Distribution and Molecular Diversity of Whitefly Species Colonizing Cassava in Kenya
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
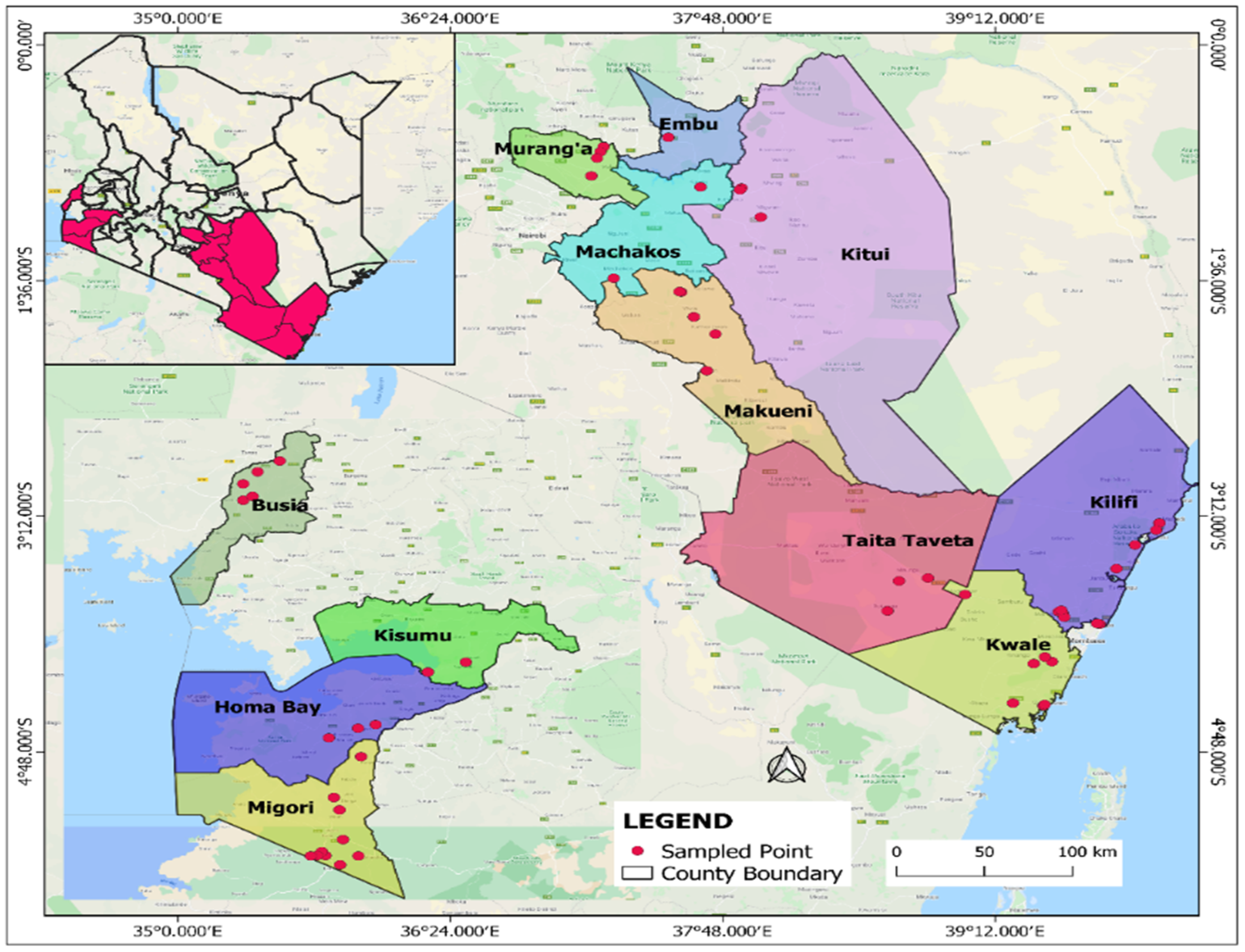
2.1. Sampling Sites, Sample Collection and Preservation
2.2. Genomic DNA Extraction and Polymerase Chain Reaction Amplification
2.3. KASP Genotyping of Whitefly Samples
2.4. Sequence Quality Control, BLAST Search and Phylogenetic Analysis
3. Results
3.1. Presense of Whiteflies in Inspected Cassava Fields
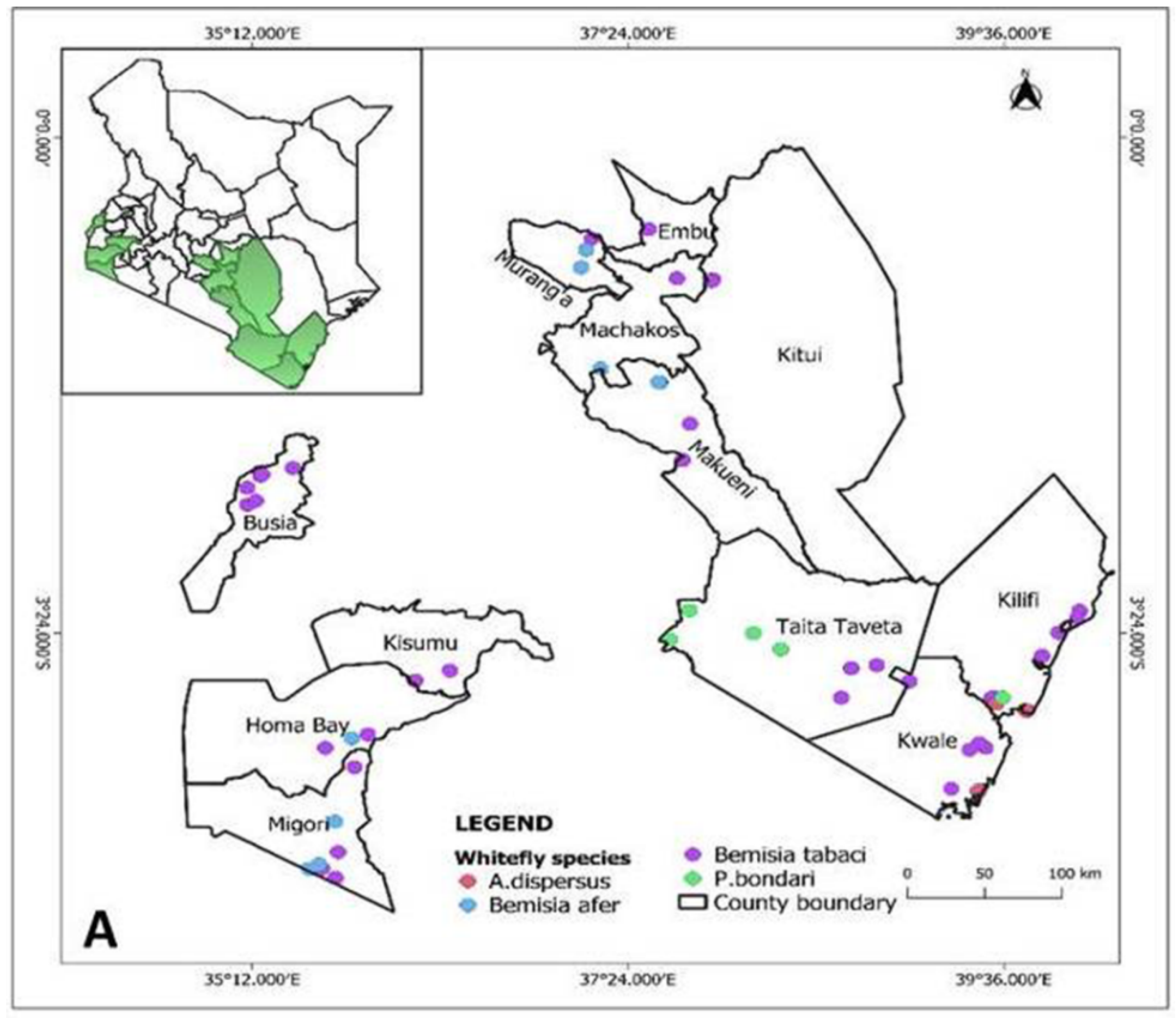
3.2. Determination of Whitefly Species and Phylogenetic Analysis
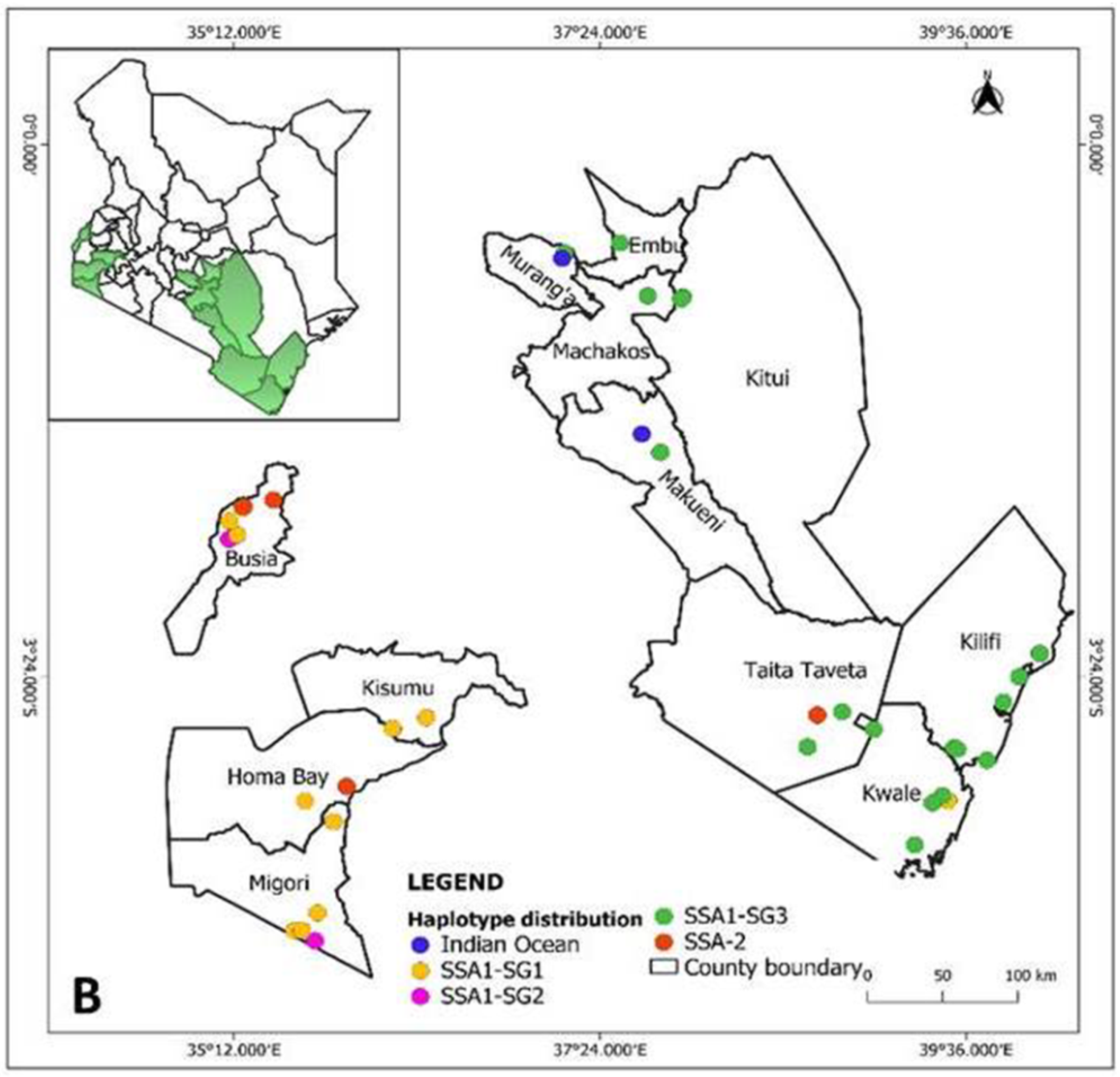
3.3. Distribution of Whitefly Species and Bemisia tabaci Haplotypes
3.4. Population Genetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aregbesola, O.Z.; Legg, J.P.; Lund, O.S.; Sigsgaard, L.; Sporleder, M.; Carhuapoma, P.; Rapisarda, C. Life history and temperature-dependence of cassava-colonising populations of Bemisia Tabaci. J. Pest. Sci. 2020, 93, 1225–1241. [Google Scholar] [CrossRef]
- Fiallo-Olivé, E.; Pan, L.L.; Liu, S.S.; Navas-Castillo, J. Transmission of begomoviruses and other whitefly-borne viruses: Dependence on the vector species. Phytopathology 2020, 110, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H. Whiteflies of Belize (Hemiptera: Aleyrodidae). Part 1-introduction and account of the subfamily Aleurodicinae Quaintance & Baker. Moscas blancas de Belice (Hemiptera: Aleyrodidae). Parte 1-introducción y descripción de la subfamilia Aleurodicinae Quaintance & Baker. Zootaxa 2004, 681, 1–119. [Google Scholar]
- Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef]
- Ouvrard, D.; Martin, J.H. The White-files–Taxonomic Checklist of the World’s Whiteflies (Insecta: Hemiptera: Aleyrodidae) (version Feb 2019). In Species 2000 & IT IS Catalpgue of Life, 2019 Annual Checklist; Roskov, Y., Ower, G., Orrell, T., Nicolson, D., Bailly, N., Kirk, P.M., Bourgoin, T., DeWalt, R.E., Decock, W., Nieukerken, E., et al., Eds.; Naturalis: Leiden, The Netherlands, 2000. [Google Scholar]
- Berry, S.D.; Fondong, V.N.; Rey, C.; Rogan, D.; Fauquet, C.M.; Brown, J.K. Molecular evidence for five distinct Bemisia tabaci (Homoptera: Aleyrodidae) geographic haplotypes associated with cassava plants in sub-Saharan Africa. Ann. Entomol. Soc. Am. 2004, 97, 852–859. [Google Scholar] [CrossRef]
