Biodiversity in and around Greenhouses: Benefits and Potential Risks for Pest Management
Abstract
:Simple Summary
Abstract
1. Introduction
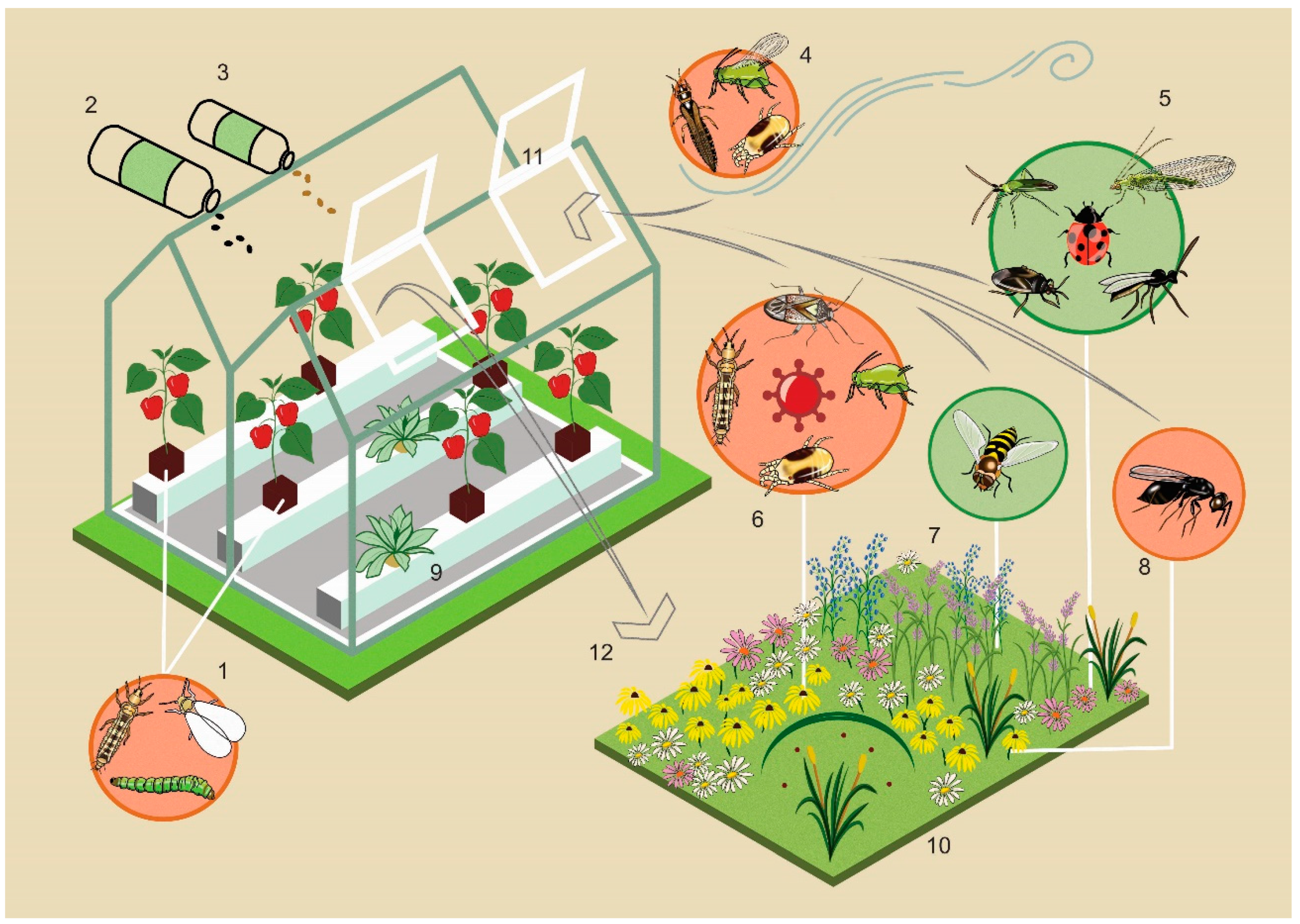
2. Where Do Pests in Greenhouses Come from?
3. Potential Risks of Plant Biodiversity around Greenhouses
4. Benefits of Biodiversity around Greenhouses
4.1. Contributing to Pest Control and Pollination inside Greenhouses
4.2. Reducing Pest Densities outside Greenhouses
4.3. Retaining Pest Species with Preferred Host Plants
5. Biodiversity in Greenhouses
6. Conclusions and Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Barbosa, P. Conservation Biological Control; Academic Press: San Diego, CA, USA, 1998; p. 396. [Google Scholar]
- Letourneau, D.K.; Jedlicka, J.A.; Bothwell, S.G.; Moreno, C.R. Effects of natural enemy biodiversity on the suppression of arthropod herbivores in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 573–592. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M.S. Habitat management to suppress pest populations: Progress and prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonsson, M.; Wratten, S.D.; Landis, D.A.; Gurr, G.M. Recent advances in conservation biological control of arthropods by arthropods. Biol. Control 2008, 45, 172–175. [Google Scholar] [CrossRef]
- Begg, G.S.; Cook, S.M.; Dye, R.; Ferrante, M.; Franck, P.; Lavigne, C.; Lovei, G.L.; Mansion-Vaquie, A.; Pell, J.K.; Petit, S.; et al. A functional overview of conservation biological control. Crop Prot. 2017, 97, 145–158. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Armbrecht, I.; Rivera, B.S.; Lerma, J.M.; Carmona, E.J.; Daza, M.C.; Escobar, S.; Galindo, V.; Gutierrez, C.; Lopez, S.D.; et al. Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl. 2011, 21, 9–21. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Luna, J.M. Multi-function agricultural biodiversity: Pest management and other benefits. Basic Appl. Ecol. 2003, 4, 107–116. [Google Scholar] [CrossRef]
- Rosenheim, J.A. Higher-order predators and the regulation of insect herbivore populations. Annu. Rev. Entomol. 1998, 43, 421–447. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, D.J.; Völkl, W. Hyperparasitism: Multitrophic ecology and behavior. Annu. Rev. Entomol. 1999, 44, 291–315. [Google Scholar] [CrossRef]
- Martin, E.A.; Dainese, M.; Clough, Y.; Baldi, A.; Bommarco, R.; Gagic, V.; Garratt, M.P.D.; Holzschuh, A.; Kleijn, D.; Kovacs-Hostyanszki, A.; et al. The interplay of landscape composition and configuration: New pathways to manage functional biodiversity and agroecosystem services across Europe. Ecol. Lett. 2019, 22, 1083–1094. [Google Scholar] [CrossRef] [Green Version]
- Chaplin-Kramer, R.; O’Rourke, M.E.; Blitzer, E.J.; Kremen, C. A meta-analysis of crop pest and natural enemy response to landscape complexity. Ecol. Lett. 2011, 14, 922–932. [Google Scholar] [CrossRef]
- Holland, J.M.; Bianchi, F.; Entling, M.H.; Moonen, A.C.; Smith, B.M.; Jeanneret, P. Structure, function and management of semi-natural habitats for conservation biological control: A review of European studies. Pest. Manag. Sci. 2016, 72, 1638–1651. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Stanghellini, C.; Van’t Ooster, B.; Heuvelink, E. Greenhouse Horticulture: Technology for Optimal Crop Production; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; p. 310. [Google Scholar]
- Kirk, W.D.J.; Terry, L.I. The spread of the western flower thrips Frankliniella occidentalis (Pergande). Agric. For. Entomol. 2003, 5, 301–310. [Google Scholar] [CrossRef]
