Sperm Production Is Reduced after a Heatwave at the Pupal Stage in the Males of the Parasitoid Wasp Microplitisrufiventris Kok (Hymenoptera; Braconidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
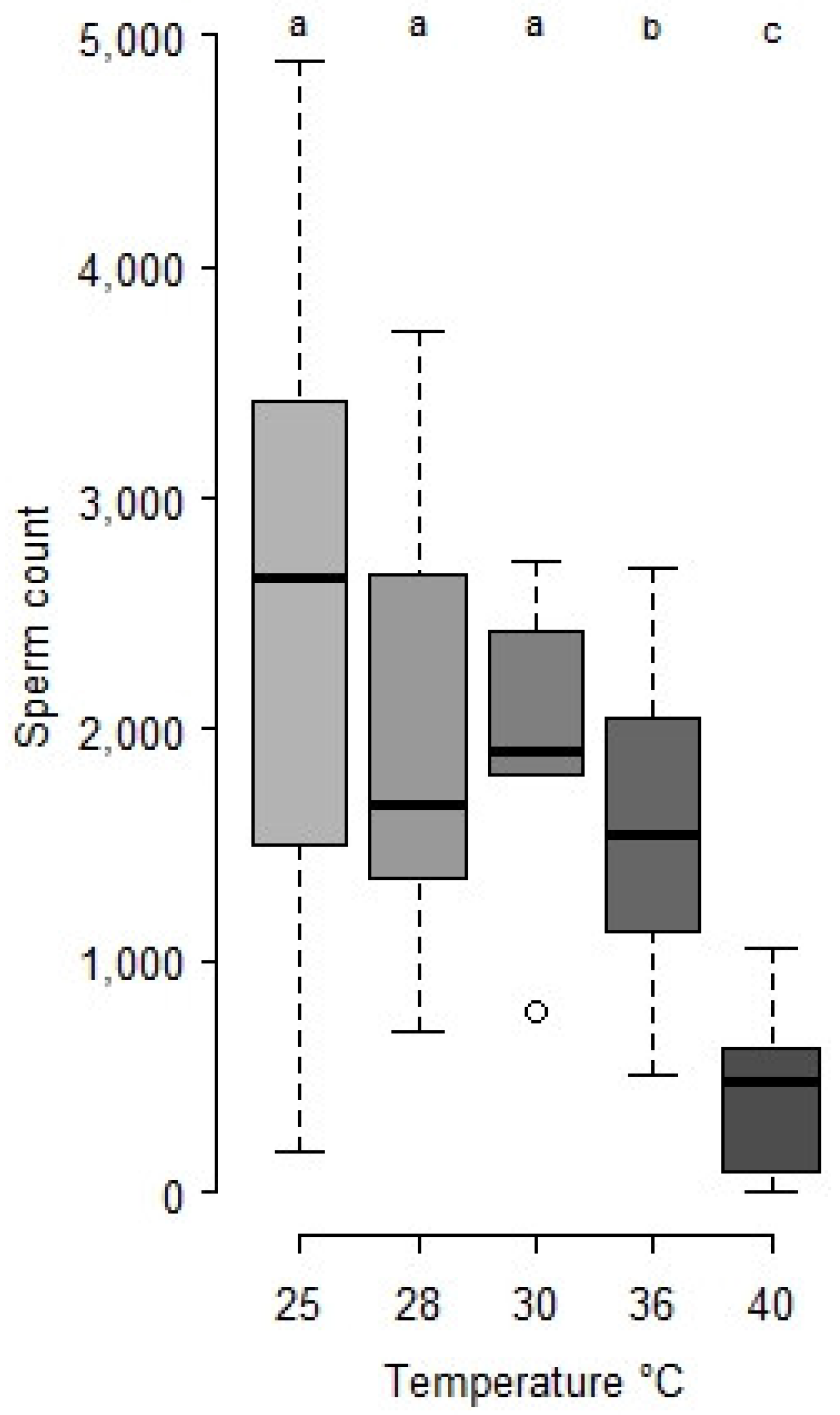
- Heat survival: Four day old cocoons were transferred to other climate chambers for 24 h at 28, 30, 36, 38, 40 and 42 °C respectively (n = 30 to 100 per treatment). Then, they were returned to the original climate chamber at 25 °C until the emergence of adult males. Emerging males were counted in each set to measure survival and sperm count in seminal vesicles (see Section 2.2).