- Brown, J.K.; Frohlich, D.E.; Rosell, R.C. The sweetpotato or silverleaf whiteflies: Biotypes of Bemisia tabaci or a species complex? Annu. Rev. Entomol. 1995, 40, 511–534. [Google Scholar] [CrossRef]
- Dinsdale, A.; Cook, L.; Riginos, C.; Buckley, Y.M.; De Barro, P. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann. Entomol. Soc. Am. 2010, 103, 196–208. [Google Scholar] [CrossRef]
- De Barro, P.J.; Liu, S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Boykin, L.M.; Armstrong, K.F.; Kubatko, L.; De Barro, P.J. Species delimitation and global biosecurity. Evol. Bioinform. 2011, 8, 1–37. [Google Scholar]
- Legg, J.P.; Shirima, R.; Tajebe, L.S.; Guastella, D.; Boniface, S.; Jeremiah, S.; Rapisarda, C. Biology and management of Bemisia whitefly vectors of cassava virus pandemics in Africa. Pest. Manag. Sci. 2014, 70, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Mugerwa, H.; Seal, S.; Wang, H.L.; Patel, M.V.; Kabaalu, R.; Omongo, C.A.; Alicai, T.; Tairo, F.; Ndunguru, J.; Sseruwagi, P.; et al. African ancestry of New World, Bemisia tabaci-whitefly species. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedford, I.D.; Pinner, M.; Liu, S.; Markham, P.G. Bemisia tabaci-Potential infestation, phytotoxicity and virus transmission within European agriculture. In Proceedings of the Brighton Crop Protection Conference, Pests and Diseases, Brighton, UK, 21–24 November 1994; Volume 2, pp. 911–916. [Google Scholar]
- Gilbertson, R.L.; Batuman, O.; Webster, C.G.; Adkins, S. Role of the insect super vectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Annu. Rev. Virol. 2015, 2, 67–93. [Google Scholar] [CrossRef]
- Compendium, C.I.S. Detailed coverage of invasive species threatening livelihoods and the environment worldwide. In Drosophila Suzukii (Spotted Wing Drosophila); CAB International: Wallingford, UK, 2020. [Google Scholar]
- CABI/EPPO. Distribution Maps of Quarantine Pests for Europe; Smith, I.M., Charles, L.M.F., Eds.; CAB International: Wallingford, UK, 1998; p. 768. [Google Scholar]
- Polston, J.E.; De Barro, P.; Boykin, L.M. Transmission specificities of plant viruses with the newly identified species of the Bemisia tabaci species complex. Pest. Manag. Sci. 2014, 70, 1547–1552. [Google Scholar] [CrossRef]
