Bioactivity of Carlina acaulis Essential Oil and Its Main Component towards the Olive Fruit Fly, Bactrocera oleae: Ingestion Toxicity, Electrophysiological and Behavioral Insights
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oil Extraction and Isolation of Carlina Oxide
2.2. Chemical Analysis of C. acaulis EO and Carlina Oxide Purification
2.3. Olive Fruit Fly Rearing
2.4. Ingestion Toxicity Assays
2.5. Electroantennography (EAG)
2.6. Behavioral Assays
2.7. Statistical Analysis
3. Results
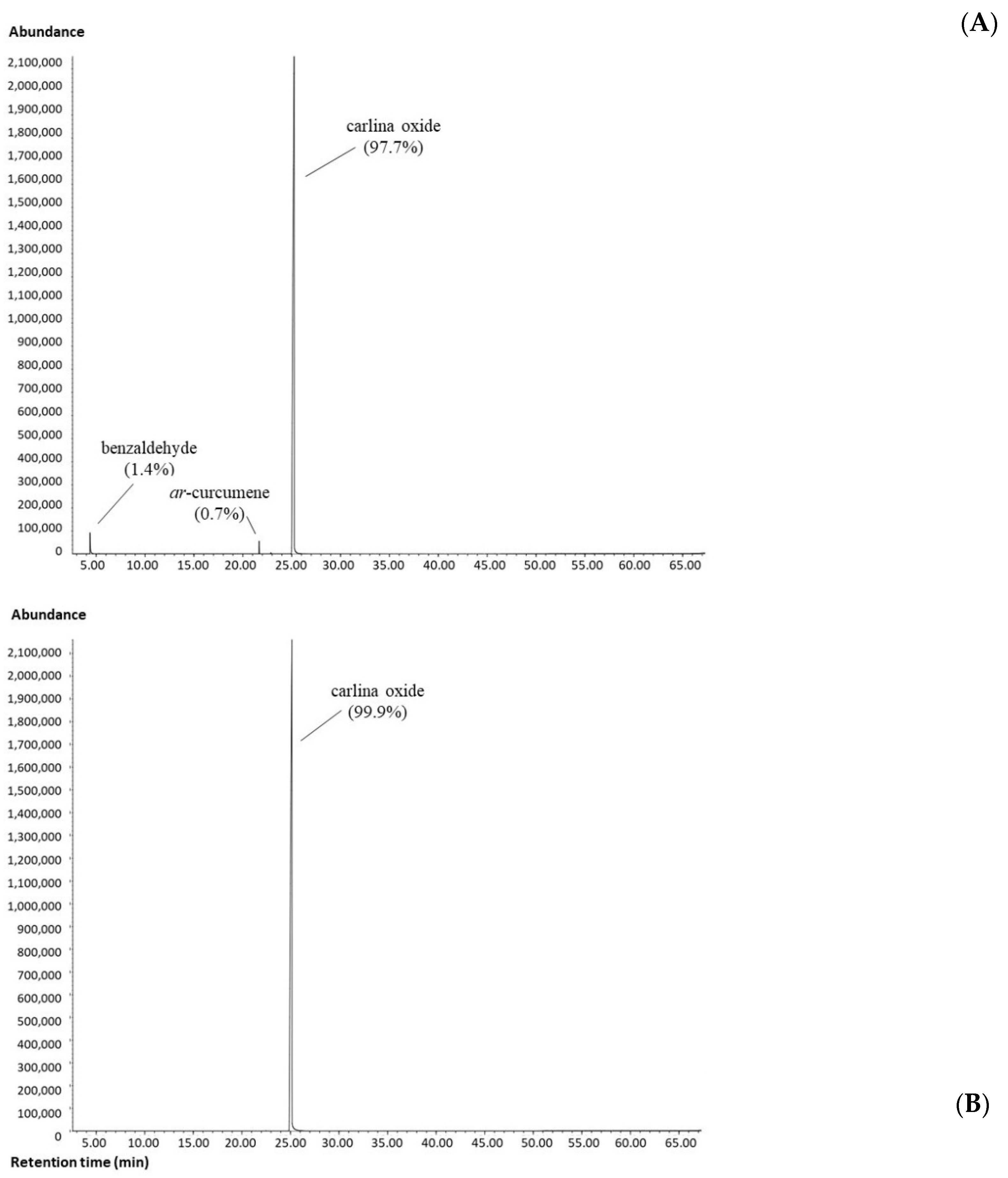
3.1. Essential Oil Chemical Analysis
3.2. Ingestion Toxicity Bioassays
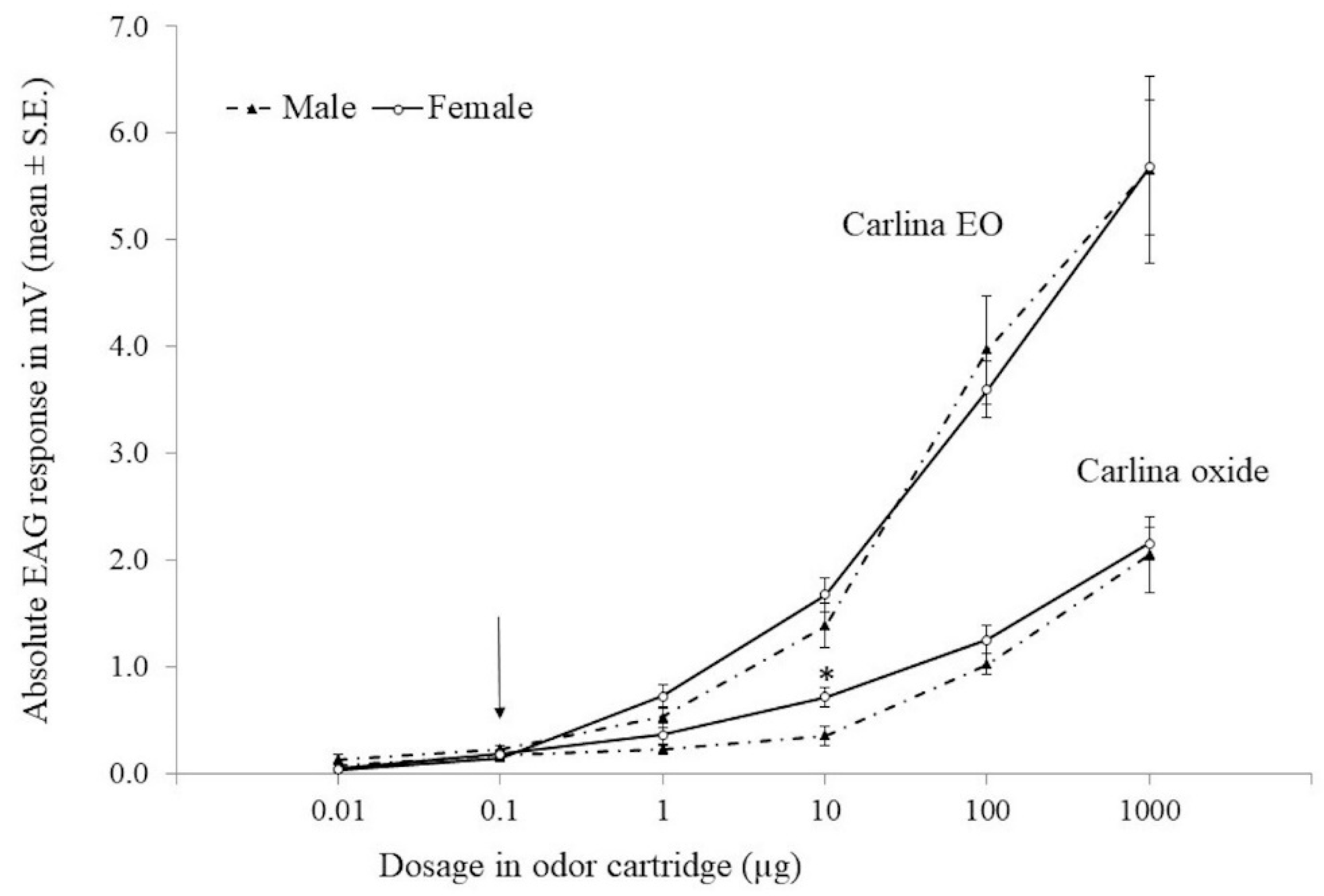
3.3. EAG Experiments
3.4. Behavioral Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Stojanović-Radić, Z.; Čomić, L.; Radulović, N.; Blagojević, P.; Mihajilov-Krstev, T.; Rajković, J. Commercial Carlinae radix herbal drug: Botanical identity, chemical composition and antimicrobial properties. Pharm. Biol. 2012, 50, 933–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Załuski, D.; Verpoorte, R. Historical and traditional medical applications of Carlina acaulis L.-A critical ethnopharmacological review. J. Ethnopharmacol. 2019, 239, 111842. [Google Scholar] [CrossRef]
- Wnorowski, A.; Wnorowska, S.; Wojas-Krawczyk, K.; Grenda, A.; Staniak, M.; Michalak, A.; Wo’zniak, S.; Matosiuk, D.; Biała, G.; Wójciak, M.; et al. Toxicity of Carlina Oxide—A Natural Polyacetylene from the Carlina acaulis Roots—In Vitro and in Vivo Study. Toxins 2020, 12, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, E.; Saukel, J. Anatomy of subterranean organs of medicinally used Cardueae and related species and its value for discrimination. Sci. Pharm. 2011, 79, 157–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negri, R. Polyacetylenes from terrestrial plants and fungi: Recent phytochemical and biological advances. Fitoterapia 2015, 106, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Wat, C.K.; Prasad, S.K.; Graham, E.A.; Partington, S.; Arnason, T.; Towers, G.H.N.; Lam, J. Photosensitization of invertebrates by natural polyacetylenes. Biochem. Syst. Ecol. 1981, 9, 59–62. [Google Scholar] [CrossRef]