- Sperm stock enumeration: Males were dissected to count sperm in both seminal vesicles, after DAPI staining (methods in [20]). Briefly, seminal vesicles were isolated with fine forceps and opened in saline solution; sperm were fixed by paraformaldehyde 4%, then by ethanol, and labeled with DAPI to be exhaustively numbered under fluorescence at a 40x objective. Counts of both vesicles were added to obtain the whole sperm stock. Pictures of the tract and spermatozoa were taken under phase contrast and fluorescence to describe the reproductive apparatus.
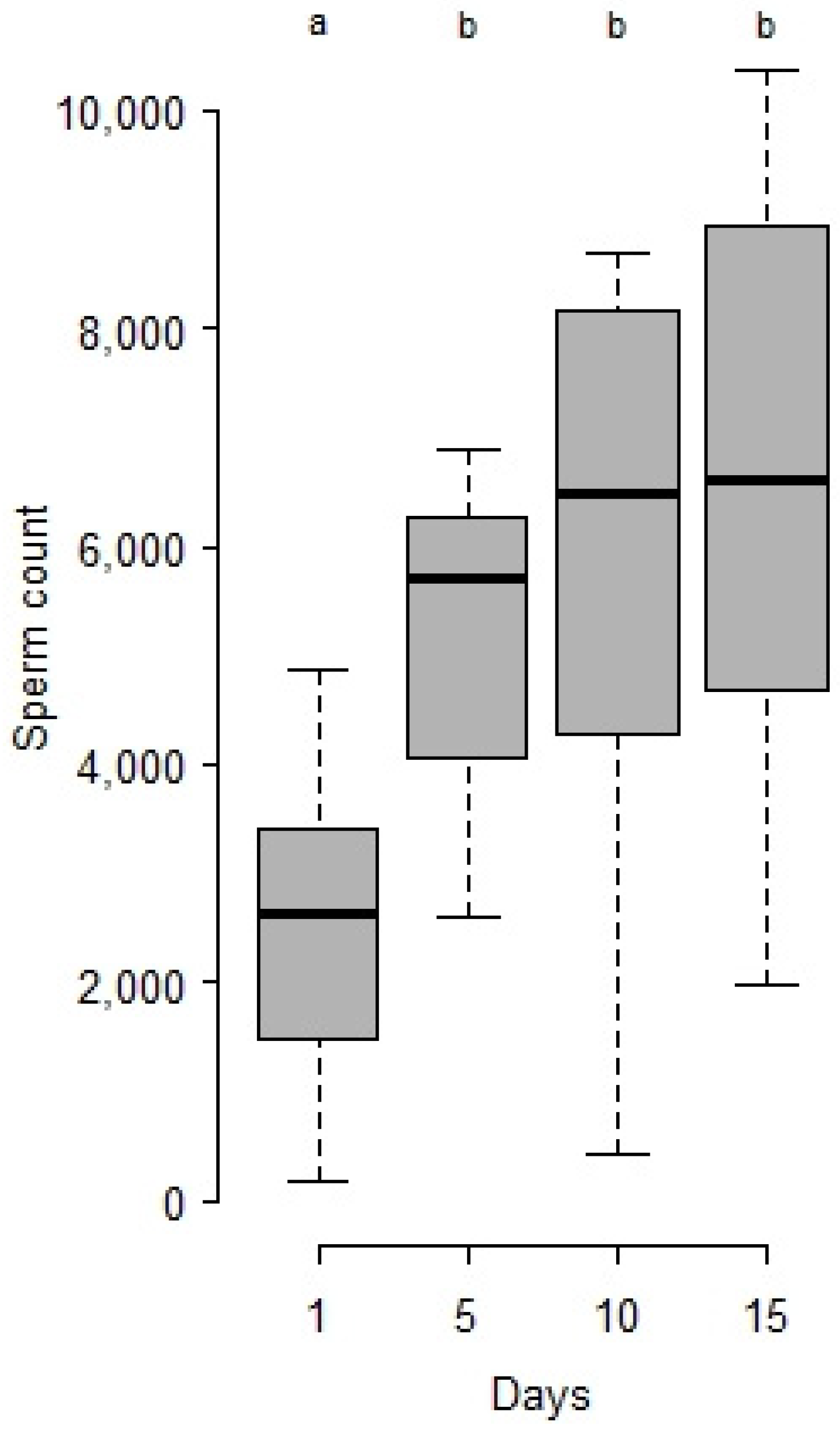
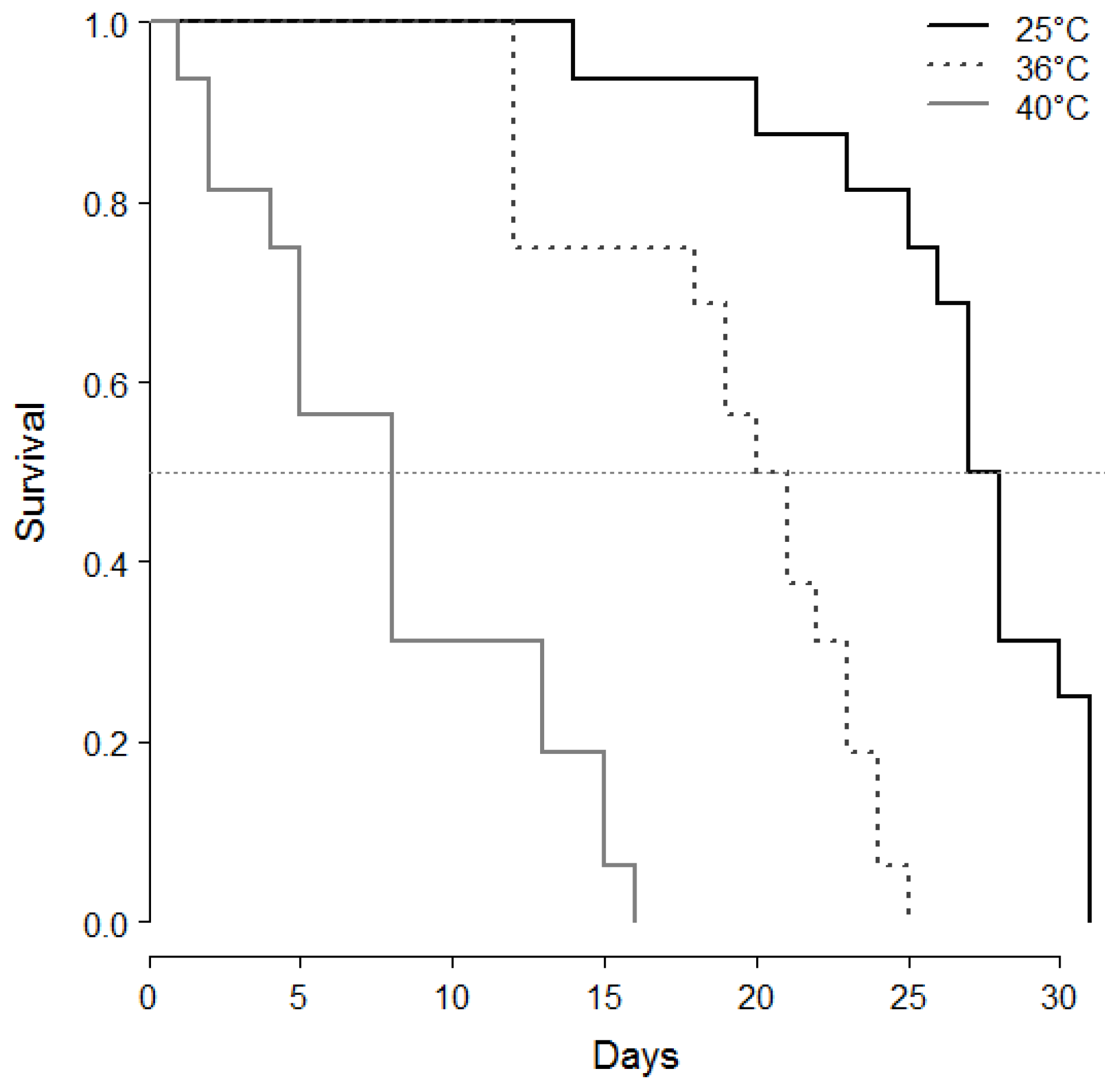
- Heat and age consequences on male sperm stock and survival: From the first series, 42 °C being a lethal temperature, and 28 and 30 °C not efficient for sperm decrease, another set of 4 day-old cocoons was maintained at 25 °C (control), or exposed at the white pupal stage for 24 h at either 36 °C and 40 °C. After, series of virgin males were isolated in a vial (plastic microtubes plugged with cotton with a drop of honey) at 25 °C continuously in the dark and dissected for sperm counts after 1 day (25 °C n = 13, 36 °C n = 13, 40 °C n = 13), 5 days (25 °C n = 13, 36 °C n = 15, 40 °C n = 10), 10 days (25 °C n = 11, 36 °C n = 10, 40 °C n = 3) and 15 days (25 °C n = 10, 36 °C n = 11, 40 °C n = 3). Others (n = 16 for each treatment) were maintained in isolation under the same conditions and checked twice a day until their death for survival recording.
- Statistical analysis: Sperm counts were presented as means ± SE. Stats were done with R studio [32]. Sperm counts along ageing and after heat periods were treated as global populations by the Kruskal-Wallis test, and, when significant, followed by Wilcoxon posthoc tests to compare series of males by age and/or temperature. Survivals after heatwaves were compared by a log-rank test.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hance, T.; van Baaren, J.; Vernon, P.; Boivin, G. Impact of extreme temperatures on parasitoids in a climate change perspective. Annu. Rev. Entomol. 2007, 52, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.S.; Ma, G.; Pincebourde, S. Survive a warming climate: Insect responses to extreme high temperatures. Annu. Rev. Entomol. 2021, 61, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.E.; Costanzo, J.P.; Mugnano, J.A. Regulation of super cooling and ice nucleation in insects. Eur. J. Entomol. 1996, 93, 405–418. [Google Scholar]
