Dose-Dependent Blood-Feeding Activity and Ovarian Alterations to PM2.5 in Aedes aegypti
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Aedes aegypti Populations
2.2. Environmental Chambers and PM2.5 Generator
2.3. Blood-Feeding Activity
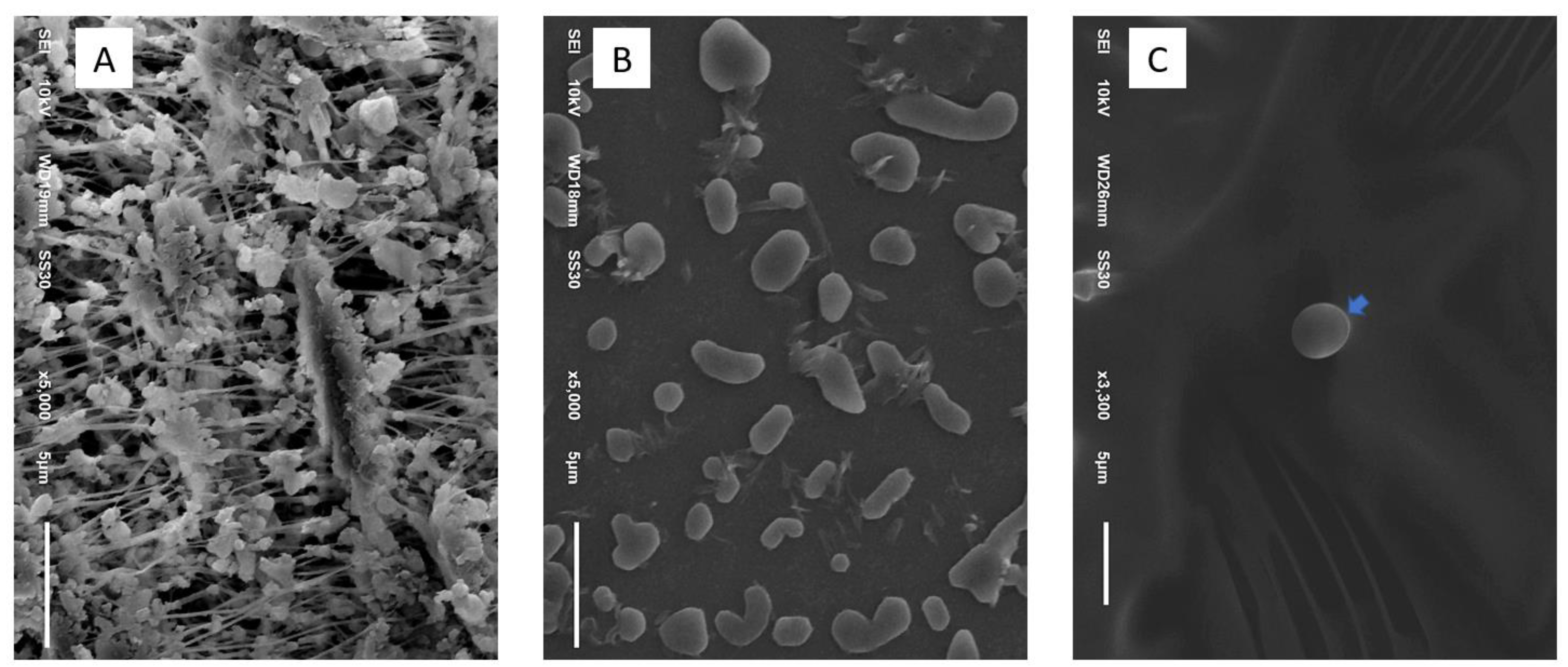
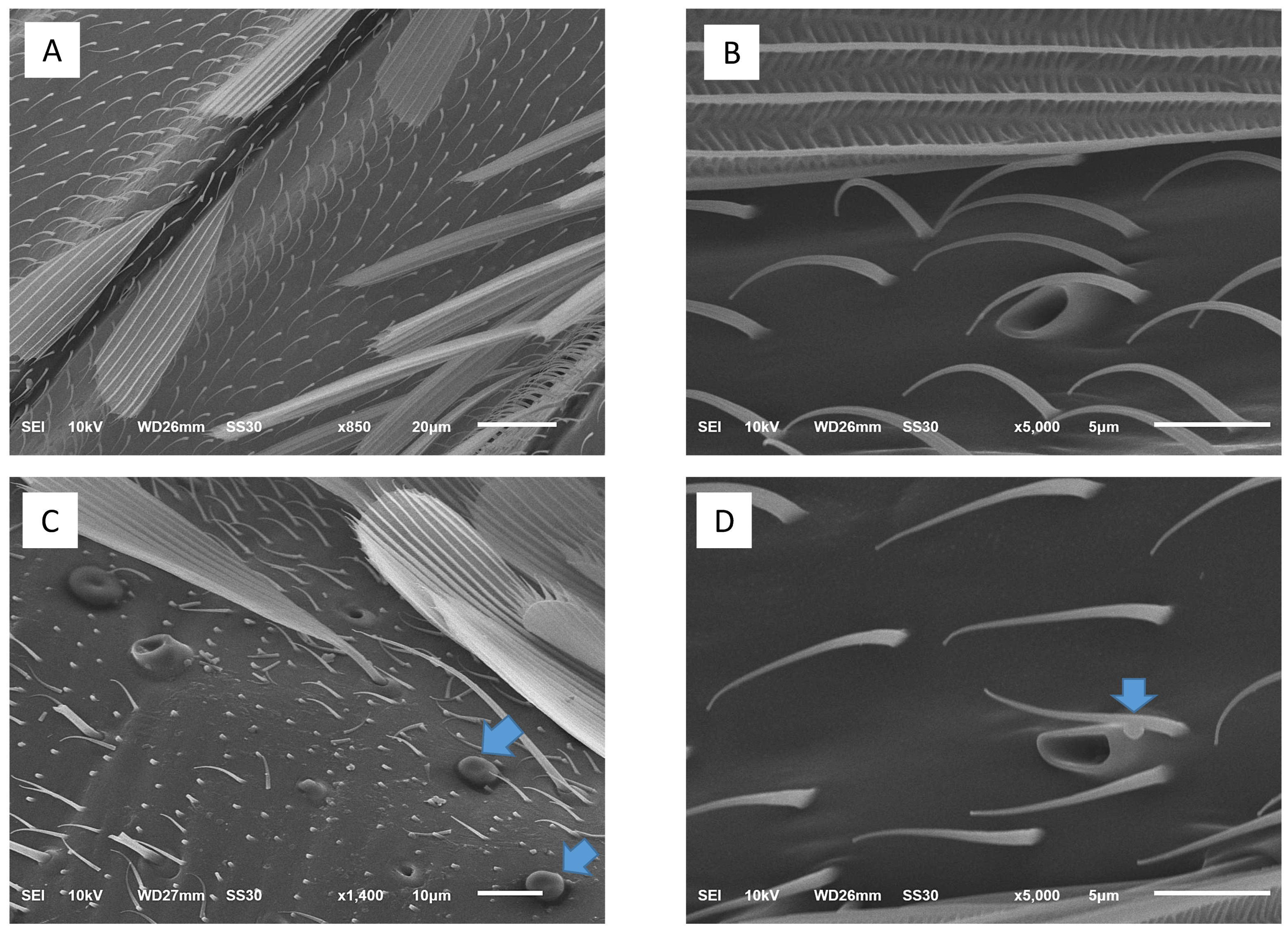
2.4. Morphological Study
2.4.1. Scanning Electron Microscopic Study