- Cao, L.J.; Gao, Y.F.; Gong, Y.J.; Chen, J.C.; Chen, M.; Hoffmann, A.; Wei, S.J. Population analysis reveals genetic structure of an invasive agricultural thrips pest related to invasion of greenhouses and suitable climatic space. Evol. Appl. 2019, 12, 1868–1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narvaez-Vasquez, C.A.; Gonzalez-Cabrera, J.; Ruescas, D.C.; Tabone, E.; Frandon, J.; et al. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest. Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Dalmon, A.; Halkett, F.; Granier, M.; Delatte, H.; Peterschmitt, M. Genetic structure of the invasive pest Bemisia tabaci: Evidence of limited but persistent genetic differentiation in glasshouse populations. Heredity 2008, 100, 316–325. [Google Scholar] [CrossRef]
- Gullino, M.L.; Albajes, R.; Nicot, P. Integrated Pest and Disease Management in Greenhouse Crops; Springer International Publishing: New York, NY, USA, 2020; p. 691. [Google Scholar]
- Van Driesche, R.G.; Heinz, K.M. An overview of biological control in protected culture. In Biocontrol in Protected Culture; Heinz, K.M., Van Driesche, R.G., Parrella, M.P., Eds.; Ball Publishing: Batavia, IL, USA, 2004; pp. 1–25. [Google Scholar]
- Vierbergen, G. Occurrence of glasshouse Thysanoptera in the open in the Netherlands. In The 7th International Symposium on Thysanoptera, Calabria, Italy, 2–7 July 2001; Marullo, R., Mound, L., Eds.; Australian National Insect Collection: Canberra, Australia, 2002; pp. 359–362. [Google Scholar]
- Sampson, C.; Bennison, J.; Kirk, W.D.J. Overwintering of the western flower thrips in outdoor strawberry crops. J. Pest. Sci. 2021, 94, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Makra, L.; Bodnar, K.; Fulop, A.; Orosz, S.; Szenasi, A.; Csepe, Z.; Jenser, G.; Tusnady, G.; Magyar, D. The first record of subtropical insects (Thysanoptera) in central Europe: Long-distance transport of airborne thrips, applying three-dimensional backward trajectories. Agric. For. Entomol. 2018, 20, 301–326. [Google Scholar] [CrossRef]
- Lewis, T. The weather and mass flights of Thysanoptera. Ann. Appl. Biol. 1964, 53, 165–170. [Google Scholar] [CrossRef]
- Johnson, C.G. Migration and Dispersal of Insects by Flight; Methuen & Co.: London, UK, 1969. [Google Scholar]
- Jenser, G. Observations on the autumn mass flight of Frankliniella intonsa Trybom (Thysanoptera, Thripidae). Acta Phytopathol. Acad. Sci. Hung. 1973, 8, 227–230. [Google Scholar]
- Bell, J.R.; Bohan, D.A.; Shaw, E.M.; Weyman, G.S. Ballooning dispersal using silk: World fauna, phylogenies, genetics and models. Bull. Entomol. Res. 2005, 95, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Clotuche, G.; Navajas, M.; Mailleux, A.C.; Hance, T. Reaching the ball or missing the flight? Collective dispersal in the two-spotted spider mite Tetranychus urticae. PLoS ONE 2013, 8, e77573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, C.B.; Williams, I.S.; Hardie, J. The role of nutrition, crowding and interspecific interactions in the development of winged aphids. Ecol. Entomol. 2001, 26, 330–340. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, M. Population dynamics of aphid species in Korean seed potato cultivation area over four decades. Entomol. Res. 2019, 49, 179–184. [Google Scholar] [CrossRef]
- Webb, S.E.; Hochmuth, R.C. Vegetable insect identification and management. In Florida Greenhouse Vegetable Production Handbook; U.S. Department of Agriculture, UF/IFAS Extension Service, University of Florida: Gainesville, FL, USA, 2016; Volume 3, pp. 1–20. [Google Scholar]