- Rinehart, J.P.; Yocum, G.D.; Denlinger, D.L. Thermotolerance and rapid cold hardening ameliorate the negative effects of brief exposures to high or low temperatures on fecundity in the flesh fly, Sarcophaga crassipalpis. Physiol. Entomol. 2000, 25, 330–336. [Google Scholar] [CrossRef] [Green Version]
- Chevrier, C.; Nguyen, T.M.; Bressac, C. Heat shock sensitivity of adult male fertility in the parasitoid wasp Anisopteromalus calandrae (Hymenoptera, Pteromalidae). J. Therm. Biol. 2019, 85, 102419. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, D.L.; Yocum, G.D. Physiology of heat sensitivity. In Temperature Sensitivity in Insects and Application in Integrated Pest Management; Hallman, G.J., Denlinger, D.L., Eds.; Westview Press: Oxford, UK, 1998; pp. 7–57. [Google Scholar]
- Mutamiswa, R.; Chidawanyika, F.; Nyamukondiwa, C. Comparative assessment of the thermal tolerance of spotted stemborer, Chilo partellus (Lepidoptera: Crambidae) and its larval parasitoid, Cotesia sesamiae (Hymenoptera: Braconidae). Insect Sci. 2017, 25, 847–860. [Google Scholar] [CrossRef]
- Fasolo, A.G.; Krebs, R.A. A comparaison of behavioural change in Drosophila during exposure to thermal stress. Biol. J. Linn. Soc. 2004, 83, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Walsh, B.S.; Paratt, S.R.; Hoffman, A.A.; Atkinson, D.; Snook, R.S.; Bretman, A.; Price, T.A.R. The impact of climate change on fertility. Tr. Ecol. Evol. 2019, 34, 249–259. [Google Scholar] [CrossRef]
- Alford, L.; Burel, F.; Baaren, J. Improving methods to measure critical thermal limits in phloem-feeding pest insects. Entomol. Exp. Appl. 2016, 159, 61–69. [Google Scholar] [CrossRef]
- Verberk, W.C.E.P.; Overgaard, J.; Ern, R.; Bayley, M.; Wang, T.; Boardman, L.; Terblanche, J.S. Does oxygen limit thermal tolerance in arthropods? A critical review of current evidence. Comp. Biochem. Physiol. Part A 2016, 192, 64–78. [Google Scholar] [CrossRef] [Green Version]