- Zerbini, F.M.; Briddon, R.W.; Idris, A.; Martin, D.P.; Moriones, E.; Navas-Castillo, J.; Rivera-Bustamante, R.; Roumagnac, P.; Varsani, A.; ICTV Report Consortium. ICTV virus taxonomy profile: Geminiviridae. J. Gen. Virol. 2017, 98, 131–133. [Google Scholar] [CrossRef]
- Ghosh, S.; Bouvaine, S.; Maruthi, M.N. Prevalence and genetic diversity of endosymbiotic bacteria infecting cassava whiteflies in Africa. BMC Microbiol. 2015, 15, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mugerwa, H.; Sseruwagi, P.; Colvin, J.; Seal, S. Is high whitefly abundance on cassava in sub-Saharan Africa driven by biological traits of a specific, cryptic Bemisia tabaci species? Insects 2021, 12, 260. [Google Scholar] [CrossRef] [PubMed]
- Mugerwa, H.; Rey, M.E.; Alicai, T.; Ateka, E.; Atuncha, H.; Ndunguru, J.; Sseruwagi, P. Genetic diversity and geographic distribution of Bemisia tabaci (Gennadius) (H emiptera: Aleyrodidae) genotypes associated with cassava in East Africa. Ecol. Evol. 2012, 2, 2749–2762. [Google Scholar] [CrossRef] [PubMed]
- Legg, J.P.; Owor, B.; Sseruwagi, P.; Ndunguru, J. Cassava mosaic virus disease in East and Central Africa: Epidemiology and management of a regional pandemic. Adv. Virus Res. 2006, 67, 355–418. [Google Scholar] [PubMed]
- Legg, J.P.; Jeremiah, S.C.; Obiero, H.M.; Maruthi, M.N.; Ndyetabula, I.; Okao-Okuja, G.; Kumar, P.L. Comparing the regional epidemiology of the cassava mosaic and cassava brown streak virus pandemics in Africa. Virus Res. 2011, 159, 161–170. [Google Scholar] [CrossRef]
- Maruthi, M.N.; Hillocks, R.J.; Mtunda, K.; Raya, M.D.; Muhanna, M.; Kiozia, H.; Rekha, A.R.; Colvin, J.; Thresh, J.M. Transmission of Cassava brown streak virus by Bemisia tabaci (Gennadius). J. Phytopathol. 2005, 153, 307–312. [Google Scholar] [CrossRef]
- Maruthi, M.N.; Jeremiah, S.C.; Mohammed, I.U.; Legg, J.P. The role of the whitefly, Bemisia tabaci (Gennadius), and farmer practices in the spread of cassava brown streak ipomoviruses. J. Phytopathol. 2017, 165, 707–717. [Google Scholar] [CrossRef] [Green Version]
- Mware, B.; Narla, R.; Amata, R.; Olubayo, F.; Songa, J.; Kyamanyua, S.; Ateka, E.M. Efficiency of cassava brown streak virus transmission by two whitefly species in coastal Kenya. J. Gen. Mol. Virol. 2009, 1, 040–045. [Google Scholar]