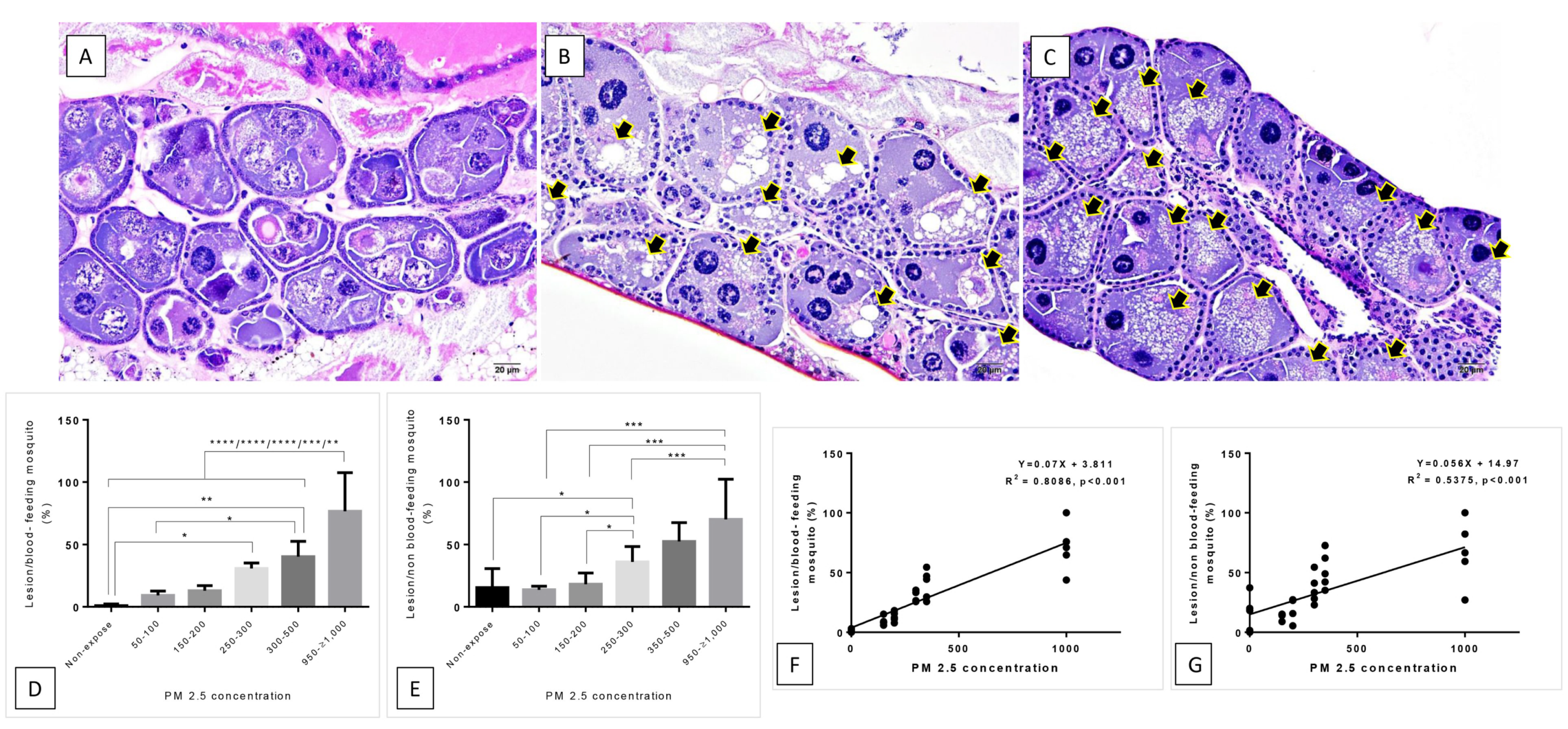
2.4.2. Histopathological Study
2.5. Statistical Analysis
3. Results
3.1. Blood-Feeding Activity
3.2. Histopathological and Morphological Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, R.; Kulkarni, M.A.; Davidson, T.M.V.; Talbot, B.; RADAM-LAC Research Team. Arbovirus vectors of epidemiological concern in the Americas: A scoping review of entomological studies on Zika, dengue and chikungunya virus vectors. PLoS ONE 2020, 15, e0220753. [Google Scholar] [CrossRef] [PubMed]
- Yuill, T.M. Overview of Arbovirus, Arenavirus, and Filovirus Infections. 2020. Available online: https://www.msdmanuals.com/professional/infectious-diseases/arboviruses,-arenaviridae,-and-filoviridae/overview-of-arbovirus,-arenavirus,-and-filovirus-infections (accessed on 16 October 2021).
