Comparative Analysis of Eight Mitogenomes of Bark Beetles and Their Phylogenetic Implications
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and DNA Extraction
2.2. Mitogenome De Novo Sequencing and Assembly
2.3. Comparative Mitochontrial Genome Analysis
2.4. Genetic Distance and Selection Pressure Analysis
2.5. Phylogenetic Analysis
3. Results
3.1. Characteristics of the Mitochondrial Genome
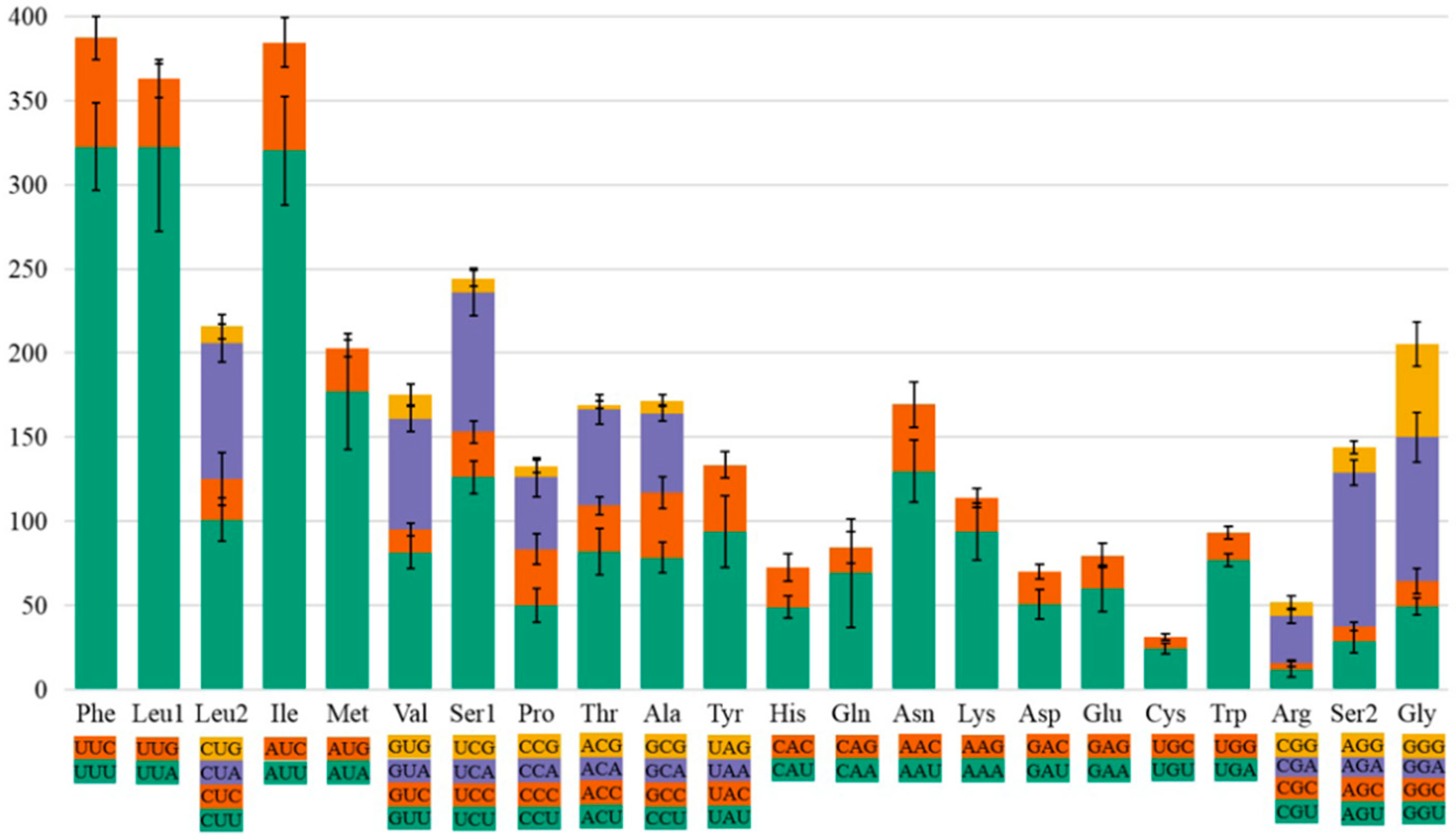
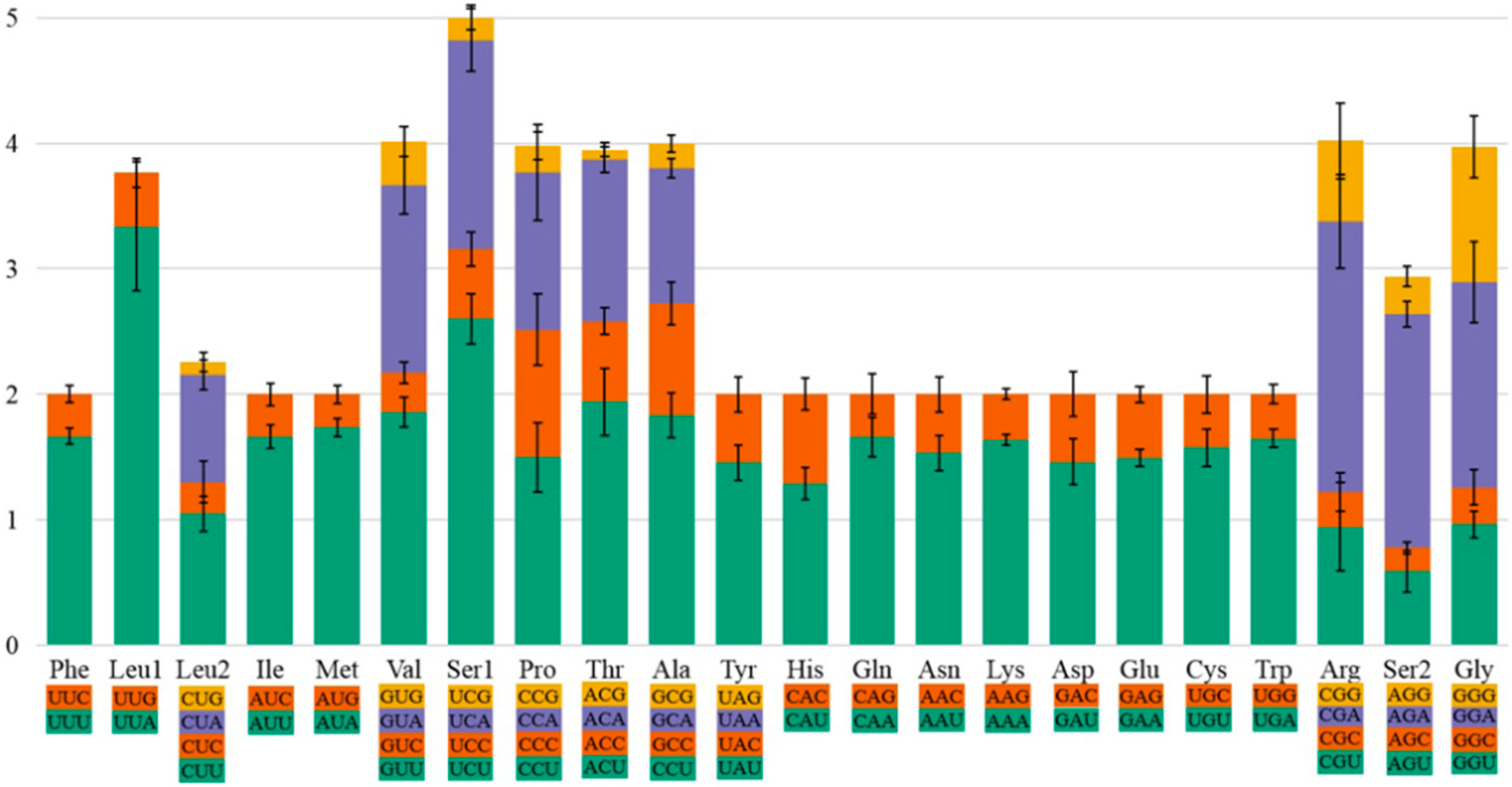
3.2. Nucleotide Composition and Condon Use
3.3. Comparison of Codon Usage in Protein-Coding Genes and tRNA Secondary Structure
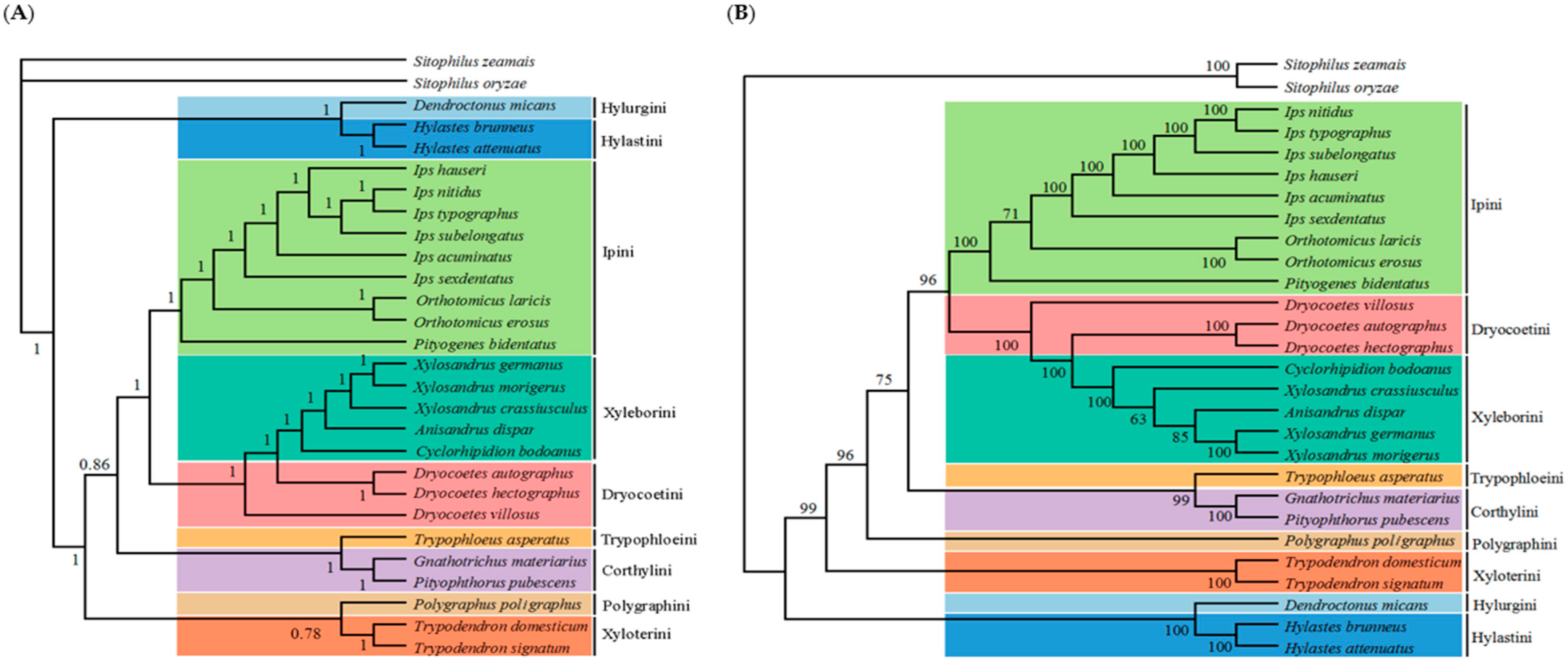
3.4. Phylogenetic Analysis
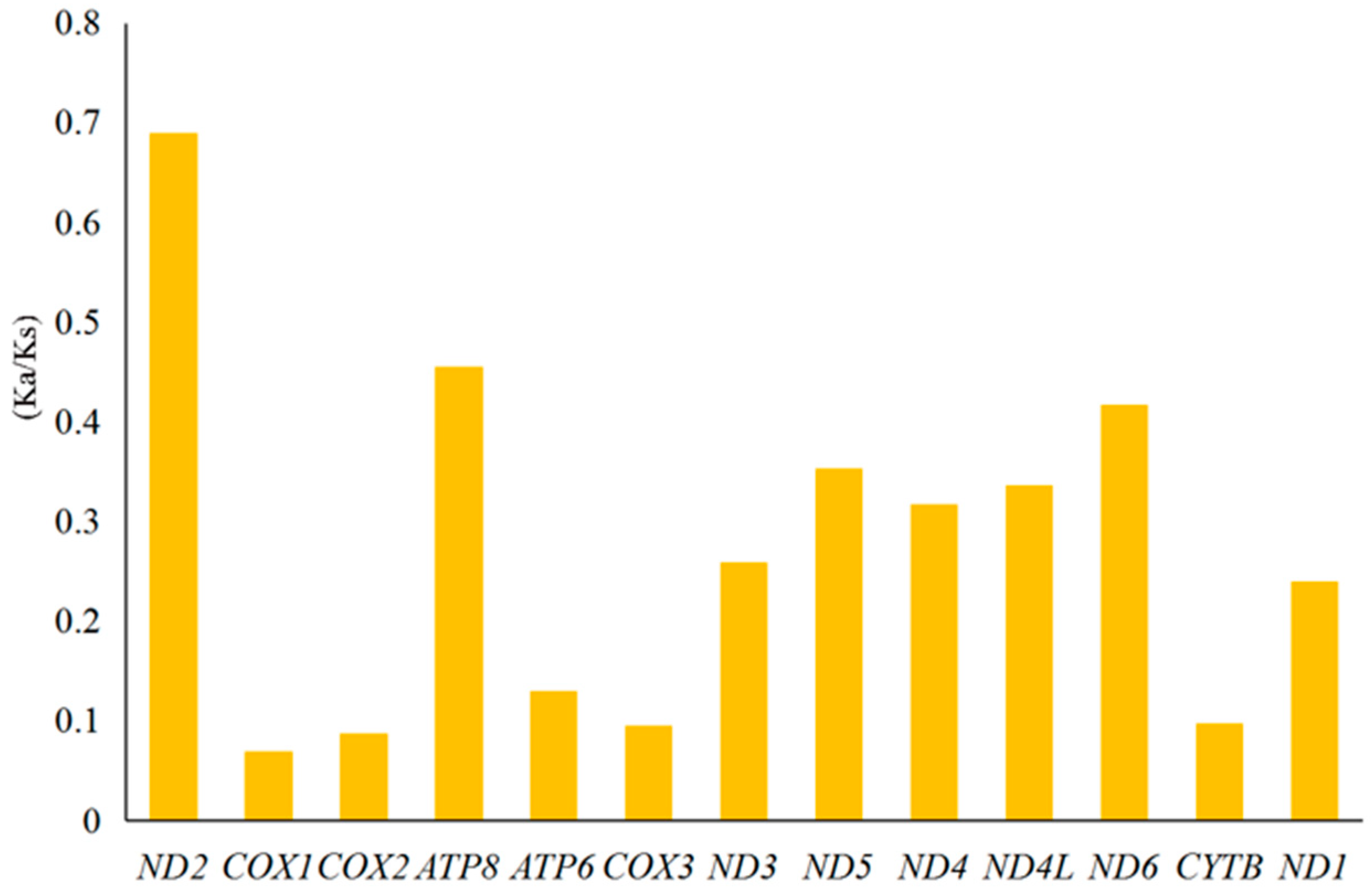
3.5. Analysis of the Genetic Distance and the Evolutionary Rate
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, J.X.; Liu, M.; Zhang, S.F.; Liu, F.; Zhang, Z.; Zhang, Q.H.; Kong, X.B. Chemical signal interactions of the bark beetle with fungal symbionts, and host/non–host trees. J. Exp. Bot. 2020, 71, 6084–6091. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, S.F.; Liu, F.; Xu, F.Y.; Zhang, F.B.; Guo, X.B.; Zhang, Z.; Kong, X.B. SEM analysis of sensilla on the mouthparts and antennae of Asian larch bark beetle Ips Subelongatus. Micron 2020, 140, 102976. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Hulcr, J.; Knížek, M.; Atkinson, T.H.; Mandelshtam, M.Y.; Smith, S.M.; Cognato, A.I.; Park, S.; Li, Y.; Jordal, B.H. Revision of the Bark Beetle Genera Within the Former Cryphalini (Curculionidae: Scolytinae). Insect Syst. Diver. 2020, 4, 1–81. [Google Scholar] [CrossRef]
- Hughes, M.A.; Riggins, J.J.; Koch, F.H.; Cognato, A.I.; Anderson, C.; Formby, J.P.; Dreaden, T.J.; Ploetz, R.C.; Smith, J.A. No rest for the laurels: Symbiotic invaders cause unprecedented damage to southern USA forests. Biol. Invasions 2017, 19, 2143–2157. [Google Scholar] [CrossRef]