- Minto, R.E.; Blacklock, B.J. Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid. Res. 2008, 47, 233–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benelli, G.; Pavela, R.; Petrelli, R.; Nzekoue, F.K.; Cappellacci, L.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Sut, S.; Dall’Acqua, S.; et al. Carlina oxide from Carlina acaulis root essential oil acts as a potent mosquito larvicide. Ind. Crop. Prod. 2019, 137, 356–366. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Petrelli, R.; Cappellacci, L.; Buccioni, M.; Palmieri, A.; Canale, A.; Benelli, G. Outstanding insecticidal activity and sublethal effects of Carlina acaulis root essential oil on the housefly, Musca domestica, with insights on its toxicity on human cells. Food Chem. Toxicol. 2020, 136, 111037. [Google Scholar] [CrossRef]
- Benelli, G.; Pavoni, L.; Zeni, V.; Ricciardi, R.; Cosci, F.; Cacopardo, G.; Gendusa, S.; Spinozzi, E.; Petrelli, R.; Cappellacci, L.; et al. Developing a Highly Stable Carlina acaulis Essential Oil Nanoemulsion for Managing Lobesia botrana. Nanomaterials 2020, 10, 1867. [Google Scholar] [CrossRef]
- Pavela, R.; Pavoni, L.; Bonacucina, G.; Cespi, M.; Cappellacci, L.; Petrelli, R.; Spinozzi, E.; Aguzzi, C.; Zeppa, L.; Ubaldi, M.; et al. Encapsulation of Carlina acaulis essential oil and carlina oxide to develop long-lasting mosquito larvicides: Microemulsions versus nanoemulsions. J. Pest Sci. 2021, 94, 819–915. [Google Scholar] [CrossRef]
- Benelli, G.; Rizzo, R.; Zeni, V.; Govigli, A.; Samková, A.; Sinacori, M.; Lo Verde, G.; Pavela, R.; Cappellacci, L.; Petrelli, R.; et al. Carlina acaulis and Trachyspermum ammi essential oils formulated in protein baits are highly toxic and reduce aggressiveness in the medfly, Ceratitis capitata. Ind. Crops Prod. 2021, 161, 113191. [Google Scholar] [CrossRef]
- Daane, K.M.; Johnson, M.W. Olive fruit fly: Managing an ancient pest in modern times. Annu. Rev. Entomol. 2010, 55, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Malheiro, R.; Casal, S.; Cunha, S.C.; Baptista, P.; Pereira, J.A. Olive volatiles from Portuguese cultivars Cobrancosa, Madural and Verdeal Transmontana: Role in oviposition preference of Bactrocera oleae (Rossi) (Diptera: Tephritidae). PLoS ONE 2015, 10, e0125070. [Google Scholar]
- Rizzo, R.; Caleca, V.; Lombardo, A. Relation of fruit color, elongation, hardness, and volume to the infestation of olive cultivars by the olive fruit fly, Bactrocera oleae. Entomol. Exp. Appl. 2012, 145, 15–22. [Google Scholar] [CrossRef]