- Phanitchat, T.; Zhao, B.; Haque, U.; Pientong, C.; Ekalaksananan, T.; Aromseree, S.; Thaewnongiew, K.; Fustec, B.; Bangs, M.J.; Alexander, N.; et al. Spatial and temporal patterns of dengue incidence in northeastern Thailand 2006–2016. BMC Infect. Dis. 2019, 19, 743. [Google Scholar] [CrossRef] [Green Version]
- Hopp, M.; Foley, J. Worldwide fluctuations in dengue fever cases related to climate variability. Clim. Res. 2003, 25, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Phanitchat, T.; Apiwathnasorn, C.; Sumroiphon, S.; Samung, Y.; Naksathit, A.; Thawornkuno, C.; Sungvornyothin, S. The influence of temperature on the developmental rate and survival of Aedes albopictus in Thailand. Southeast Asian J. Trop. Med. Public Health 2017, 48, 799–808. [Google Scholar]
- Wilder-Smith, A.; Earnest, A.; Tan, S.B.; Ooi, E.E.; Gubler, D.J. Lack of association of dengue activity with haze. Epidemiol. Infect. 2010, 138, 962–967. [Google Scholar] [CrossRef]
- Descloux, E.; Mangeas, M.; Menkes, C.; Lengaigne, M.; Leroy, A.; Tehei, T.; Guillaumot, L.; Teurlai, M.; Gourinat, A.-C.; Benzler, J.; et al. Climate-Based Models for Understanding and Forecasting Dengue Epidemics. PLoS Neglected Trop. Dis. 2012, 6, e1470. [Google Scholar] [CrossRef] [Green Version]
- Gould, E.A.; Higgs, S. Impact of climate change and other factors on emerging arbovirus diseases. Trans. R Soc. Trop. Med. Hyg. 2009, 103, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Sang, S.; Gu, S.; Bi, P.; Yang, W.; Yang, Z.; Xu, L.; Yang, J.; Liu, X.; Jiang, T.; Wu, H.; et al. Predicting Unprecedented Dengue Outbreak Using Imported Cases and Climatic Factors in Guangzhou, 2014. PLoS Neglected Trop. Dis. 2015, 9, e0003808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdock, C.C.; Evans, M.V.; McClanahan, T.D.; Miazgowicz, K.L.; Tesla, B. Fine-scale variation in microclimate across an urban landscape shapes variation in mosquito population dynamics and the potential of Aedes albopictus to transmit arboviral disease. PLoS Neglected Trop. Dis. 2017, 11, e0005640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, L.D. Complexity of virus-vector interactions. Curr. Opin. Virol. 2016, 21, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, D.M.; Whitmire, R.E.; Burke, D.S.; Nisalak, A.; Harrison, B.A. Effect of Temperature on the Vector Efficiency of Aedes aegypti for Dengue 2 Virus. Am. J. Trop. Med. Hyg. 1987, 36, 143–152. [Google Scholar] [CrossRef]
- Rohani, A.; Wong, Y.C.; Zamre, I.; Lee, H.L.; Zurainee, M.N. The effect of extrinsic incubation temperature on development of dengue serotype 2 and 4 viruses in Aedes aegypti (L.). Southeast Asian J. Trop. Med. Public Health 2009, 40, 942. [Google Scholar] [PubMed]
- Thongthammachart, T.; Jinsart, W. Estimating PM2.5 concentrations with statistical distribution techniques for health risk assessment in Bangkok, Human and Ecological Risk Assessment. Int. J. 2020, 26, 1848–1863. [Google Scholar] [CrossRef]
- What is Haze? Available online: https://www.earth.com/earthpedia-articles/haze/ (accessed on 16 October 2021).
- EPA. Particle Pollution and Your Patients’ Health. 2020. Available online: https://www.epa.gov/pmcourse/what-particle-pollution (accessed on 16 October 2021).
- Nussbaumer, T. Aerosols from Biomass Combustion. 2001. Available online: https://www.osti.gov/etdeweb/servlets/purl/20371223P.13 (accessed on 16 October 2021).
- Schwartz, J.; Laden, F.; Zanobetti, A. The concentration-response relation between PM2.5 and daily deaths. Environ. Health Perspect. 2002, 110, 1025–1029. [Google Scholar] [CrossRef] [Green Version]
- PM 2.5 Exposure and Health Management Measures for the Thai Population, Faculty of Engineering, Mahidol University. 2020. Available online: https://www.eg.mahidol.ac.th/egmu_eng/about/news-events/previous-news/218-pm-2-5-exposure-and-health-management-measures-for-the-thai-population (accessed on 16 October 2021).