- Inward, D.J.G. Three new species of ambrosia beetles established in Great Britain illustrate unresolved risks from imported wood. J. Pest Sci. 2020, 93, 117–126. [Google Scholar] [CrossRef]
- Biedermann, P.H.W.; Müller, J.; Grégoire, J.C.; Gruppe, A.; Hagge, J.; Hammerbacher, A.; Hofstetter, R.W.; Kandasamy, D.; Kolarik, M.; Kostovcik, M.; et al. Bark beetle population dynamics in the Anthropocene: Challenges and solution. Trends Ecol. Evol. 2019, 34, 914–924. [Google Scholar] [CrossRef] [Green Version]
- Lv, F.; Yang, W.Y.; Chen, Z.T.; Xu, Q.; Zhou, Y.J.; Du, Y.Z. Three partial mitochondrial genomes from Ips (Coleoptera: Cruculionidae, Scolytinae) contribute to the phylogeny of Scolytinae. J. Asia-Pac. Entomol. 2017, 20, 1007–1013. [Google Scholar]
- Avtzis, D.N.; Lakatos, F.; Gallego, D.; Pernek, M.; Faccoli, M.; Wegensteiner, R.; Stauffer, C. Shallow Genetic Structure among the European Populations of the Six-Toothed Bark Beetle Ips sexdentatus (Coleoptera, Curculionidae, Scolytinae). Forests 2019, 10, 136. [Google Scholar] [CrossRef] [Green Version]
- Pistone, D.; Gohli, J.; Jordal, B.H. Molecular phylogeny of bark and ambrosia beetles (Curculionidae: Scolytinae) based on 18 molecular markers. Syst. Entomol. 2017, 43, 387–406. [Google Scholar] [CrossRef]
- Jordal, B.H.; Kaidel, J. Phylogenetic analysis of Micracidini bark beetles (Coleoptera: Curculionidae) demonstrates a single trans-Atlantic disjunction and inclusion of Cactopinus in the New World clade. Can. Entomol. 2016, 149, 8–25. [Google Scholar] [CrossRef]
- Li, X.Y.; Yan, L.P.; Pape, T.; Gao, Y.Y.; Zhang, D. Evolutionary insight into bot flies (Insecta: Diptera: Oestridae) from comparative analysis of the mitochondrial genomes. Int. J. Biol. Macromol. 2020, 149, 371–380. [Google Scholar] [CrossRef]
- Song, F.; Li, H.; Liu, G.H.; Wang, W.; James, P.; Colwell, D.D.; Tran, A.; Gong, S.; Cai, W.; Shao, R. Mitochondrial genome fragmentation unites the parasitic lice of Eutherian mammals. Syst. Biol. 2019, 68, 430–440. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Song, F.; Jiang, P.; Wilson, J.J.; Cai, W.; Li, H. Compositional heterogeneity in true bug mitochondrial phylogenomics. Mol. Phylogenet. Evol. 2018, 118, 135–144. [Google Scholar] [CrossRef]