- Knapp, M.; Palevsky, E.; Rapisarda, C. Insect and mite pests. In Integrated Pest and Disease Management in Greenhouse Crops; Gullino, M.L., Albajes, R., Nicot, P.C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 101–146. [Google Scholar]
- Rodriguez, E.; Gonzalez, M.; Paredes, D.; Campos, M.; Benitez, E. Selecting native perennial plants for ecological intensification in Mediterranean greenhouse horticulture. Bull. Entomol. Res. 2018, 108, 694–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirk, W.D.J. Pollen-feeding and the host specificity and fecundity of flower thrips (Thysanoptera). Ecol. Entomol. 1985, 10, 281–289. [Google Scholar] [CrossRef]
- Li, S.; Jaworski, C.C.; Hatt, S.; Zhang, F.; Desneux, N.; Wang, S. Flower strips adjacent to greenhouses help reduce pest populations and insecticide applications inside organic commercial greenhouses. J. Pest. Sci. 2021, 94, 679–689. [Google Scholar] [CrossRef]
- Easterbrook, M.A.; Tooley, J.A. Assessment of trap plants to regulate numbers of the European tarnished plant bug, Lygus rugulipennis, on late-season strawberries. Entomol. Exp. Appl. 1999, 92, 119–125. [Google Scholar] [CrossRef]
- Ondiaka, S.; Migiro, L.; Rur, M.; Birgersson, G.; Porcel, M.; Ramert, B.; Tasin, M. Sunflower as a trap crop for the European tarnished plant bug (Lygus rugulipennis). J. Appl. Entomol. 2016, 140, 453–461. [Google Scholar] [CrossRef]
- Winkler, K.; Waeckers, F.L.; Termorshuizen, A.J.; van Lenteren, J.C. Assessing risks and benefits of floral supplements in conservation biological control. BioControl 2010, 55, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Balzan, M.V.; Bocci, G.; Moonen, A.C. Utilisation of plant functional diversity in wildflower strips for the delivery of multiple agroecosystem services. Entomol. Exp. Appl. 2016, 158, 304–319. [Google Scholar] [CrossRef]
- Parrella, G.; Gognalons, P.; Gebre-Selassie, K.; Vovlas, C.; Marchoux, G. An update of the host range of tomato spotted wilt virus. J. Plant. Pathol. 2003, 85, 227–264. [Google Scholar]
- Stobbs, L.W.; Broadbent, A.B.; Allen, W.R.; Stirling, A.L. Transmission of Tomato Spotted Wilt Virus by the western flower thrips to weeds and native plants found in southern Ontario. Plant Dis. 1992, 76, 23–29. [Google Scholar] [CrossRef]
- Rotenberg, D.; Jacobson, A.L.; Schneweis, D.J.; Whiffleld, A.E. Thrips transmission of tospoviruses. Curr. Opin. Virol. 2015, 15, 80–89. [Google Scholar] [CrossRef]
- Ng, J.C.K.; Falk, B.W. Virus-vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Annu. Rev. Phytopathol. 2006, 44, 183–212. [Google Scholar] [CrossRef]
- Lecoq, H. Epidemiology of Cucurbit aphid-borne yellows virus. In The Luteoviridae; Smith, H.G., Barker, H., Eds.; CAB International: Wallingford, UK, 1999; pp. 243–248. [Google Scholar]
- Navas-Castillo, J.; Fiallo-Olive, E.; Sanchez-Campos, S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef]
- Velasco, L.; Simon, B.; Janssen, D.; Cenis, J.L. Incidences and progression of tomato chlorosis virus disease and tomato yellow leaf curl virus disease in tomato under different greenhouse covers in southeast Spain. Ann. Appl. Biol. 2008, 153, 335–344. [Google Scholar] [CrossRef]
- Albrecht, M.; Kleijn, D.; Williams, N.M.; Tschumi, M.; Blaauw, B.R.; Bommarco, R.; Campbell, A.J.; Dainese, M.; Drummond, F.A.; Entling, M.H.; et al. The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: A quantitative synthesis. Ecol. Lett. 2020, 23, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Gerling, D.; Alomar, O.; Arno, J. Biological control of Bemisia tabaci using predators and parasitoids. Crop. Prot. 2001, 20, 779–799. [Google Scholar] [CrossRef]