- Sentis, A.; Hemptinne, J.-L.; Brodeur, J. Effects of simulated heat waves on an experimental plant-herbivore-predator food chain. Glob. Chang. Biol. 2013, 19, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in Fluctuating Thermal Environments. An. Rev. Entomol. 2015, 60, 123–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegazi, E.M.; Kolaib, M.O.; Fattah el, M.I.A. Development of Microplitis rufiventris Kok at different temperatures. J. Agric. Sci. Camb. 1980, 94, 739–740. [Google Scholar] [CrossRef]
- Chevrier, C.; Bressac, C. Sperm storage and use after multiple mating in Dinarmus basalis (Hymenoptera: Pteromalidae). J. Insect Behav. 2002, 15, 385–398. [Google Scholar] [CrossRef]
- Dallai, R. Overview on spermatogenesis and sperm structure of Hexapoda. Arth. Struct. Dev. 2014, 43, 257–290. [Google Scholar] [CrossRef]
- Poidatz, J.; Bressac, C.; Bonnard, O.; Thiery, D. Delayed sexual maturity in males of Vespa velutina. Insect Sci. 2018, 25, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Chirault, M.; Lucas, C.; Goubault, M.; Chevrier, C.; Lécureuil, C.; Bressac, C. A combined approach to heat stress effect on male fertility in Nasonia vitripennis: From the physiological consequences on spermatogenesis to the reproductive adjustment of females mated with stressed males. PLoS ONE 2015, 10, e0120656. [Google Scholar] [CrossRef]
- Bressac, C.; Do Thi Khanh, H.; Chevrier, C. Effects of age and repeated mating on male sperm supply and paternity in a parasitoid wasp. Ent. Exp. Appl. 2009, 130, 207–213. [Google Scholar] [CrossRef]
- Bredlau, J.P.; El-Sabrout, A.M.; Bressac, C. Reproductive context of extremely short sperm in the parasitic wasp Cotesia congregata (Hymenoptera: Braconidae). Biol. J. Linn. Soc. 2020, 131, 384–395. [Google Scholar] [CrossRef]
- Boivin, G.; Sebastien, J.; Damiens, D. Spermatogeny as a life-history index in parasitoid wasps. Oecologia 2005, 143, 198–202. [Google Scholar] [CrossRef]
- Iossa, G. Sex specific differences in thermal fertility limits. Trends Ecol. Evol. 2019, 34, 490–492. [Google Scholar] [CrossRef]