- Tzanakakis, M.E. Insects and Mites Feeding on Olive: Distribution, Importance, Habits, Seasonal Development and Dormancy; Brill Academic: Leiden, The Netherlands, 2006. [Google Scholar]
- Gucci, R.; Caruso, G.; Canale, A.; Loni, A.; Raspi, A.; Urbani, S.; Taticchi, A.; Esposto, S.; Servili, M. Qualitative changes of olive oils obtained from fruits damaged by Bactrocera oleae (Rossi). HortScience 2012, 47, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Caleca, V.; Antista, G.; Campisi, G.; Caruso, T.; Lo Verde, G.; Maltese, M.; Rizzo, R.; Planeta, D. High quality extra virgin olive oil from olives attacked by the olive fruit fly, Bactrocera oleae (Rossi) (Diptera Tephritidae): Which is the tolerable limit? Data from experimental ‘Nocellara del Belice’ and ‘Cerasuola’ olive groves in Sicily. Chem. Eng. Trans. 2017, 58, 451–456. [Google Scholar]
- Canale, A.; Germinara, S.G.; Carpita, A.; Benelli, G.; Bonsignori, G.; Stefanini, C.; Raspi, A.; Rotundo, G. Behavioural and electrophysiological responses of the olive fruit fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae), to male- and female-borne sex attractants. Chemoecology 2013, 23, 155–164. [Google Scholar] [CrossRef]
- Rizzo, R.; Lo Verde, G.; Sinacori, M.; Maggi, F.; Cappellacci, L.; Petrelli, R.; Vittori, S.; Reza Morshedloo, M.; Yvette Fofiee, N.G.B.; Benelli, G. Developing green insecticides to manage olive fruit flies? Ingestion toxicity of four essential oils in protein baits on Bactrocera oleae. Ind. Crops Prod. 2020, 143, 111884. [Google Scholar] [CrossRef]
- Canale, A.; Benelli, G. Impact of mass-rearing on the host seeking behaviour and parasitism by the fruit fly parasitoid Psyttalia concolor (Szépligeti) (Hymenoptera: Braconidae). J. Pest Sci. 2012, 85, 65–74. [Google Scholar] [CrossRef]
- Canale, A.; Benelli, G.; Conti, B.; Lenzi, G.; Flamini, G.; Francini, A.; Cioni, P.L. Ingestion toxicity of three Lamiaceae essential oils incorporated in protein baits against the olive fruit fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae). Nat. Prod. Res. 2013, 27, 2091–2209. [Google Scholar] [CrossRef]
- Rotundo, G.R.; Germinara, S.; De Cristofaro, A.; Rama, F. Identificazione di composti volatili in estratti da diverse cultivar di Olea europaea L. biologicamente attivi su Bactrocera olae (Gmelin) (Diptera: Tephritidae). Boll. Lab. Entomol. Agrar. Filippo Silvestri 2001, 57, 25–34. [Google Scholar]