- Castañé, C.; Alomar, O.; Goula, M.; Gabarra, R. Colonization of tomato greenhouses by the predatory mirid bugs Macrolophus caliginosus and Dicyphus tamaninii. Biol. Control 2004, 30, 591–597. [Google Scholar] [CrossRef]
- Gabarra, R.; Alomar, O.; Castañé, C.; Goula, M.; Albajes, R. Movement of greenhouse whitefly and its predators between in- and outside of Mediterranean greenhouses. Agric. Ecosyst. Environ. 2004, 102, 341–348. [Google Scholar] [CrossRef]
- Ingegno, B.L.; Pansa, M.G.; Tavella, L. Tomato colonization by predatory bugs (Heteroptera: Miridae) in agroecosystems of NW Italy. IOBC WPRS Bull. 2009, 49, 287–291. [Google Scholar]
- Perdikis, D.; Fantinou, A.; Lykouressis, D. Enhancing pest control in annual crops by conservation of predatory Heteroptera. Biol. Control 2011, 59, 13–21. [Google Scholar] [CrossRef]
- Lambion, J. Functional biodiversity in southern France: A method to enhance predatory mirid bug populations. Acta Hortic. 2011, 915, 165–170. [Google Scholar] [CrossRef]
- Bosco, L.; Tavella, L. Distribution and abundance of species of the genus Orius in horticultural ecosystems of northwestern Italy. Bull. Insectol. 2013, 66, 297–307. [Google Scholar]
- Postic, E.; Le Ralec, A.; Buchard, C.; Granado, C.; Outreman, Y. Variations in community assemblages and trophic networks of aphids and parasitoids in protected crops. Ecosphere 2020, 11, e03126. [Google Scholar] [CrossRef]
- Naselli, M.; Biondi, A.; Garzia, G.T.; Desneux, N.; Russo, A.; Siscaro, G.; Zappala, L. Insights into food webs associated with the South American tomato pinworm. Pest. Manag. Sci. 2017, 73, 1352–1357. [Google Scholar] [CrossRef]
- Grosman, A.; Bloemhard, C. Nieuwe Sluipwespen Tegen Turkse Mot, Chrysodeixis Chalcites, in Paprika; GTB-1306; Wageningen University & Research: Bleiswijk, The Netherlands, 2013; p. 42. [Google Scholar]
- Abe, Y.; Takeuchi, T.; Tokumaru, S.; Kamata, J. Comparison of the suitability of three pest leafminers (Diptera: Agromyzidae) as hosts for the parasitoid Dacnusa sibirica (Hymenoptera: Bracenidae). Eur. J. Entomol. 2005, 102, 805–807. [Google Scholar] [CrossRef] [Green Version]
- Woets, J.; van der Linden, A. On the occurrence of Opius pallipes Wesmael and Dacnusa sibirica Telenga (Braconidae) in cases of natural control of the tomato leafminer Liriomyza bryoniae Kalt. (Agromyzidae) in some large greenhouses in the Netherlands. Med. Fac. Landbouw. Rijksuniv. Gent 1982, 47, 533–540. [Google Scholar]
- Chailleux, A.; Mohl, E.K.; Alves, M.T.; Messelink, G.J.; Desneux, N. Natural enemy-mediated indirect interactions among prey species: Potential for enhancing biocontrol services in agroecosystems. Pest. Manag. Sci. 2014, 70, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Zhang, Y.J.; Wu, K.M.; Wyckhuys, K.A.G.; Guo, Y.Y. Flight potential of Microplitis mediator, a parasitoid of various lepidopteran pests. BioControl 2009, 54, 183–193. [Google Scholar] [CrossRef]
- Winkler, K.; Wäckers, F.; Bukovinszkine-Kiss, G.; van Lenteren, J. Sugar resources are vital for Diadegma semiclausum fecundity under field conditions. Basic Appl. Ecol. 2006, 7, 133–140. [Google Scholar] [CrossRef]
- Heimpel, G.E. Linking parasitoid nectar feeding and dispersal in conservation biological control. Biol. Control 2019, 132, 36–41. [Google Scholar] [CrossRef]
- Araj, S.E.; Wratten, S.; Lister, A.; Buckley, H. Adding floral nectar resources to improve biological control: Potential pitfalls of the fourth trophic level. Basic Appl. Ecol. 2009, 10, 554–562. [Google Scholar] [CrossRef]
