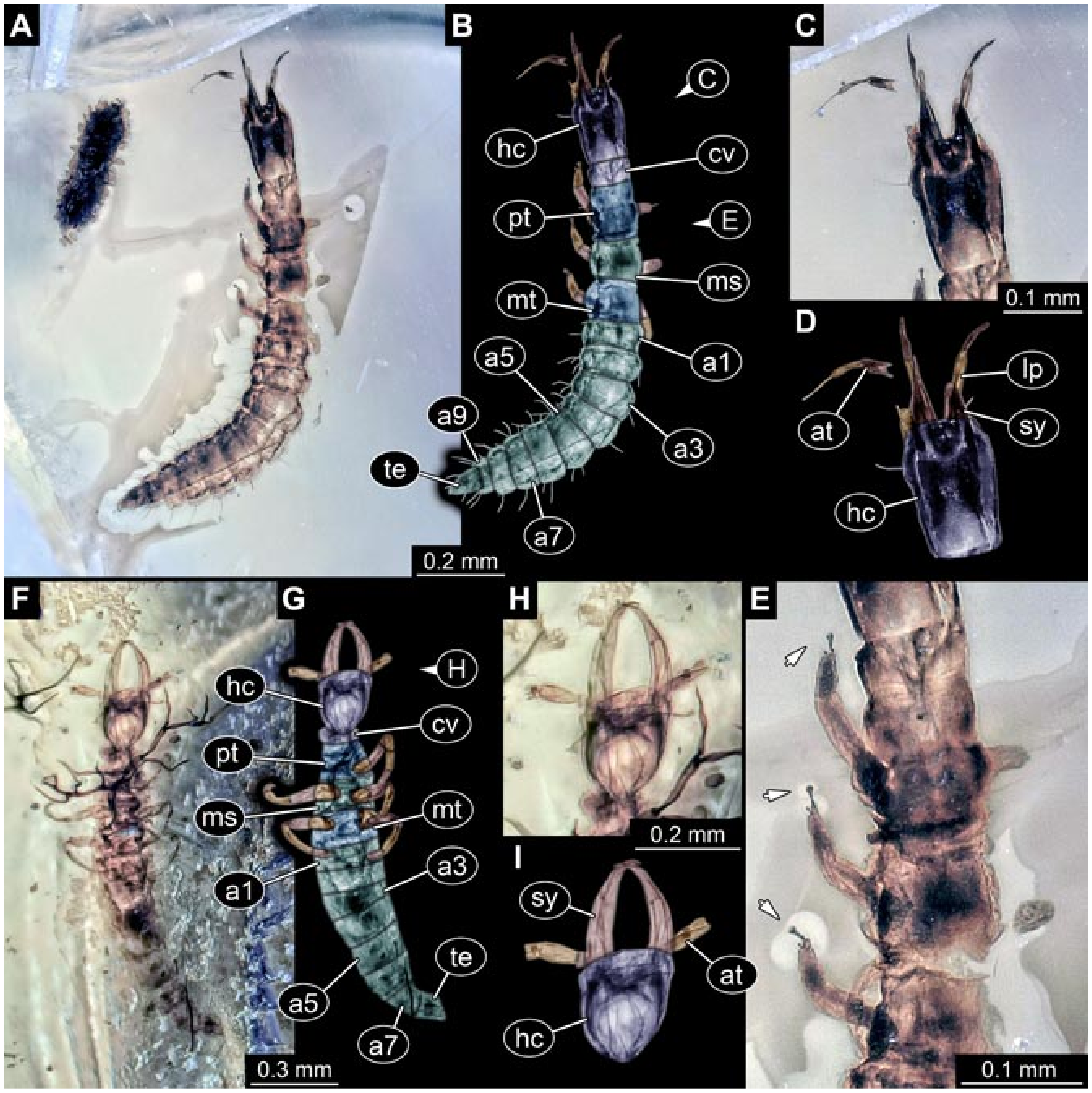
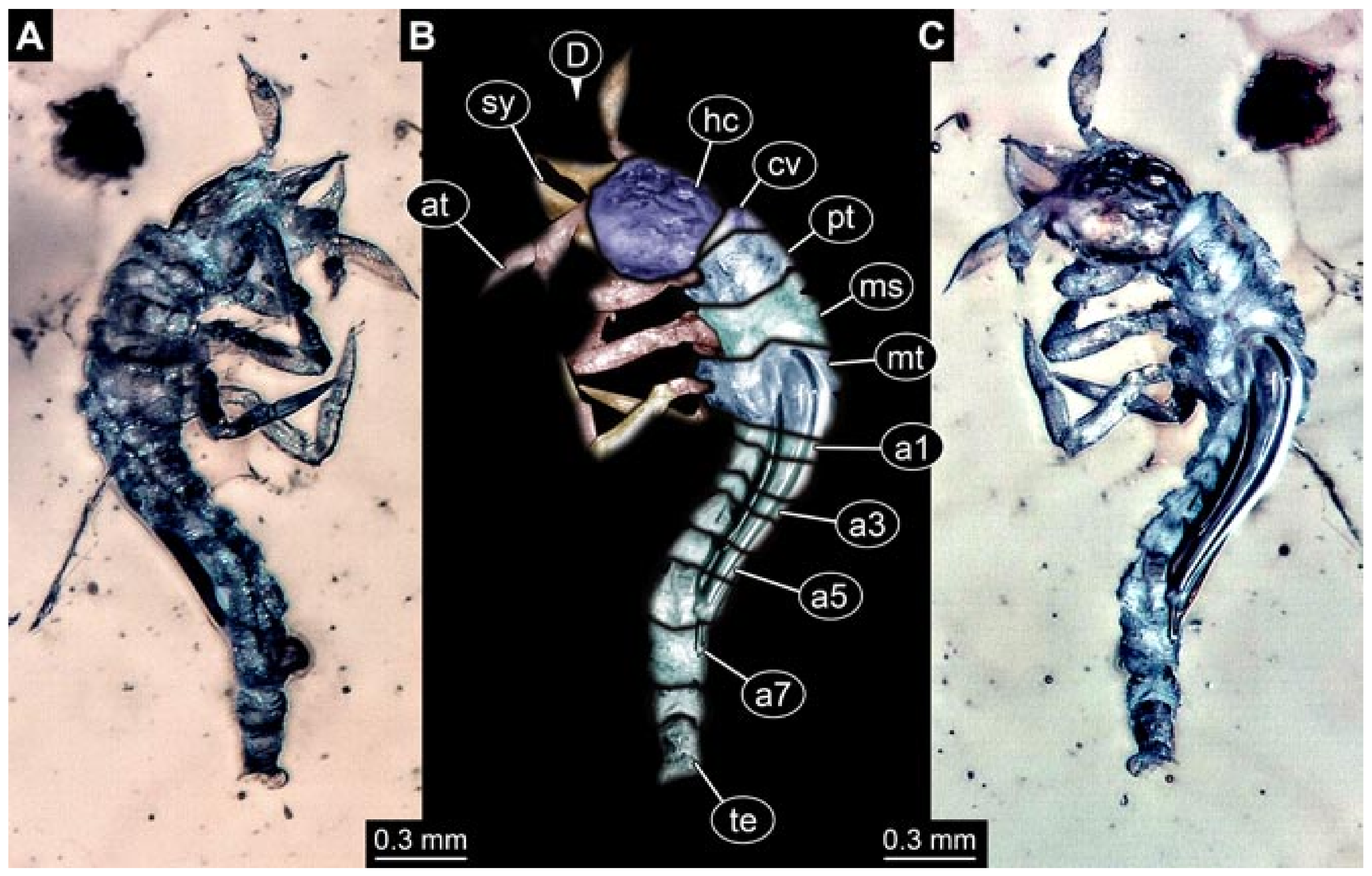
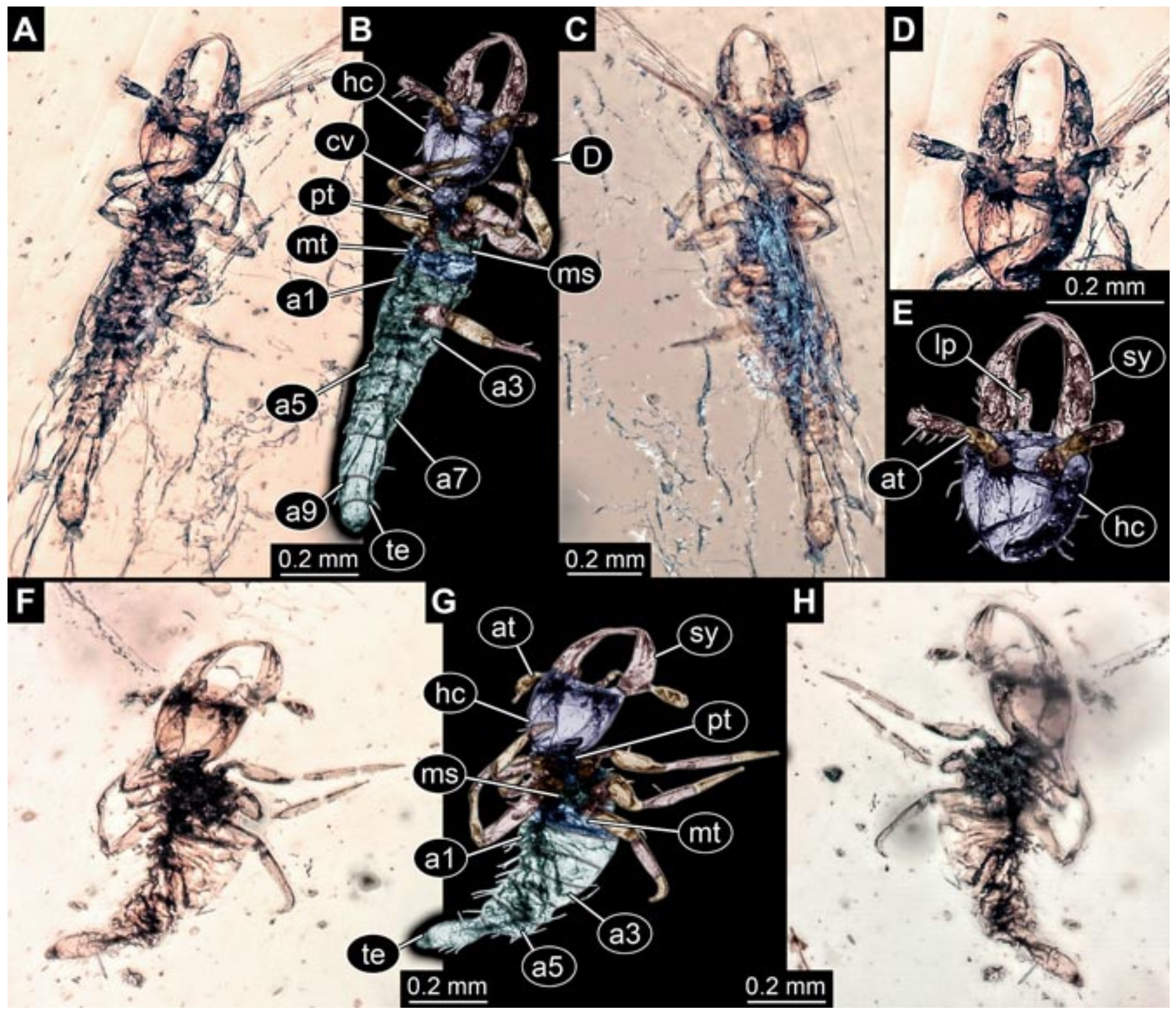

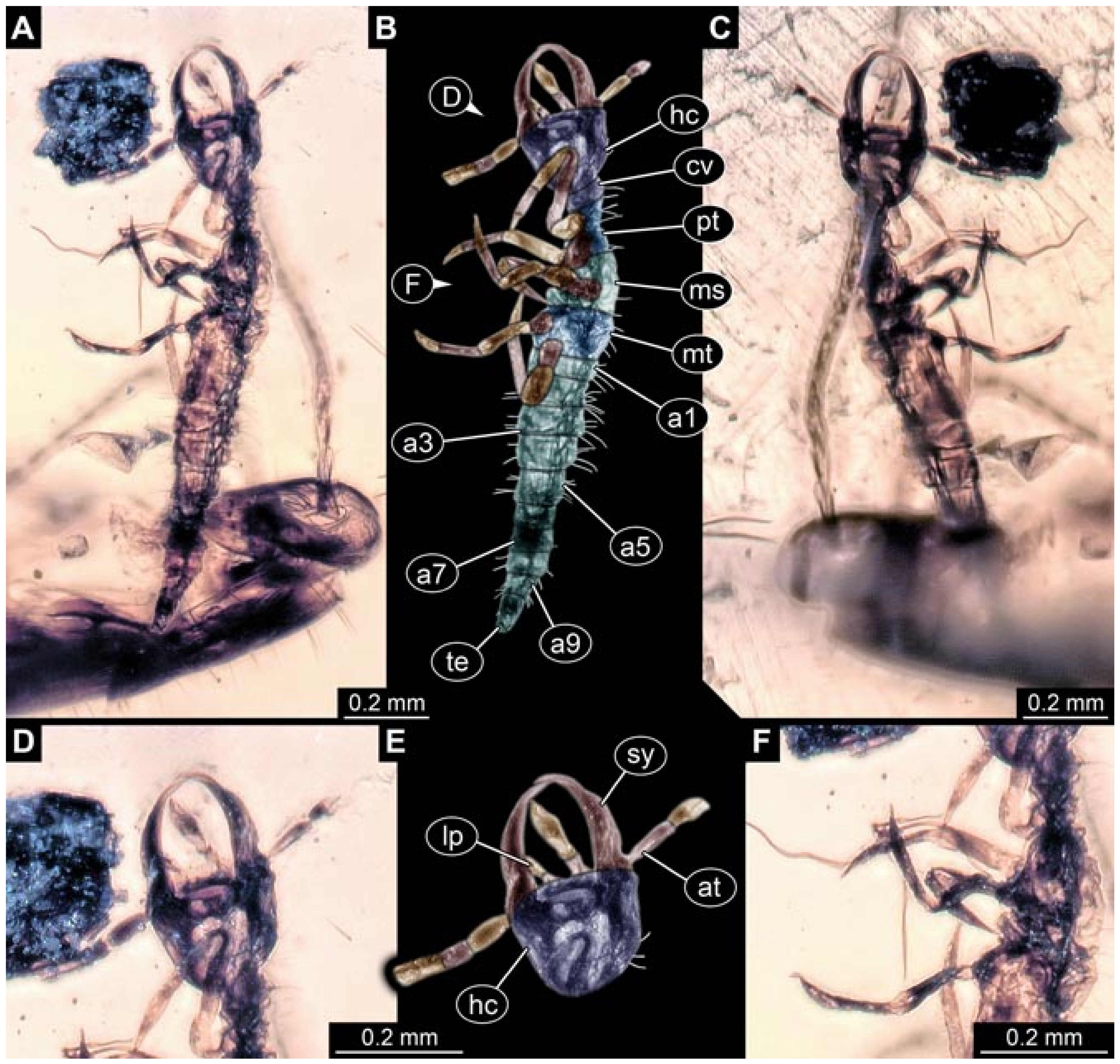

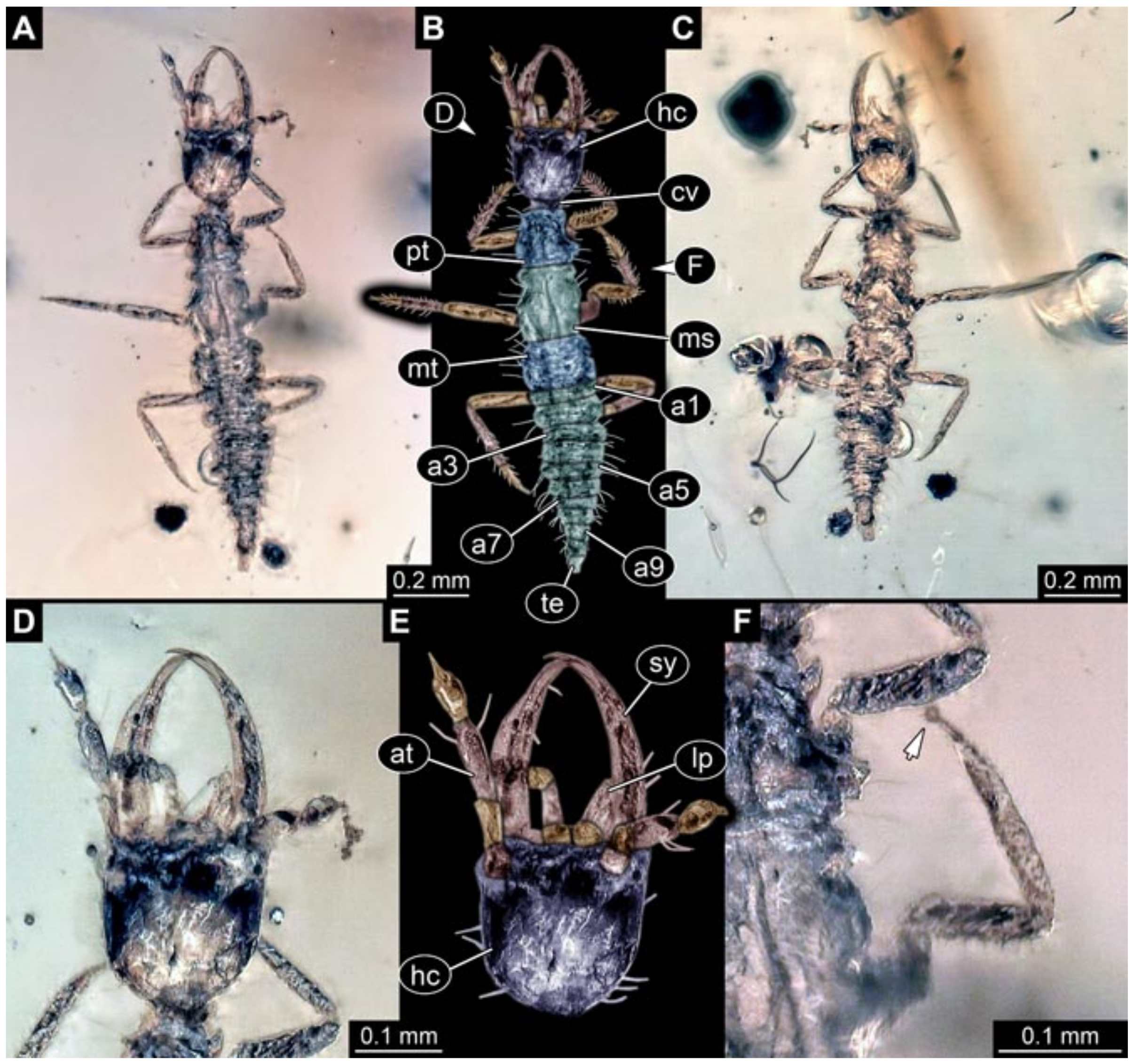
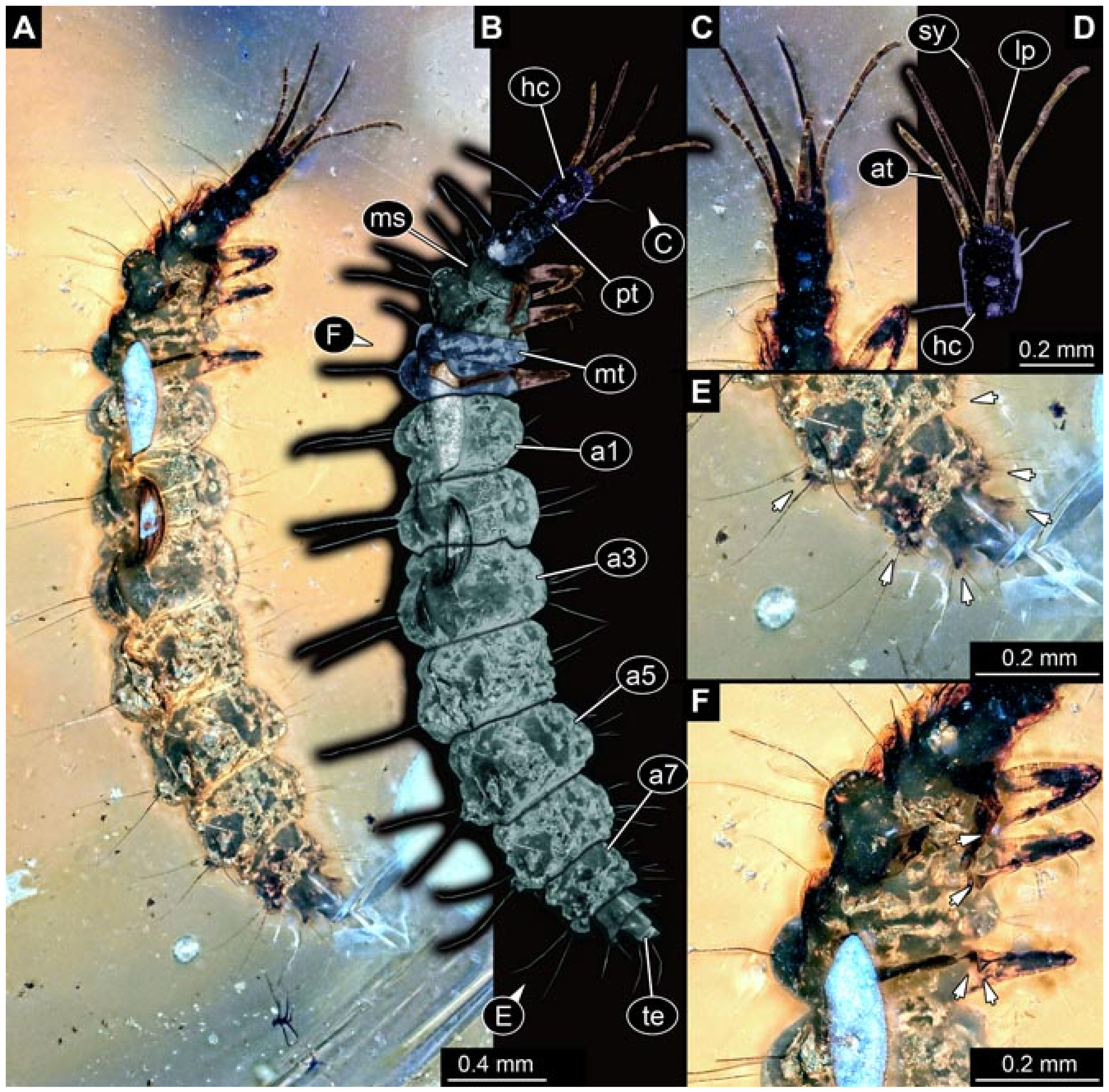