- Njoroge, M.K.; Kilalo, D.C.; Miano, D.W.; Mutisya, D.L. Whiteflies species distribution and abundance on cassava crop in different agro-ecological zones of Kenya. J. Entomol. Zool. Stud. 2016, 4, 258–262. [Google Scholar]
- Legg, J.P.; French, R.; Rogan, D.; Okao-Okuja, G.; Brown, J.K. A distinct Bemisia tabaci (Gennadius) (Hemiptera: Sternorrhyncha: Aleyrodidae) genotype cluster is associated with the epidemic of severe cassava mosaic virus disease in Uganda. Mol. Ecol. 2002, 11, 1219–1229. [Google Scholar] [CrossRef]
- Njoroge, M.K.; Mutisya, D.L.; Miano, D.W.; Kilalo, D.C. Whitefly species efficiency in transmitting cassava mosaic and brown streak virus diseases. Cog. Biol. 2017, 3, 1311499. [Google Scholar] [CrossRef]
- Mware, B.; Olubayo, F.; Narla, R.; Songa, J.; Amata, R.; Kyamanywa, S.; Ateka, E.M. First record of spiraling whitefly in coastal Kenya: Emergence, host range, distribution and association with cassava brown streak virus disease. Int. J. Agric. Biol. 2010, 12, 411–415. [Google Scholar]
- Khamis, F.M.; Ombura, F.L.; Ajene, I.J.; Akutse, K.S.; Subramanian, S.; Mohamed, S.A.; Ekesi, S. Mitogenomic analysis of diversity of key whitefly pests in Kenya and its implication to their sustainable management. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Omongo, C.A.; Namuddu, A.; Okao-Okuja, G.; Alicai, T.; van Brunschot, S.; Ouvrard, D.; Colvin, J. Occurrence of Bondar’s nesting whitefly, Paraleyrodes bondari (Hemiptera: Aleyrodidae), on cassava in Uganda. Rev. Bras. Entomol. 2018, 62, 257–259. [Google Scholar] [CrossRef]
- De Barro, P.J. The Bemisia tabaci species complex: Questions to guide future research. J. Integr. Agric. 2012, 11, 187–196. [Google Scholar] [CrossRef]
- Tocko-Marabena, B.K.; Silla, S.; Simiand, C.; Zinga, I.; Legg, J.; Reynaud, B.; Delatte, H. Genetic diversity of Bemisia tabaci species colonizing cassava in Central African Republic characterized by analysis of cytochrome c oxidase subunit I. PLoS ONE 2017, 12, e0182749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wosula, E.N.; Chen, W.; Fei, Z.; Legg, J.P. Unravelling the genetic diversity among cassava Bemisia tabaci whiteflies using NextRAD sequencing. Genome Biol. Evol. 2017, 9, 2958–2973. [Google Scholar] [CrossRef] [Green Version]