- Bloemhard, C.M.J.; van der Wielen, M.; Messelink, G.J. Seasonal abundance of aphid hyperparasitoids in organic greenhouse crops in the Netherlands. IOBC WPRS Bull. 2014, 102, 15–19. [Google Scholar]
- Dong, Z.K.; Men, X.Y.; Liu, S.; Zhang, Z.Y. Food web structure of parasitoids in greenhouses is affected by surrounding landscape at different spatial scales. Sci. Rep. 2019, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Hatt, S.; Uytenbroeck, R.; Lopes, T.; Mouchon, P.; Osawa, N.; Piqueray, J.; Monty, A.; Francis, F. Identification of flower functional traits affecting abundance of generalist predators in perennial multiple species wildflower strips. Arthropod Plant. Interact. 2019, 13, 127–137. [Google Scholar] [CrossRef]
- Martin, C.D.; Fountain, M.T.; Brown, M.J.F. Varietal and seasonal differences in the effects of commercial bumblebees on fruit quality in strawberry crops. Agric. Ecosyst. Environ. 2019, 281, 124–133. [Google Scholar] [CrossRef]
- Dunn, L.; Lequerica, M.; Reid, C.R.; Latty, T. Dual ecosystem services of syrphid flies (Diptera: Syrphidae): Pollinators and biological control agents. Pest. Manag. Sci. 2020, 76, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Jay, C.N.; Fountain, M.T.; Linka, J.; Fitzgerald, J.D. Development and validation of a model forecasting the phenology of European tarnished plant bug Lygus rugulipennis in the UK. Agric. For. Entomol. 2014, 16, 265–272. [Google Scholar] [CrossRef]
- Pansa, M.G.; Guidone, L.; Tavella, L. Distribution and abundance of nymphal parasitoids of Lygus rugulipennis and Adelphocoris lineolatus in northwestern Italy. Bull. Insectol. 2012, 65, 81–87. [Google Scholar]
- Cotes, B.; Gonzalez, M.; Benitez, E.; De Mas, E.; Clemente-Orta, G.; Campos, M.; Rodriguez, E. Spider communities and biological control in native habitats surrounding greenhouses. Insects 2018, 9, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rijn, P.C.J.; Wäckers, F.L. Nectar accessibility determines fitness, flower choice and abundance of hoverflies that provide natural pest control. J. Appl. Ecol. 2016, 53, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Cocuzza, G.E.; DeClercq, P.; VandeVeire, M.; DeCock, A.; Degheele, D.; Vacante, V. Reproduction of Orius laevigatus and Orius albidipennis on pollen and Ephestia kuehniella eggs. Entomol. Exp. Appl. 1997, 82, 101–104. [Google Scholar] [CrossRef]
- Pumariño, L.; Alomar, O.; Lundgren, J.G. Effects of floral and extrafloral resource diversity on the fitness of an omnivorous bug, Orius insidiosus. Entomol. Exp. Appl. 2012, 145, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Hulshof, J.; Ketoja, E.; Vänninen, I. Life history characteristics of Frankliniella occidentalis on cucumber leaves with and without supplemental food. Entomol. Exp. Appl. 2003, 108, 19–32. [Google Scholar] [CrossRef]
- Atakan, E.; Tunc, I. Seasonal abundance of hemipteran predators in relation to western flower thrips Frankliniella occidentalis (Thysanoptera: Thripidae) on weeds in the eastern Mediterranean region of Turkey. Biocontrol Sci. Technol. 2010, 20, 821–839. [Google Scholar] [CrossRef]
- Riudavets, J.; Castañé, C. Identification and evaluation of native predators of Frankliniella occidentalis (Thysanoptera: Thripidae) in the Mediterranean. Environ. Entomol. 1998, 27, 86–93. [Google Scholar] [CrossRef]
- Montserrat, M.; Albajes, R.; Castañé, C. Functional response of four Heteropteran predators preying on greenhouse whitefly (Homoptera: Aleyrodidae) and western flower thrips (Thysanoptera: Thripidae). Environ. Entomol. 2000, 29, 1075–1082. [Google Scholar] [CrossRef]