- Sompornrattanaphan, M.; Thongngarm, T.; Tantilipikorn, P.; Kreetapirom, P.; Foo, J. The Contribution of Outdoor Fine Particulate Matter to Indoor Air Quality in Bangkok Metropolitan Region, Thailand—Are Indoor Dwellers Safe? Siriraj Med. J. 2018, 70, 265–271. [Google Scholar]
- Jiang, X.Q.; Mei, X.D.; Feng, D. Air pollution and chronic airway diseases: What should people know and do? J. Thorac. Dis. 2016, 8, E31–E40. [Google Scholar] [PubMed]
- Gavett, S.H.; Haykal-Coates, N.; Copeland, L.B.; Heinrich, J.; Gilmour, M.I. Metal composition of ambient PM2.5 influences severity of allergic airways disease in mice. Environ. Health Perspect. 2003, 111, 1471–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Xu, X.; Chu, M.; Guo, Y.; Wang, J. Air particulate matter and cardiovascular disease: The epidemiological, biomedical and clinical evidence. J. Thorac. Dis. 2016, 8, E8–E19. [Google Scholar] [CrossRef] [PubMed]
- Massad, E.; Coutinho, F.A.B.; Ma, S.; Burattini, M.N. A hypothesis for the 2007 dengue outbreak in Singapore. Epidemiol. Infect. 2009, 138, 951–957. [Google Scholar] [CrossRef]
- Narita, D.; Oanh, N.; Sato, K.; Huo, M.; Permadi, D.; Chi, N.; Ratanajaratroj, T.; Pawarmart, I. Pollution Characteristics and Policy Actions on Fine Particulate Matter in a Growing Asian Economy: The Case of Bangkok Metropolitan Region. Atmosphere 2019, 10, 227. [Google Scholar] [CrossRef] [Green Version]
- Stabile, L.; Trassierra, C.V.; Dell’Agli, G.; Buonanno, G. Ultrafine Particle Generation through Atomization Technique: The Influence of the Solution. Aerosol Air Qual. Res. 2013, 13, 1667–1677. [Google Scholar] [CrossRef] [Green Version]
- Yawootti, A.; Wimonthanasit, P.; Chaithanu, K.; Sampattagul, S. Comparison of Particulate Matter Monitoring Using Beta Attenuation Monitor and Light Scattering Method in Bangkok Thailand. ITC-CSCC 2018. In Proceedings of the 33rd International Technical Conference on Circuits/Systems, Computers and Communications, Chulalongkorn University and Mandarin Hotel, Bangkok, Thailand, 4–7 July 2018. [Google Scholar]
- Yawootti, A.; Wannachai, S.; Wimonthanasit, P.; Thinnakorn, J.; Sampattagul, S. Development of PM Monitor by Light Scattering Principle and Comparison with Standard Device. In Proceedings of the 42nd Electrical Engineering Conference (EECON42), Nakhonratchasima, Thailand, 31 October–1 November 2019. [Google Scholar]
- Yawootti, A.; Wiriya, W.; Chantara, S.; Wannachai, S.; Wimonthanasit, P. A Comparison of Light-Scattering PM2.5 Monitor between Low-Cost and Standard Sensor. In Proceedings of the 43rd Electrical Engineering Conference (EECON-43), Phitsanulok, Thailand, 28–30 October 2020. [Google Scholar]
- Timinao, L.; Vinit, R.; Katusele, M.; Schofield, L.; Burkot, T.R.; Karl, S. Optimization of the feeding rate of Anopheles farauti s.s. colony mosquitoes in direct membrane feeding assays. Parasites Vectors 2021, 14, 1–9. [Google Scholar] [CrossRef]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: New York, NY, USA, 2015. [Google Scholar]
- Hurvich, C.M.; Tsai, C.L. Regression and time series model selection in small samples. Biometrika 1989, 76, 297–307. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multi model Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Lehane, M.J. Biology of Blood-Sucking Insects, 2nd ed.; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Jung, J.W.; Baeck, S.-J.; Perumalsamy, H.; Hansson, B.S.; Ahn, Y.-J.; Kwon, H.W. A novel olfactory pathway is essential for fast and efficient blood-feeding in mosquitoes. Sci. Rep. 2015, 5, srep13444. [Google Scholar] [CrossRef] [PubMed]
- Valzania, L.; Mattee, M.T.; Strand, M.R.; Brown, M.R. Blood feeding activates the vitellogenic stage of oogenesis in the mosquito Aedes aegypti through inhibition of glycogen synthase kinase 3 by the insulin and TOR pathways. Dev. Biol. 2019, 454, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.; Suesdek, L. Effects of Wolbachia on ovarian apoptosis in Culex quinquefasciatus (Say, 1823) during the previtellogenic and vitellogenic periods. Parasites Vectors 2017, 10, 398. [Google Scholar] [CrossRef] [Green Version]
- Department, P.C. Thailand’s Air Quality and Situation Reports. 2021. Available online: http://air4thai.pcd.go.th/webV2/index.php (accessed on 16 October 2021).