- Du, H.C.; Wang, Y.; Fang, J.X.; Zhang, Z.Y.; Zhang, S.F.; Liu, F.; Zhang, Z.; Kong, X.B. Sequencing and analysis of the complete mitochondrial genome of Dendrolimus punctatus (Lepidoptera: Lasiocampidae). Sci. Silvae Sin. 2019, 12, 162–172. [Google Scholar]
- Du, H.C.; Liu, M.; Zhang, S.F.; Liu, F.; Zhang, Z.; Kong, X.B. Lineage divergence of Dendrolimus punctatus in Southern China based on mitochondrial genome. Front. Genet. 2020, 11, 65. [Google Scholar] [CrossRef] [Green Version]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef] [Green Version]
- Curole, J.P.; Kocher, T.D. Mitogenomics: Digging deeper with complete mitochondrial genomes. Trends Ecol. Evol. 1999, 14, 394–398. [Google Scholar] [CrossRef]
- Du, Z.Y.; Hasegawa, H.; Cooley, J.R.; Simon, C.; Yoshimura, J.; Cai, W.Z.; Li, H. Mitochondrial genomics reveals shared phylogeographic patterns and demographic history among three periodical cicada species groups. Mol. Biol. Evol. 2019, 36, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Siva, C.; Ali, S.; Sahoo, P.K.; Nath, R.; Laskar, M.A.; Sarma, D. The complete mitochondrial genome of the medicinal fish, Cyprinion semiplotum: Insight into its structural features and phylogenetic implications. Int. J. Biol. Macromol. 2020, 164, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Y.Q.; Wu, Y.F.; Song, F.; Cai, W.Z.; Li, H. Novel tRNA gene rearrangements in the mitochondrial genome of Camarochiloides weiweii (Hemiptera: Pachynomidae). Int. J. Biol. Macromol. 2020, 165, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, W.; Ma, Z.X.; Zhou, C.F. Novel gene rearrangement pattern in the mitochondrial genomes of Torleya mikhaili and Cincticostella fusca (Ephemeroptera: Ephemerellidae). Int. J. Biol. Macromol. 2020, 165, 3106–3114. [Google Scholar] [CrossRef]