- Urbaneja, A.; Monton, H.; Molla, O. Suitability of the tomato borer Tuta absoluta as prey for Macrolophus pygmaeus and Nesidiocoris tenuis. J. Appl. Entomol. 2009, 133, 292–296. [Google Scholar] [CrossRef]
- Ingegno, B.L.; Bodino, N.; Leman, A.; Messelink, G.J.; Tavella, L. Predatory efficacy of Dicyphus errans on different prey. Acta Hortic. 2017, 1164, 425–430. [Google Scholar] [CrossRef]
- Messelink, G.J.; Bloemhard, C.M.J.; Hoogerbrugge, H.; van Schelt, J.; Ingegno, B.L.; Tavella, L. Evaluation of mirid predatory bugs and release strategy for aphid control in sweet pepper. J. Appl. Entomol. 2015, 139, 333–341. [Google Scholar] [CrossRef]
- Ingegno, B.L.; Goula, M.; Navone, P.; Tavella, L. Distribution and host plants of the genus Dicyphus in the Alpine valleys of NW Italy. Bull. Insectol. 2008, 61, 139–140. [Google Scholar]
- Ingegno, B.L.; Pansa, M.G.; Tavella, L. Plant preference in the zoophytophagous generalist predator Macrolophus pygmaeus (Heteroptera: Miridae). Biol. Control 2011, 58, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Ingegno, B.L.; Candian, V.; Psomadelis, I.; Bodino, N.; Tavella, L. The potential of host plants for biological control of Tuta absoluta by the predator Dicyphus errans. Bull. Entomol. Res. 2017, 107, 340–348. [Google Scholar] [CrossRef]
- Walter, D.E. Living on leaves: Mites, tomenta, and leaf domatia. Annu. Rev. Entomol. 1996, 41, 101–114. [Google Scholar] [CrossRef]
- Thierry, D.; Rat-Morris, E.; Caldumbide, C. Selective attractivity of artificial overwintering chambers for the common green lacewing species of the Chrysoperla carnea (Stephens) complex in western Europe (Neuroptera: Chrysopidae). Acta Zool. Acad. Sci. Hung. 2002, 48, 351–357. [Google Scholar]
- Xu, Q.C.; Fujiyama, S.; Xu, H.L. Pest control by enriching natural enemies under artificial habitat management along sidewalls of greenhouse in organic farming systems. J. Food Agric. Environ. 2012, 10, 449–458. [Google Scholar]
- Balzan, M.V.; Moonen, A.C. Field margin vegetation enhances biological control and crop damage suppression from multiple pests in organic tomato fields. Entomol. Exp. Appl. 2014, 150, 45–65. [Google Scholar] [CrossRef]
- Swezey, S.L.; Nieto, D.J.; Hagler, J.R.; Pickett, C.H.; Bryer, J.A.; Machtley, S.A. Dispersion, distribution, and movement of Lygus spp. (Hemiptera: Miridae) in trap-cropped organic strawberries. Environ. Entomol. 2013, 42, 770–778. [Google Scholar] [CrossRef]
- Hagler, J.R.; Nieto, D.J.; Machtley, S.A.; Swezey, S.L. Predator demographics and dispersal in alfalfa trap-cropped strawberry. Entomol. Exp. Appl. 2020, 168, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Messelink, G.J.; Bennison, J.; Alomar, O.; Ingegno, B.L.; Tavella, L.; Shipp, L.; Palevsky, E.; Wäckers, F.L. Approaches to conserving natural enemy populations in greenhouse crops: Current methods and future prospects. BioControl 2014, 59, 377–393. [Google Scholar] [CrossRef]
- Pijnakker, J.; Vangansbeke, D.; Duarte, M.; Moerkens, R.; Wäckers, F.L. Predators and parasitoids-in-first: From inundative releases to preventative biological control in greenhouse crops. Front. Sustain. Food Syst. 2020, 4, 38. [Google Scholar] [CrossRef]
- Huang, N.X.; Enkegaard, A.; Osborne, L.S.; Ramakers, P.M.J.; Messelink, G.J.; Pijnakker, J.; Murphy, G. The banker plant method in biological control. Crit. Rev. Plant Sci. 2011, 30, 259–278. [Google Scholar] [CrossRef]