- Sseruwagi, P.; Legg, J.P.; Maruthi, M.N.; Colvin, J.; Rey, M.E.C.; Brown, J.K. Genetic diversity of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) populations and presence of the B biotype and a non-B biotype that can induce silverleaf symptoms in squash, in Uganda. Ann. Appl. Biol. 2005, 147, 253–265. [Google Scholar] [CrossRef]
- Tajebe, L.S.; Guastella, D.; Cavalieri, V.; Kelly, S.E.; Hunter, M.S.; Lund, O.S.; Legg, J.P.; Rapisarda, C. Diversity of symbiotic bacteria associated with Bemisia tabaci (Homoptera: Aleyrodidae) in cassava mosaic disease pandemic areas of Tanzania. Ann. Appl. Biol. 2015, 166, 297–310. [Google Scholar] [CrossRef]
- Esterhuizen, L.L.; Mabasa, K.G.; Van Heerden, S.W.; Czosnek, H.; Brown, J.K.; Van Heerden, H.; Rey, M.E. Genetic identification of members of the Bemisia tabaci cryptic species complex from South Africa reveals native and introduced haplotypes. J. Appl. Entomol. 2013, 137, 122–135. [Google Scholar] [CrossRef]
- Bickford, D.; Lohman, D.J.; Sodhi, N.S.; Ng, P.K.; Meier, R.; Winker, K.; Ingram, K.K.; Das, I. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 2007, 22, 148–155. [Google Scholar] [CrossRef]
- Costa, H.S.; Brown, J.K. Variation in biological characteristics and esterase patterns among populations of Bemisia tabaci, and the association of one population with silverleaf symptom induction. Entomol. Exp. Appl. 1991, 61, 211–219. [Google Scholar] [CrossRef]
- Ovalle, T.M.; Parsa, S.; Hernández, M.P.; Becerra Lopez-Lavalle, L.A. Reliable molecular identification of nine tropical whitefly species. Ecol. Evol. 2014, 4, 3778–3787. [Google Scholar] [CrossRef]
- Frohlich, D.R.; Torres-Jerez, I.; Bedford, I.D.; Markham, P.G.; Brown, J.K. A phylogeographical analysis of the Bemisia tabaci species complex based on mitochondrial DNA markers. Mol. Ecol. 1999, 8, 1683–1691. [Google Scholar] [CrossRef]
- Boykin, L.M.; Shatters, R.G., Jr.; Rosell, R.C.; McKenzie, C.L.; Bagnall, R.A.; De Barro, P.; Frohlich, D.R. Global relationships of Bemisia tabaci (Hemiptera: Aleyrodidae) revealed using Bayesian analysis of mitochondrial COI DNA sequences. Mol. Phylogenet. Evol. 2007, 44, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Shatters, R.G., Jr.; Powell, C.A.; Boykin, L.M.; Liansheng, H.E.; McKenzie, C.L. Improved DNA barcoding method for Bemisia tabaci and related Aleyrodidae: Development of universal and Bemisia tabaci biotype-specific mitochondrial cytochrome c oxidase I polymerase chain reaction primers. J. Econ. Entomol. 2009, 102, 750–758. [Google Scholar] [CrossRef]