- Cognato, A.I.; Sperling, F.A.H. Phylogeny of Ips DeGeer species (Coleoptera: Scolytidae) inferred from mitochondrial cytochrome oxidase I DNA sequence. Mol. Phylogenet. Evol. 2000, 14, 445–460. [Google Scholar] [CrossRef]
- Ramirez–Rios, V.; Franco–Sierra, N.D.; Alvarez, J.C.; Saldamando–Benjumea, C.I.; Villanueva–Mejia, D.F. Mitochondrial genome characterization of Tecia solanivora (Lepidoptera: Gelechiidae) and its phylogenetic relationship with other lepidopteran insects. Gene 2016, 581, 107–116. [Google Scholar] [CrossRef]
- Huang, F.S.; Lu, J. The Classification Outline of Scolytidae from China; Tongji University Press: Shanghai, China, 2015. [Google Scholar]
- Dmitry, A.; Anton, K.; McLean, J.S.; Pevzner, P.A. HYBRIDSPADES: An algorithm for hybrid assembly of short and long reads. Bioinformatics 2016, 7, 1009–1015. [Google Scholar]
- Meng, G.; Li, Y.; Yang, C.; Liu, S. MitoZ: A toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 2019, 47, e63. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Chan, P.P. tRNAscan–SE On–line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, 54–57. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer–Mata, A.; Sánchez–DelBarrio, J.C.; Guirao–Rico, S.; Librado, P.; Ramos–Onsins, S.E.; Sánchez–Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Ojo, J.A.; Valero, M.C.; Sun, W.; Coates, B.S.; Omoloye, A.A.; Pittendrigh, B.R. Comparision of full mitochondrial genomes for the rice weevil, Sitophilus oryzae and the maize weevil, Sitophilus zeamais (Coleoptera: Curculionidae). Agri Gene 2016, 2, 29–37. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clary, D.O.; Wolstenholme, D.R. The ribosomal RNA genes of Drosophila mitochondrial DNA. Nucleic Acids Res. 1985, 13, 4029–4045. [Google Scholar] [CrossRef] [Green Version]
- Huelsenbeck, J.P.; Ronquist, F.; Nielsen, R.; Bollback, J.P. Bayesian inference of phylogeny and its impact on evolutionary biology. Science 2001, 294, 2310–2314. [Google Scholar] [CrossRef] [Green Version]