- David, J.R.; Araripe, L.O.; Chakir, M.; Legout, H.; Lemos, B.; Petavy, G.; Rohmer, C.; Joly, D.; Moreteau, B. Male sterility at extreme temperatures: A significant but neglected phenomenon for understanding Drosophila climatic adaptations. J. Evol. Biol. 2005, 18, 838–846. [Google Scholar] [CrossRef]
- Porcelli, D.; Gaston, K.J.; Butlin, R.K.; Snook, R.R. Local adaptation of reproductive performance during thermal stress. J. Evol. Biol. 2016, 30, 422–429. [Google Scholar] [CrossRef]
- Sales, K.; Vasudeva, R.; Gage, M.J.G. Fertility and mortality impacts of thermal stress from experimental heatwaves on different life stages and their recovery in a model insect. R. Soc. Open Sci. 2021, 8, 201717. [Google Scholar] [CrossRef]
- N’guyen, T.-M.; Bressac, C.; Chevrier, C. Heat stress affects male reproduction in a parasitoid wasp. J. Insect Physiol. 2013, 59, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Lacoume, S.; Bressac, C.; Chevrier, C. Sperm production and mating potential of males after a cold shock on pupae of the parasitoid wasp Dinarmus basalis (Hymenoptera: Pteromalidae). J. Insect Physiol. 2007, 53, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Lacoume, S.; Bressac, C.; Chevrier, C. Male hypofertility induced by Paraquat consumption in the non-target parasitoid Anisopteromalus calandrae (Hymenoptera:Pteromalidae). Biol. Control 2009, 49, 214–218. [Google Scholar] [CrossRef]
- Zhang, W.; Chang, X.Q.; Hoffmann, A.; Zhang, S.; Ma, C.S. Impact of hot events at different developmental stages of a moth: The closer to adult stage, the less reproductive output. Sci. Rep. 2015, 5, 10436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lelieveld, J.; Proestos, Y.; Hadjinicolaou, P.; Tanarhte, M.; Tyrlis, E.; Zittis, G. Strongly increasing heat extremes in the Middle East and North Africa (MENA) in the 21st century. Clim. Chang. 2016, 137, 245–260. [Google Scholar] [CrossRef] [Green Version]
- Uzbekov, R.; Burlaud-Gaillard, J.; Garanina, A.S.; Bressac, C. The length of a short sperm: Elongation and shortening during spermiogenesis in Cotesia congregata (Hymenoptera, Braconidae). Arth. Struct. Dev. 2017, 46, 265–273. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R. RStudio, 2020. PBC, Boston, MA, USA. Available online: http://www.rstudio.com/ (accessed on 17 September 2020).