- Germinara, G.S.; De Cristofaro, A.; Rotundo, G. Antennal olfactory responses to individual cereal volatiles in Theocolax elegans (Westwood) (Hymenoptera: Pteromalidae). J. Stored Prod. Res. 2009, 45, 195–200. [Google Scholar] [CrossRef]
- Canale, A.; Benelli, G.; Germinara, G.S.; Fusini, G.; Romano, D.; Rapalini, F.; Desneux, N.; Rotundo, G.; Raspi, A.; Carpita, A. Behavioural and electrophysiological responses to overlooked female pheromone components in the olive fruit fly, Bactrocera oleae (Diptera: Tephritidae). Chemoecology 2015, 25, 147–157. [Google Scholar] [CrossRef]
- Carpita, A.; Canale, A.; Raffaelli, A.; Saba, A.; Benelli, G.; Raspi, A. (Z)-9-tricosene identified in rectal gland extracts of Bactrocera oleae males: First evidence of a male-produced female attractant in olive fruit fly. Naturwissenschaften 2012, 99, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Ngumbi, E.; Jordan, M.; Fadamiro, H. Comparison of associative learning of host-related plant volatiles in two parasitoids with different degrees of host specificity, Cotesia marginiventris and Microplitis croceipes. Chemoecology 2012, 22, 207–215. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Finney, D.J. Statistical Method in Biological Assay; Griffin: London, UK, 1978. [Google Scholar]
- Raguso, R.A.; Light, D.M. Electroantennogram responses of male Sphinx perelegans hawkmoths to floral and “green-leaf volatiles”. Entomol. Exp. Appl. 1998, 86, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Den Otter, C.J.; Tchicaya, T.; Schutte, A.M. Effects of age, sex and hunger on the antennal olfactory sensitivity of tsetse flies. Physiol. Entomol. 1991, 16, 173–182. [Google Scholar] [CrossRef]
- Germinara, G.S.; Pistillo, M.; Griffo, R.; Garonna, A.P.; Di Palma, A. Electroantennographic Responses of Aromia bungii (Faldermann, 1835) (Coleoptera, Cerambycidae) to a Range of Volatile Compounds. Insects 2019, 10, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scolari, F.; Valerio, F.; Benelli, G.; Papadopoulos, N.T.; Vaníčková, L. Tephritid fruit fly semiochemicals: Current knowledge and future perspectives. Insects 2021, 12, 408. [Google Scholar] [CrossRef]