- Frank, S.D. Biological control of arthropod pests using banker plant systems: Past progress and future directions. Biol. Control 2010, 52, 8–16. [Google Scholar] [CrossRef]
- Bennison, J. Biological control of aphids on cucumbers use of open rearing systems or ‘banker plants’ to aid establishment of Aphidius matricariae and Aphidoletes aphidimyza. Med. Fac. Landbouw. Rijksuniv. Gent 1992, 57, 457–466. [Google Scholar]
- Pineda, A.; Marcos-García, M.A. Use of selected flowering plants in greenhouses to enhance aphidophagous hoverfly populations (Diptera: Syrphidae). Ann. Soc. Entomol. Fr. 2008, 44, 487–492. [Google Scholar] [CrossRef]
- Waite, M.O.; Scott-Dupree, C.D.; Brownbridge, M.; Buitenhuis, R.; Murphy, G. Evaluation of seven plant species/cultivars for their suitability as banker plants for Orius insidiosus (Say). BioControl 2014, 59, 79–87. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, X.J.; Tan, X.L.; Desneux, N.; Zappala, L.; Zhang, F.; Wang, S. Using Calendula officinalis as a floral resource to enhance aphid and thrips suppression by the flower bug Orius sauteri (Hemiptera: Anthocoridae). Pest. Manag. Sci. 2017, 73, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Parolin, P.; Bresch, C.; Ruiz, G.; Desneux, N.; Poncet, C. Testing banker plants for biological control of mites on roses. Phytoparasitica 2013, 41, 249–262. [Google Scholar] [CrossRef]
- Kakimoto, K.; Inoue, H.; Yamaguchi, T.; Fukamachi, S.; Shima, K.; Taguchi, Y.; Saiki, Y.; Ohno, K. Simultaneous release of Orius strigicollis (Poppius) eggs and adults to improve its establishment in greenhouses. Jpn. J. Appl. Entomol. Zool. 2007, 51, 29–37. [Google Scholar] [CrossRef]
- Van Rijn, P.C.J.; van Houten, Y.M.; Sabelis, M.W. How plants benefit from providing food to predators even when it is also edible to herbivores. Ecology 2002, 83, 2664–2679. [Google Scholar] [CrossRef]
- Leman, A.; Messelink, G.J. Supplemental food that supports both predator and pest: A risk for biological control? Exp. Appl. Acarol. 2015, 65, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Vangansbeke, D.; Nguyen, D.T.; Audenaert, J.; Verhoeven, R.; Gobin, B.; Tirry, L.; De Clercq, P. Supplemental food for Amblyseius swirskii in the control of thrips: Feeding friend or foe? Pest. Manag. Sci. 2016, 72, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Loughner, R.; Nyrop, J.; Wentworth, K.; Sanderson, J. Effects of supplemental pollen and fibers on canopy abundance of Amblyseius swirskii. IOBC WPRS Bull. 2011, 68, 105–109. [Google Scholar]
- Bresch, C.; Carlesso, L.; Suay, R.; Van Oudenhove, L.; Touzeau, S.; Fatnassi, H.; Ottenwaelder, L.; Paris, B.; Poncet, C.; Mailleret, L.; et al. In search of artificial domatia for predatory mites. Biocontrol Sci. Technol. 2019, 29, 131–148. [Google Scholar] [CrossRef] [Green Version]
- Manandhar, R.; Wang, K.H.; Hooks, C.R.R.; Wright, M.G. Effects of strip-tilled cover cropping on the population density of thrips and predatory insects in a cucurbit agroecosystem. J. Asia Pac. Entomol. 2017, 20, 1254–1259. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Altieri, M.A. Abundance patterns of a predator, Orius tristicolor (Hemiptera, Anthocoridae), and its prey, Frankliniella occidentalis (Thysanoptera, Thripidae)—Habitat attraction in polycultures versus monocultures. Environ. Entomol. 1983, 12, 1464–1469. [Google Scholar] [CrossRef]
- Lambion, J.; van Rijn, P. Flower Strips: A tool for Pest Control in Greenhouses. Organic E-Prints Document 38705. p. 4. 2021. Available online: https://orgprints.org/id/eprint/38705/1/FlowerStrips_GreenResilient.pdf (accessed on 1 July 2021).