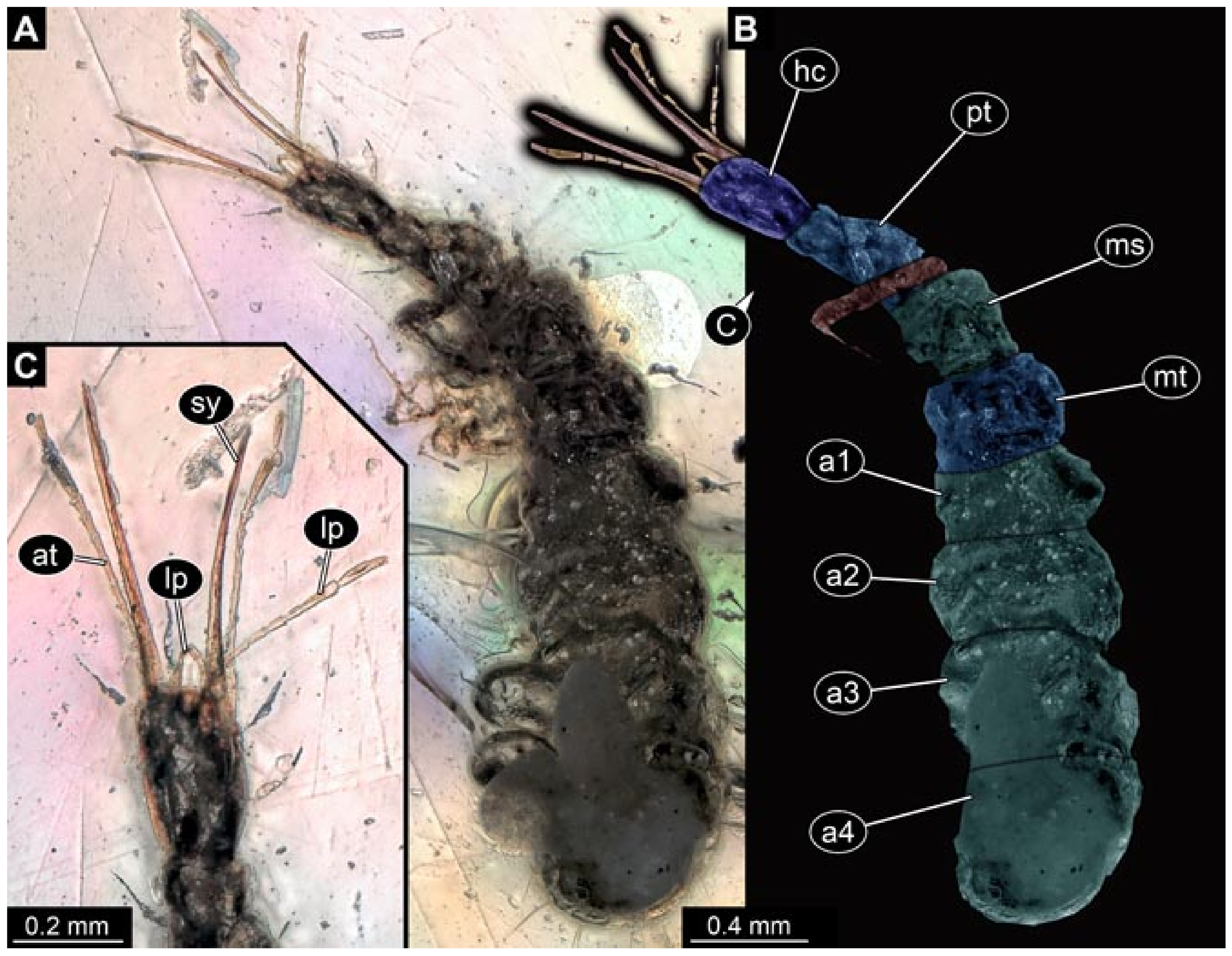


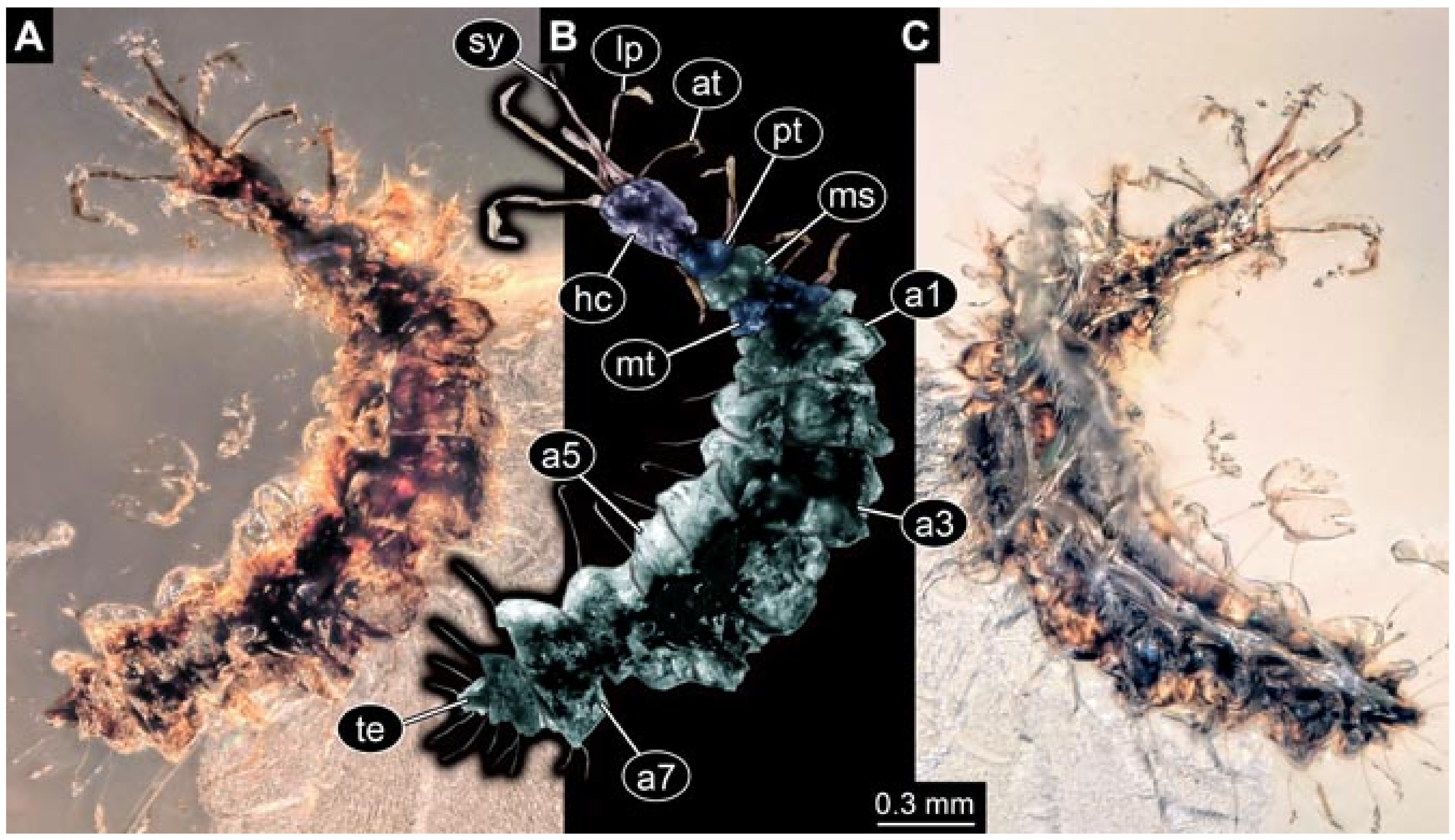
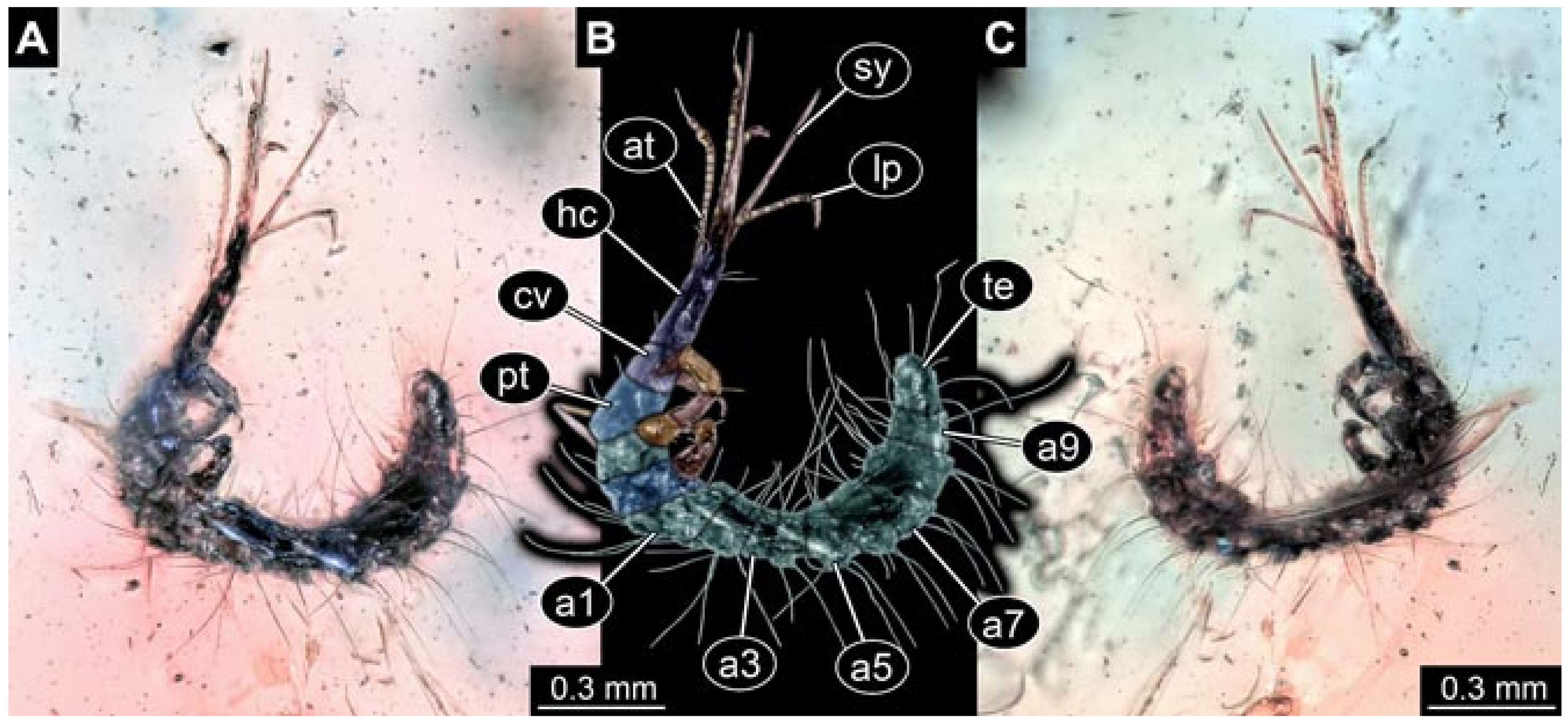
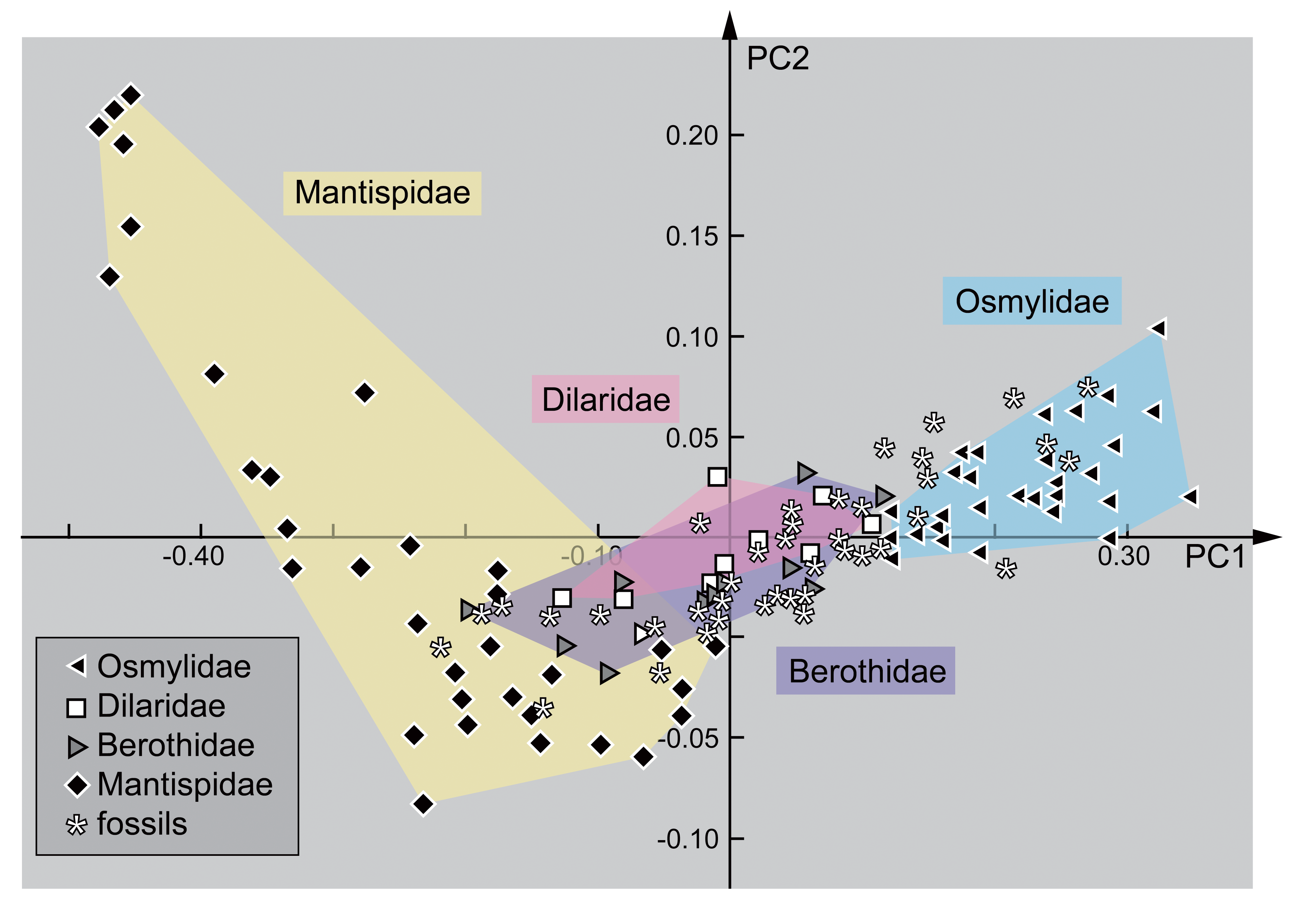
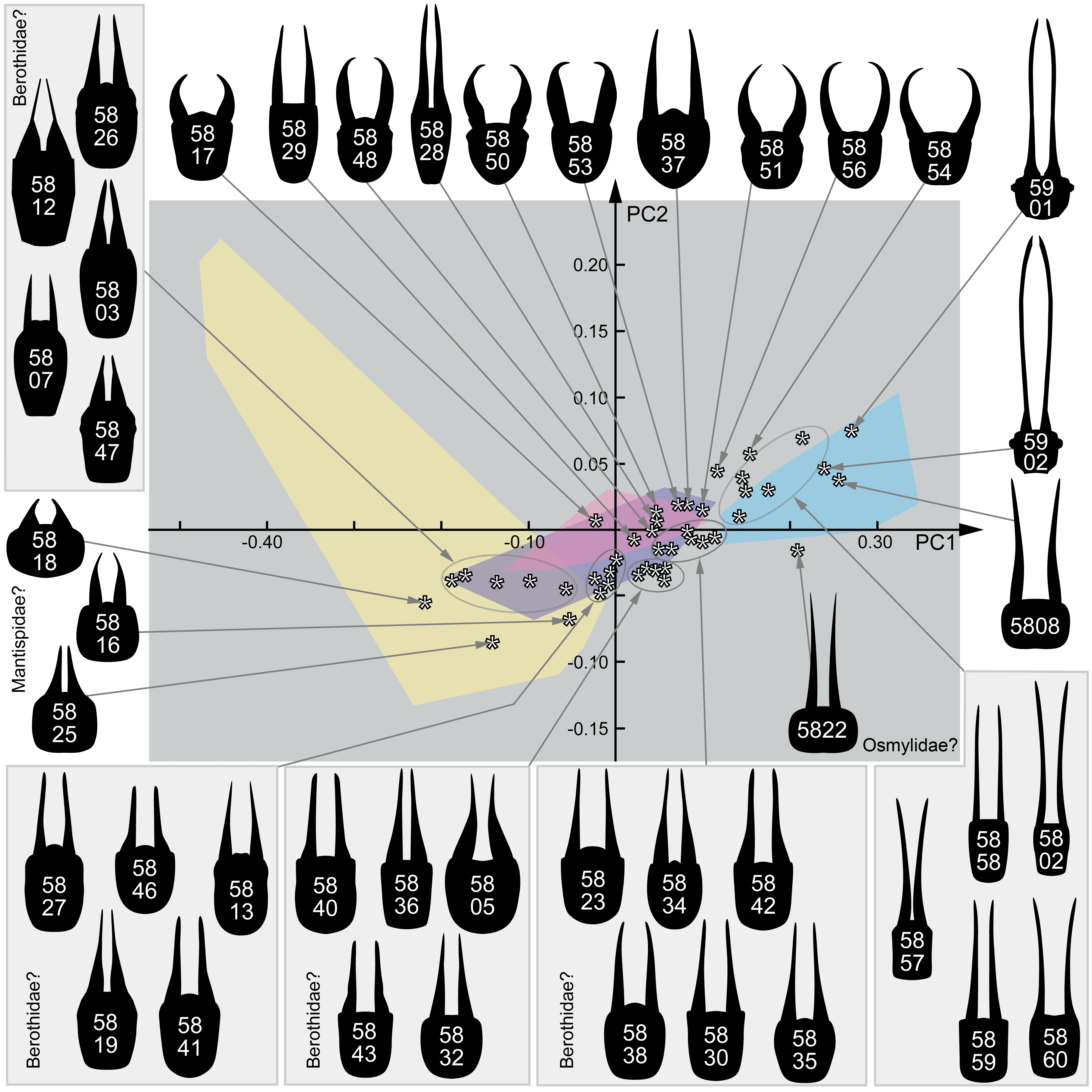