- Arnason, T.; Swain, T.; Wat, C.K.; Graham, E.A.; Partington, S.; Towers, G.H.N.; Lam, J. Mosquito larvicidal activity of polyacetylenes from species in the Asteraceae. Biochem. Syst. Ecol. 1981, 9, 63–68. [Google Scholar] [CrossRef]
- Waksmundzka-Hajnos, M.; Sherma, J.; Kowalska, T. (Eds.) Thin Layer Chromatography in Phytochemistry; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Wink, M. Medicinal plants: A source of anti-parasitic secondary metabolites. Molecules 2012, 17, 12771–12791. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, F.; Hamoud, R.; Sporer, F.; Tahrani, A.; Wink, M. Carlina oxide—A natural polyacetylene from Carlina acaulis (Asteraceae) with potent antitrypanosomal and antimicrobial properties. Planta Med. 2011, 77, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D. Electrophysiological investigations on the olfactory specificity of sexual attracting substances in different species of moth. J. Insect Physiol. 1962, 8, 15–30. [Google Scholar] [CrossRef]
- Malheiro, R.; Ortiz, A.; Casal, S.; Baptista, P.; Pereira, J.A. Electrophysiological response of Bactrocera oleae (Rossi) (Diptera: Tephritidae) adults to olive leaves essential oils from different cultivars and olive tree volatiles. Ind. Crops Prod. 2015, 77, 81–88. [Google Scholar] [CrossRef]
- Anfora, G.; Vitagliano, S.; Germinara, G.S.; Latella, C.; Mazzoni, V.; Rotundo, G.; De Cristofaro, A. Electrophysiological and behavioural activity of plant volatile terpenes in three tephritid flies. IOBC/WPRS Bull. 2012, 74, 166. [Google Scholar]
- Strzemski, M.; Dresler, S.; Sowa, I.; Czubacka, A.; Agacka-Mołdoch, M.; Płachno, B.J.; Granica, S.; Feldo, M.; Wójciak-Kosior, M. The impact of different cultivation systems on the content of selected secondary metabolites and antioxidant activity of Carlina acaulis plant material. Molecules 2020, 25, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strzemski, M.; Dzida, K.; Dresler, S.; Sowa, I.; Kurzepa, J.; Szymczak, G.; Wójciak, M. Nitrogen fertilisation decreases the yield of bioactive compounds in Carlina acaulis L. grown in the field. Ind. Crops Prod. 2021, 170, 113698. [Google Scholar] [CrossRef]