- Ballard, J.W.; Whitlock, M.C. The incomplete natural history of mitochondria. Mol. Ecol. 2004, 13, 729–744. [Google Scholar] [CrossRef] [Green Version]
- White, J.A.; Kelly, S.E.; Perlman, S.J.; Hunter, M.S. Cytoplasmic incompatibility in the parasitic wasp Encarsia inaron: Disentangling the roles of Cardinium and Wolbachia symbionts. Heredity 2009, 102, 483–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folmer, O.; Hoeh, W.R.; Black, M.B.; Vrijenhoek, R.C. Conserved primers for PCR amplification of mitochondrial DNA from different invertebrate phyla. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Wosula, E.N.; Chen, W.; Amour, M.; Fei, Z.; Legg, J.P. KASP Genotyping as a Molecular Tool for Diagnosis of Cassava-Colonizing Bemisia tabaci. Insects 2020, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighing, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [Green Version]
- Sseruwagi, P.; Maruthi, M.N.; Colvin, J.; Rey, M.E.C.; Brown, J.K.; Legg, J.P. Colonization of non-cassava plant species by cassava whiteflies (Bemisia tabaci) in Uganda. Entomol. Exp. Appl. 2006, 119, 145–153. [Google Scholar] [CrossRef]
- Manani, D.M.; Ateka, E.M.; Nyanjom, S.R.; Boykin, L.M. Phylogenetic relationships among whiteflies in the Bemisia tabaci (Gennadius) species complex from major cassava growing areas in Kenya. Insects 2017, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Tajebe, L.S.; Boni, D.; Guastella, V.; Cavalieri, O.S.; Lund, C.; Rugumamu, C.; Rapisarda, C.; Legg, J.P. Abundance, diversity and geographic distribution of cassava mosaic disease pandemic-associated Bemisia tabaci in Tanzania. J. Appl. Entomol. 2015, 139, 627–637. [Google Scholar] [CrossRef]
- Chen, W.; Wosula, E.N.; Hasegawa, D.K.; Casinga, C.; Shirima, R.R.; Fiaboe, K.K.M.; Hanna, R.; Fosto, A.; Goergen, G.; Tamò, M.; et al. Genome of the African cassava whitefly Bemisia tabaci and distribution and genetic diversity of cassava-colonizing whiteflies in Africa. Insect Biochem. Mol. Biol. 2019, 110, 112–120. [Google Scholar] [CrossRef]