- Carmelo, A.; Paula, A.; Benjamin, L.; Robin, K.; Ladislav, B.; Alfried, P. The mitochondrial genome of iberobaenia (Coleoptera: Iberobaeniidae): First rearrangement of protein-coding genes in the beetles. Mitochondrial DNA Part A 2016, 28, 156–158. [Google Scholar] [CrossRef]
- Boyce, T.M.; Zwick, M.E.; Aquadro, C.F. Mitochondrial DNA in the bark weevils: Size, structure and heteroplasmy. Genetics 1989, 123, 825–836. [Google Scholar] [CrossRef]
- Zhang, F.; Hong, B.; Wang, Y.Z.; Li, Y.M.; Chen, Z.J. Sequencing and phylogenetic analysis of the comple mitochondrial genome of Scythropus yasumatsui (Coleoptera: Curculionidae). Acta Entomol. Sin. 2019, 62, 1305–1314. [Google Scholar]
- Timmermans, M.J.T.N.; Vogler, A.P. Phylogenetically informative rearrangements in mitochondrial genomes of Coleoptera, and monophyly of aquatic elateriform beetles (Dryopoidea). Mol. Phylogenet. Evol. 2013, 63, 229–304. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.; Ye, W.T.; Dai, M.; Lu, Z.T.; Li, M.X.; Fang, Y.L.; Qu, J.W.; Su, W.J.; Li, F.C.; Sun, H.N.; et al. Phylogenetic relationships of Limacodidae and insights into the higher phylogeny of Lepidoptera. Int. J. Biol. Macromol. 2020, 159, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.P.; Zheng, F.Y.; Bai, J.; Wang, J.M.; Lv, C.Y.; Li, X.; Zhi, Y.C.; Li, X.J. Comparative analysis of mitogenomes among six species of grasshoppers (Orthoptera: Acridoidea: Catantopidae) and their phylogenetic implications in wing–type evolution. Int. J. Biol. Macromol. 2020, 159, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Foerstner, K.U.; Mering, C.V.; Hooper, S.D.; Bork, P. Environments shape the nucleotide composition of genomes. EMBO Rep. 2005, 6, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Lu, G.; Bork, P.; Hu, S.; Lercher, M.J. Energy effificiency trade–offs drive nucleotide usage in transcribed regions. Nat. Commun. 2016, 7, 11334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, E.P.C.; Danchin, A. Base composition bias might result from competition for metabolic resources. Trends Genet. 2002, 18, 291–294. [Google Scholar] [CrossRef]