- Malina, R.; Praslicka, J. Effect of temperature on the developmental rate, longevity and parasitism of Aphidius ervi Haliday. Plant Prot. Sci. 2008, 44, 19–24. [Google Scholar] [CrossRef]
- Jerbi-Elayed, M.; Lebdi-Grissa, K.; Foray, V.; Muratori, F.; Hance, T. Using multiple traits to estimate the effects of heat shock on the fitness of Aphidius colemani. Ent. Exp. App. 2015, 155, 18–27. [Google Scholar] [CrossRef]
- Quicke, D.J.L. Parasitic Wasps; Chapman & Hall: London, UK, 1997; 470p. [Google Scholar]
- Haberl, M.; Tautz, D. Sperm usage in honey bees. Behav. Ecol. Sociobiol. 1998, 42, 247–255. [Google Scholar] [CrossRef]
- Yi, S.J.; Hopkins, R.J.; Chen, X.Y.; Chen, Z.M.; Wang, X.; Huang, G.H. Effects of temperature on the development and fecundity of Microplitis similis (Hymenoptera: Braconidae), a parasitoid of Spodoptera litura (Lepidoptera: Noctuidae). Physiol. Entomol. 2020, 45, 95–102. [Google Scholar] [CrossRef]
- Chihrane, J.; Laugé, G. Incidences de chocs de températures élevées sur la lignée germinale mâle de Trichogramma brassicae Bezdenko (Hym.: Trichogrammatidae). Entomophaga 1994, 39, 11–20. [Google Scholar] [CrossRef]
- Krebs, R.A.; Loeschcke, V. Effects of exposure to short-term heat stress on fitness components in Drosophila melanogaster. J. Evol. Biol. 1994, 7, 39–49. [Google Scholar] [CrossRef]
- Damiens, D.; Bressac, C.; Chevrier, C. The effect of age on sperm stock and egg laying in the parasitoid wasp, Dinarmus basalis. J. Insect Sci. 2003, 3, 22–27. [Google Scholar]
- Damiens, D.; Boivin, G. Male reproductive strategy in Trichogramma evanescens: Sperm production and allocation to females. Physiol. Entomol. 2005, 30, 241–247. [Google Scholar] [CrossRef]
- Moore, M.E.; Hill, C.A.; Kingsolver, J.G. Differing thermal sensitivities in a host-parasitoid interaction: High, fluctuating developmental temperatures produce dead wasps and giant caterpillars. Funct. Ecol. 2021, 35, 675–685. [Google Scholar] [CrossRef]