- Bale, J.S.; van Lenteren, J.C.; Bigler, F. Biological control and sustainable food production. Philos. Trans. R. Soc. B 2008, 363, 761–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Pest Group and Order | Common Name | Scientific Name | Origin |
|---|---|---|---|
| Thrips | Western flower thrips | Frankliniella occidentalis Pergande | Exotic, USA |
| (Thysanoptera) | Onion thrips | Trips tabaci Lindeman | Indigenous |
| Japanese flower thrips | Thrips setosus Moulton | Exotic, Japan | |
| Poinsettia thrips | Echinothrips americanus Morgan | Exotic, USA | |
| Orchid thrips | Chaetanaphothrips Orchidii (Moulton) | Exotic, tropical | |
| Vanda thrips | Dichromothrips corbetti (Priesner) | Exotic, tropical | |
| Palm thrips | Parthenothrips dracaenae (Heeger) | Exotic, tropical | |
| Banded greenhouse thrips | Hercinothrips femoralis (Reuter) | Exotic, tropical | |
| Tobacco thrips | Thrips parvispinus (Karny) | Exotic, Asia | |
| Rose thrips | Thrips fuscipennis Haliday | Indigenous | |
| European flower thrips | Frankliniella intonsa (Trybom) | Indigenous | |
| Whiteflies | Greenhouse whitefly | Trialeurodes vaporariorum (Westwood) | Exotic, South America |
| (Hemiptera) | Tobacco whitefly | Bemisia tabaci (Gennadius) | Exotic, Mediterranean |
| Aphids | Green peach aphid | Myzus persicae (Sulzer) | Indigenous |
| (Hemiptera) | Foxglove aphid | Aulacorthum solani (Kaltenbach) | Indigenous |
| Potato aphid | Macrosiphum euphorbiae (Thomas) | Indigenous | |
| Cotton aphid | Aphis gosyppii Glover | Indigenous | |
| Mealybugs | Citrus mealybug | Planacoccus citri (Risso) | Exotic, Mediterranean |
| (Hemiptera) | Long-tailed mealybug | Pseudococcus longispinus (Targioni-Tozzetti) | Exotic, Mediterranean |
| Obscure mealybug | Pseudococcus viburni (Signoret) | Exotic, South America | |
| Armoured scales | Boisduval scale | Diaspis boisduvalii Signoret | Exotic, tropical |
| (Hemiptera) | Rose scale | Aulacaspis rosae (Bouché) | Exotic, subtropical |
| Spider mites | Two-spotted spider mite | Tetranychus urticae Koch | Indigenous |
| (Trombidiformes) | |||
| Tarsonemid mites | Bulb scale mite | Steneotarsonemus laticeps (Halbert) | Exotic |
| (Trombidiformes) | Broad mite | Polyphagotarsonemus latus (Banks) | Exotic, tropical, subtropical |
| Eriophyid mites (Trombidiformes) | Tomato russet mite | Aculops lycopersici (Tryon) | Exotic, Mediterranean |
| Bugs | Nesidiocoris tenuis (Reuter) | Exotic, Mediterranean | |
| (Hemiptera) | Southern green stink bug | Nezara viridula (Linnaeus) | Exotic, Mediterranean |
| Tarnished plant bug | Lygus rugulipennis Poppius | Indigenous | |
| Common nettle bug | Liocoris tripustulatus (Fabricius) | Indigenous | |
| Caterpillars | South American tomato pinworm | Tuta absoluta (Meyrick) | Exotic, South-America |
| (Lepidoptera) | Golden twin-spot moth | Chrysodeixis chalcitis (Esper) | Exotic, Mediterranean |
| Southern European marshland pyralid | Duponchelia fovealis Zeller | Exotic, Mediterranean | |
| Cabbage leafroller | Clepsis spectrana (Treitschke) | Indigenous | |
| Carnation tortrix | Cacoecimorpha pronubana (Hübner) | Indigenous | |
| Flies and midges | Cabbage fly | Delia radicum (Linnaeus) | Indigenous |
| (Diptera) | Lyprauta | Lyprauta chacoensis (Edwards) Lyprauta cambria Chandler & Pijnakker | Exotic, tropical |
| Inside Greenhouses | Adjacent to Greenhouses | Landscape around Greenhouses |
|---|---|---|
| Releases of mass-produced natural enemies (augmentative biological control) | flowering strips and shrubs with year-round successive
flowering periods that support natural enemies (conservation biological control) * | Connection with ecological
structures like hedgerows |
| Providing supplemental food sources for natural enemies (mites, pollen, eggs) | Shrubs and plants that are hosts for non-pest herbivores, which serve as food for natural enemies | |
| Banker plants, oviposition plants for natural enemies | Shelters for overwintering of natural enemies: woody shrubs, dead wood, nest structures | |
| Strips of flowering plants |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messelink, G.J.; Lambion, J.; Janssen, A.; van Rijn, P.C.J. Biodiversity in and around Greenhouses: Benefits and Potential Risks for Pest Management. Insects 2021, 12, 933. https://doi.org/10.3390/insects12100933
Messelink GJ, Lambion J, Janssen A, van Rijn PCJ. Biodiversity in and around Greenhouses: Benefits and Potential Risks for Pest Management. Insects. 2021; 12(10):933. https://doi.org/10.3390/insects12100933
Chicago/Turabian StyleMesselink, Gerben J., Jérôme Lambion, Arne Janssen, and Paul C. J. van Rijn. 2021. "Biodiversity in and around Greenhouses: Benefits and Potential Risks for Pest Management" Insects 12, no. 10: 933. https://doi.org/10.3390/insects12100933
APA StyleMesselink, G. J., Lambion, J., Janssen, A., & van Rijn, P. C. J. (2021). Biodiversity in and around Greenhouses: Benefits and Potential Risks for Pest Management. Insects, 12(10), 933. https://doi.org/10.3390/insects12100933