- Boykin, L.M.; Savill, A.; De Barro, P. Updated mtCOI reference dataset for the Bemisia tabaci species complex. F1000Research 2017, 16, 1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milenovic, M.; Wosula, E.N.; Rapisarda, C.; Legg, J.P. Impact of host plant species and whitefly species on feeding behavior of Bemisia tabaci. Front. Plant. Sci. 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Were, M.N.; Mukoye, B.; Osogo, A.K.; Mangeni, B.C.; Nyamwamu, P.A.A.; Ogemah, V.K.; Were, H.K. Occurrence and distribution of begomoviruses infecting cassava in Western Kenya. Plant 2016, 4, 108–113. [Google Scholar] [CrossRef] [Green Version]
- Osogo, A.K.; Muoma, J.; Nyamwamu, P.; Omuse, C.N.; Were, H.K. Occurrence and distribution of cassava brown streak viruses in Western Kenya. J. Agri-Food Appl. Sci. 2014, 2, 184–190. [Google Scholar]
- Campo, B.V.H.; Hyman, G.; Bellotti, A. Threats to cassava production: Known and potential geographic distribution of four key biotic constraints. Food Secur. 2011, 3, 329–345. [Google Scholar] [CrossRef]
- Gamarra, H.A.; Fuentes, S.; Morales, F.J.; Glover, R.; Malumphy, C.; Barker, I. Bemisia afer sensu lato, a vector of Sweet potato chlorotic stunt virus. Plant. Dis. 2010, 94, 510–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josephrajkumar, A.; Mohan, C.; Babu, M.; Krishna, A.; Krishnakumar, V.; Hegde, V.; Chowdappa, P. First record of the invasive Bondar’s nesting whitefly, Paraleyrodes bondari Peracchi on coconut from India. Phytoparasitica 2019, 47, 333–339. [Google Scholar] [CrossRef]
- Dickey, A.M.; Stocks, I.C.; Smith, T.; Osborne, L.; McKenzie, C.L. DNA barcode development for three recent exotic whitefly (Hemiptera: Aleyrodidae) invaders in Florida. Florida Entomol. 2015, 98, 473–478. [Google Scholar] [CrossRef] [Green Version]
- Vidya, C.V.; Sundararaj, R.; Dubey, A.K.; Bhaskar, H.; Chellappan, M.; Henna, M.K. Invasion and establishment of Bondar’s nesting whitefly, Paraleyrodes bondari Peracchi (Hemiptera: Aleyrodidae) in Indian mainland and Andaman and Nicobar Islands. Entomon 2019, 44, 149–153. [Google Scholar] [CrossRef]