- Foray, V.; Desouhant, E.; Gibert, P. The impact of thermal fluctuations on reaction norms in specialist and generalist parasitic wasps. Funct. Ecol. 2014, 28, 411–423. [Google Scholar] [CrossRef]
- Woods, H.A.; Dillon, M.E.; Pincebourde, S. The roles of microclimatic diversity and of behavior in mediating the responses of ectotherms to climate change. J. Therm. Biol. 2015, 54, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Moiroux, J.; Brodeur, J.; Boivin, G. Sex ratio variations with temperature in an egg parasitoid: Behavioural adjustment and physiological constraint. Anim. Behav. 2014, 91, 61–66. [Google Scholar] [CrossRef]
- Moore, M.P.; Martin, R.A. On the evolution of carry-over effects. J. Anim. Ecol. 2019, 88, 1832–1844. [Google Scholar] [CrossRef]
- Sentinella, A.T.; Crean, A.J.; Bonduriansky, R. Dietary protein mediates a trade-off between larval survival and the development of male secondary sexual traits. Funct. Ecol. 2013, 27, 1134–1144. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sabrout, A.M.; Hegazi, E.; Khafagi, W.; Bressac, C. Sperm Production Is Reduced after a Heatwave at the Pupal Stage in the Males of the Parasitoid Wasp Microplitisrufiventris Kok (Hymenoptera; Braconidae). Insects 2021, 12, 862. https://doi.org/10.3390/insects12100862
El-Sabrout AM, Hegazi E, Khafagi W, Bressac C. Sperm Production Is Reduced after a Heatwave at the Pupal Stage in the Males of the Parasitoid Wasp Microplitisrufiventris Kok (Hymenoptera; Braconidae). Insects. 2021; 12(10):862. https://doi.org/10.3390/insects12100862
Chicago/Turabian StyleEl-Sabrout, Ahmed M., Esmat Hegazi, Wedad Khafagi, and Christophe Bressac. 2021. "Sperm Production Is Reduced after a Heatwave at the Pupal Stage in the Males of the Parasitoid Wasp Microplitisrufiventris Kok (Hymenoptera; Braconidae)" Insects 12, no. 10: 862. https://doi.org/10.3390/insects12100862
APA StyleEl-Sabrout, A. M., Hegazi, E., Khafagi, W., & Bressac, C. (2021). Sperm Production Is Reduced after a Heatwave at the Pupal Stage in the Males of the Parasitoid Wasp Microplitisrufiventris Kok (Hymenoptera; Braconidae). Insects, 12(10), 862. https://doi.org/10.3390/insects12100862