- Yao, L.; Lu, N.; Yue, X.; Du, J.; Yang, C. Comparison of Hourly PM2.5 Observations Between Urban and Suburban Areas in Beijing, China. Int. J. Environ. Res. Public Health 2015, 12, 12264–12276. [Google Scholar] [CrossRef] [Green Version]
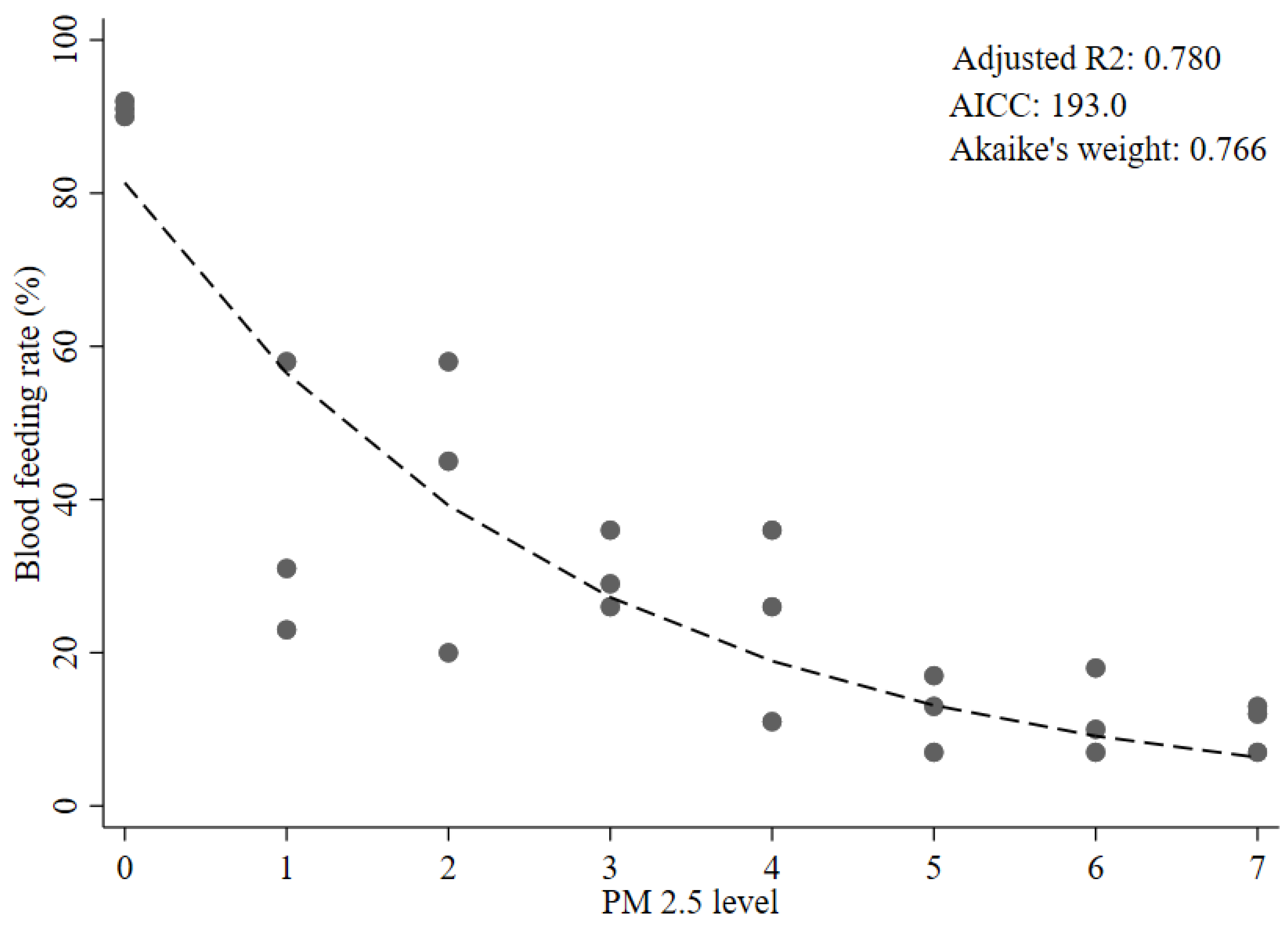


| Conc. of PM2.5 (µg/m3) | PM2.5 Level Category | Total Number of Mosquitoes | Blood-Feeding Rate, %, Mean ± SD (Range) |
|---|---|---|---|
| a 0–5 | 0 | 100 | 91.0 ± 1.00 (90, 92) |
| 50–100 | 1 | 100 | 37.3 ± 18.3 (23, 58) |
| 150–200 | 2 | 100 | 41.0 ± 19.3 (20, 58) |
| 250–300 | 3 | 100 | 30.3 ± 5.1 (26, 36) |
| 350–500 | 4 | 100 | 24.3 ± 12.6 (11, 36) |
| 550–700 | 5 | 100 | 12.3 ± 5.0 (7, 17) |
| 750–900 | 6 | 100 | 11.7 ± 5.7 (7, 18) |
| 950–≥1000 | 7 | 100 | 10.7 ± 3.2 (7, 13) |
| PM2.5 Level Category | β | SE | p-Value | Adjusted R2 | AICc | a ΔAICc | Akaike’s Weight [26] | b Evidence Ratio | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Linear | ||||||||||
| b Model 1 | B0 | 64.9 | 0.642 | 203.4 | 10.4 | 0.004 | 191.5 | |||
| X1 | −9.32 | 1.43 | <0.0001 | |||||||
| Piecewise linear | ||||||||||
| c Model 2 (2 segments) | B0 | 81.3 | 0.755 | 196.3 | 3.3 | 0.147 | 5.21 | |||
| X1 | 0–1 | −24.5 | 4.68 | <0.0001 | ||||||
| X2 | 2–7 | −4.89 | 1.78 | 0.012 | ||||||
| d Model 3 (3 segments) | B0 | 81.0 | 0.747 | 198.9 | 5.9 | 0.042 | 18.2 | |||
| X1 | 0–1 | −23.5 | 5.06 | <0.0001 | ||||||
| X2 | 2–4 | −6.32 | 3.22 | 0.064 | ||||||
| X3 | 5–7 | −2.34 | 5.06 | 0.649 | ||||||
| Non-linear exponential decay | ||||||||||