- Hassanin, A.; Leger, N.; Deutsch, J. Evidence for multiple reversals of asymmetric mutational constraints during the evolution of the mitochondrial genome of metazoa, and consequences for phylogenetic inferences. Syst. Biol. 2005, 54, 277–298. [Google Scholar] [CrossRef]
- Bogenhagen, D.F.; Clayton, D.A. The mitochondrial DNA replication bubble has not burst. Trends Biochem. Sci. 2003, 28, 357–360. [Google Scholar] [CrossRef]
- Brown, T.A.; Cecconi, C.; Tkachuk, A.N.; Bustamante, C.; Clayton, D.A. Replication of mitochondrial DNA occurs by strand displacement with alternative light—strand origins, not via a strand—coupled mechanism. Gene. Dev. 2005, 19, 2466–2476. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Luo, C.B.; Li, Y.Q.; Yang, Y.J. Mitochondrial genome characteristics and phylogenetic analysis of the Curculionidae. J. Environ. Entomol. 2019, 41, 129–1310. [Google Scholar]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; Bruijn, M.H.L.D.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, Z. The complete mitochondrial genome sequence of the cutlassfish Trichiurus japonicus (Perciformes: Trichiuridae): Genome characterization and phylogenetic considerations. Mar. Genom. 2009, 2, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, Y.; Nishida, M. Sequence evolution of mitochondrial tRNA genes and deep–branch animal phylogenetics. J. Mol. Evol. 1993, 37, 380–398. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Q.; Ma, C.; Chen, J.Y.; Yang, D.R. The complete mitochondrial genomes of two ghost moths, Thitarodes renzhiensis and Thitarodes yunnanensis: The ancestral gene arrangement in Lepidoptera. BMC Genom. 2012, 13, 276. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.L.; Sun, X.Y.; Chen, M.; Gai, Y.H.; Hao, J.S.; Yang, Q. Complete mitochondrial genome of the five–dot sergeant Parathyma sulpitia (Nymphalidae: Limenitidinae) and its phylogenetic implications. Zool. Res. 2012, 33, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokobori, S.; Paabo, S. Transfer RNA editing in land snail mitochondria. Proc. Natl. Acad. Sci. USA 1995, 92, 10432–10435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordal, B.H.; Kambestad, M. DNA barcoding of bark and ambrosia beetles reveals excessive NUMTs and consistent east-west divergence across Palearctic forests. Mol. Ecol. Resour. 2014, 14, 7–17. [Google Scholar] [CrossRef]
- Dole, S.A.; Jordal, B.H.; Cognato, A.I. Polyphyly of Xylosandrus Reitter inferred from nuclear and mitochondrial genes (Coleoptera: Curculionidae: Scolytinae). Mol. Phylogenet. Evol. 2010, 54, 773–782. [Google Scholar] [CrossRef]
- Cognato, A.I.; Smith, S.M.; Beaver, R.A. Two new genera of Oriental xyleborine ambrosia beetles (Coleoptera, Curculionidae: Scolytinae). Zootaxa 2020, 6, 540–554. [Google Scholar] [CrossRef]
- Smith, S.M.; Beaver, R.A.; Cognato, A.I. A monograph of the Xyleborini (Coleoptera, Curculionidae, Scolytinae) of the Indochinese Peninsula (except Malaysia) and China. ZooKeys 2020, 983, 1–442. [Google Scholar] [CrossRef]
- Skelton, J.; Johnson, A.J.; Jusino, M.A.; Bateman, C.C.; Li, Y.; Hulcr, J. A selective fungal transport organ (mycangium) maintains coarse phylogenetic congruence between fungus-farming ambrosia beetles and their symbionts. Proc. R. Soc. B 2019, 286, 20182127. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; McKenna, D.D.; Jordal, B.H.; Cognato, A.I.; Smith, S.M.; Lemmon, A.R.; Lemmon, E.M.; Hulcr, J. Phylogenomics clarifies repeated evolutionary origins of in breeding and fungus farming in bark beetles (Curculionidar, Scolytinae). Mol. Phylogent. Evol. 2018, 127, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Dole, S.A.; Cognato, A.I. Revision of Xylosandrus Reitter (Curculionidae: Scolytinae). Proc. Calif. Sci. 2010, 61, 451–545. [Google Scholar]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; Waard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.; Hao, D.J.; Xiao, R.T.; Liu, Y.; Qian, L.; An, Y.L.; Yang, X.J. DNA barcoding based on the mitochondrial COI gene sequences for Ips species (Coleoptera: Scolytinae). Acta Entomol. Sin. 2012, 55, 1075–1081. [Google Scholar]