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haug, J.T.; Haug, G.T.; Zippel, A.; van der Wal, S.; Müller, P.; Gröhn, C.; Wunderlich, J.; Hoffeins, C.; Hoffeins, H.-W.; Haug, C. Changes in the Morphological Diversity of Larvae of Lance Lacewings, Mantis Lacewings and Their Closer Relatives over 100 Million Years. Insects 2021, 12, 860. https://doi.org/10.3390/insects12100860
Haug JT, Haug GT, Zippel A, van der Wal S, Müller P, Gröhn C, Wunderlich J, Hoffeins C, Hoffeins H-W, Haug C. Changes in the Morphological Diversity of Larvae of Lance Lacewings, Mantis Lacewings and Their Closer Relatives over 100 Million Years. Insects. 2021; 12(10):860. https://doi.org/10.3390/insects12100860
Chicago/Turabian StyleHaug, Joachim T., Gideon T. Haug, Ana Zippel, Serita van der Wal, Patrick Müller, Carsten Gröhn, Jörg Wunderlich, Christel Hoffeins, Hans-Werner Hoffeins, and Carolin Haug. 2021. "Changes in the Morphological Diversity of Larvae of Lance Lacewings, Mantis Lacewings and Their Closer Relatives over 100 Million Years" Insects 12, no. 10: 860. https://doi.org/10.3390/insects12100860
APA StyleHaug, J. T., Haug, G. T., Zippel, A., van der Wal, S., Müller, P., Gröhn, C., Wunderlich, J., Hoffeins, C., Hoffeins, H.-W., & Haug, C. (2021). Changes in the Morphological Diversity of Larvae of Lance Lacewings, Mantis Lacewings and Their Closer Relatives over 100 Million Years. Insects, 12(10), 860. https://doi.org/10.3390/insects12100860