- Benelli, G.; Ceccarelli, C.; Zeni, V.; Rizzo, R.; Lo Verde, G.; Sinacori, M.; Boukouvala, M.C.; Kavalieratos, N.G.; Ubaldi, M.; Tomassoni, D.; et al. Lethal and behavioural effects of a green insecticide against an invasive polyphagous fruit fly pest and its safety to mammals. Chemosphere 2022, 287, 132089. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, L.; Pavela, R.; Cespi, M.; Bonacucina, G.; Maggi, F.; Zeni, V.; Canale, A.; Lucchi, A.; Bruschi, F.; Benelli, G. Green micro-and nanoemulsions for managing parasites, vectors and pests. Nanomaterials 2019, 9, 1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Tested Product | LC10 (95%CI) (ppm) | LC30 (95%CI) (ppm) | LC50 (95% CI) (ppm) | LC90 (95% CI) (ppm) | Intercept ± SE | Slope ± SE | Goodness of Fit χ2 (d.f.) |
|---|---|---|---|---|---|---|---|
| C. acaulis EO | 190.823 (96.319–288.602) | 413.400 (268.900–547.482) | 706.155 (530.213–880.887) | 2613.202 (1991.810–3939.835) | 2.595 ± 0.318 | 2.255 ± 0.309 | 12.838 (7) p = 0.076 n.s. |
| Carlina oxide | 352.606 (240.896–463.244) | 672.744 (521.039–821.618) | 1052.376 (865.805–1254.674) | 3140.863 (2530.428–4178.891) | 2.638 ± 0.259 | 2.698 ± 0.271 | 7.446 (6) p = 0.282 n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, R.; Pistillo, M.; Germinara, G.S.; Lo Verde, G.; Sinacori, M.; Maggi, F.; Petrelli, R.; Spinozzi, E.; Cappellacci, L.; Zeni, V.; et al. Bioactivity of Carlina acaulis Essential Oil and Its Main Component towards the Olive Fruit Fly, Bactrocera oleae: Ingestion Toxicity, Electrophysiological and Behavioral Insights. Insects 2021, 12, 880. https://doi.org/10.3390/insects12100880
Rizzo R, Pistillo M, Germinara GS, Lo Verde G, Sinacori M, Maggi F, Petrelli R, Spinozzi E, Cappellacci L, Zeni V, et al. Bioactivity of Carlina acaulis Essential Oil and Its Main Component towards the Olive Fruit Fly, Bactrocera oleae: Ingestion Toxicity, Electrophysiological and Behavioral Insights. Insects. 2021; 12(10):880. https://doi.org/10.3390/insects12100880
Chicago/Turabian StyleRizzo, Roberto, Marco Pistillo, Giacinto Salvatore Germinara, Gabriella Lo Verde, Milko Sinacori, Filippo Maggi, Riccardo Petrelli, Eleonora Spinozzi, Loredana Cappellacci, Valeria Zeni, and et al. 2021. "Bioactivity of Carlina acaulis Essential Oil and Its Main Component towards the Olive Fruit Fly, Bactrocera oleae: Ingestion Toxicity, Electrophysiological and Behavioral Insights" Insects 12, no. 10: 880. https://doi.org/10.3390/insects12100880