| e Model 4 | B0 | 81.3 | 6.46 | <0.0001 | 0.780 | 193.0 | Ref. | 0.766 | Ref. | |
| B1 | −0.365 | 0.0509 | <0.0001 | |||||||
| Restricted cubic spline | ||||||||||
| f Model 5 | B0 | 76.5 | 0.729 | 198.8 | 5.8 | 0.042 | 18.2 | |||
| Spline 1 | −17.7 | 3.19 | <0.0001 | |||||||
| Spline 2 | 11.2 | 3.91 | 0.010 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phanitchat, T.; Ampawong, S.; Yawootti, A.; Denpetkul, T.; Wadmanee, N.; Sompornrattanaphan, M.; Sivakorn, C. Dose-Dependent Blood-Feeding Activity and Ovarian Alterations to PM2.5 in Aedes aegypti. Insects 2021, 12, 948. https://doi.org/10.3390/insects12100948
Phanitchat T, Ampawong S, Yawootti A, Denpetkul T, Wadmanee N, Sompornrattanaphan M, Sivakorn C. Dose-Dependent Blood-Feeding Activity and Ovarian Alterations to PM2.5 in Aedes aegypti. Insects. 2021; 12(10):948. https://doi.org/10.3390/insects12100948
Chicago/Turabian StylePhanitchat, Thipruethai, Sumate Ampawong, Artit Yawootti, Thammanitchpol Denpetkul, Napid Wadmanee, Mongkhon Sompornrattanaphan, and Chaisith Sivakorn. 2021. "Dose-Dependent Blood-Feeding Activity and Ovarian Alterations to PM2.5 in Aedes aegypti" Insects 12, no. 10: 948. https://doi.org/10.3390/insects12100948
APA StylePhanitchat, T., Ampawong, S., Yawootti, A., Denpetkul, T., Wadmanee, N., Sompornrattanaphan, M., & Sivakorn, C. (2021). Dose-Dependent Blood-Feeding Activity and Ovarian Alterations to PM2.5 in Aedes aegypti. Insects, 12(10), 948. https://doi.org/10.3390/insects12100948