- Isabirye, Z. The Distribution and Abundance of Bondar’s Nesting Whitefly (Paraleyrodes bondari) on Cassava in Busukuma Sub County, Wakiso District, Uganda. Master’s Thesis, Makerere University, Kampala, Uganda, 2021. [Google Scholar]
- Neuenschwander, P. Spiralling whitefly, Aleurodicus dispersus, a recent invader and new cassava pest. Afr. Crop. Sci. J. 1994, 2, 419–421. [Google Scholar]
- Lambkin, T.A. A host list for Aleurodicus dispersus Russell (Hemiptera: Aleyrodidae) in Australia. Aust. J. Entomol. 1999, 38, 373–376. [Google Scholar] [CrossRef]
- Kumarasinghe, N.C.; Salim, N.; Wijayarathne, W. Identification and biology of two whitefly species on cassava in Sri Lanka. J. Plant. Prot. Res. 2009, 49, 373–377. [Google Scholar] [CrossRef]



| Parameter | All B. tabaci | SSA1-SG1 | SSA1-SG3 | B. afer |
|---|---|---|---|---|
| Sample size | 56 | 17 | 31 | 10 |
| Number of haplotypes | 6 | 1 | 2 | 2 |
| Polymorphic sites (S) | 162 | 0 | 1 | 5 |
| Average no of nucleotide differences (k) | 19.24091 | 0.0000 | 0.3226 | 1.77778 |
| Nucleotide diversity (Pi) | 0.0245 | 0.0000 | 0.0004 | 0.0023 |
| Haplotype diversity (Hd) | 0.702 | 0 | 0.323 | 0.356 |
| Variance of Hd | 0.0017 | 0.000 | 0.0078 | 0.0253 |
| Standard deviation of Hd | 0.0079 | 0.000 | 0.088 | 0.159 |
| Theta per sequence | 39.1849 | - | 0.2503 | 1.7674 |
| Theta per site | 0.0499 | - | 0.0003 | 0.0023 |
| Fu’s Fs statistic | 25.868 | - | 0.864 | 3.636 |
| Tajima’s D | −1.80960 | - | 0.44525 | 0.02348 |
| p | p < 0.05 | - | p > 0.10 | p > 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munguti, F.M.; Kilalo, D.C.; Nyaboga, E.N.; Wosula, E.N.; Macharia, I.; Mwango’mbe, A.W. Distribution and Molecular Diversity of Whitefly Species Colonizing Cassava in Kenya. Insects 2021, 12, 875. https://doi.org/10.3390/insects12100875
Munguti FM, Kilalo DC, Nyaboga EN, Wosula EN, Macharia I, Mwango’mbe AW. Distribution and Molecular Diversity of Whitefly Species Colonizing Cassava in Kenya. Insects. 2021; 12(10):875. https://doi.org/10.3390/insects12100875
Chicago/Turabian StyleMunguti, Florence M., Dora C. Kilalo, Evans N. Nyaboga, Everlyne N. Wosula, Isaac Macharia, and Agnes W. Mwango’mbe. 2021. "Distribution and Molecular Diversity of Whitefly Species Colonizing Cassava in Kenya" Insects 12, no. 10: 875. https://doi.org/10.3390/insects12100875