| Species | Location (District, Province) | Latitude (°) | Longitude (°) | GenBank Numbers |
|---|---|---|---|---|
| Dendroctonus micans | Maixiu, Qinghai | 35.27 | 101.91 | MZ768861 |
| Polygraphus poligraphus | Qilian, Qinghai | 38.18 | 100.32 | OK110248 |
| Dryocoetes hectographus | Qilian, Qinghai | 38.18 | 100.32 | MZ766132 |
| Orthotomicus erosus | Yuxi, Yunnan | 24.13 | 102.10 | MZ823388 |
| Ips typographus | Habahe, Xinjiang | 48.47 | 86.68 | MZ766131 |
| Ips subelongatus | Yichun, Heilongjiang | 48.65 | 126.63 | MZ766130 |
| Ips hauseri | Tianshan, Xinjiang | 43.18 | 82.85 | MZ768860 |
| Ips nitidus | Maixiu, Qinghai | 35.26 | 101.89 | MZ748471 |
| Species | Amino Acid Acceptor Arm | DHU Arm | TΨC Arm | Anticodon Arm | Sum |
|---|---|---|---|---|---|
| Ips nitidus | 13 | 7 | 1 | 3 | 24 |
| Ips subelongatus | 13 | 7 | 2 | 1 | 23 |
| Ips hauseri | 15 | 7 | 3 | 3 | 28 |
| Ips typographus | 10 | 8 | 1 | 3 | 22 |
| Orthotomicus erosus | 5 | 4 | 2 | 3 | 14 |
| Dryocoetes hectographus | 9 | 6 | 1 | 5 | 21 |
| Polygraphus poligraphus | 10 | 4 | 4 | 4 | 22 |
| Dendroctonus micans | 6 | 4 | 1 | 5 | 12 |
| Mean | 10 | 6 | 2 | 3 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Fang, J.; Shi, X.; Zhang, S.; Liu, F.; Yu, C.; Zhang, Z.; Kong, X. Comparative Analysis of Eight Mitogenomes of Bark Beetles and Their Phylogenetic Implications. Insects 2021, 12, 949. https://doi.org/10.3390/insects12100949
Du H, Fang J, Shi X, Zhang S, Liu F, Yu C, Zhang Z, Kong X. Comparative Analysis of Eight Mitogenomes of Bark Beetles and Their Phylogenetic Implications. Insects. 2021; 12(10):949. https://doi.org/10.3390/insects12100949
Chicago/Turabian StyleDu, Huicong, Jiaxing Fang, Xia Shi, Sufang Zhang, Fu Liu, Chunmei Yu, Zhen Zhang, and Xiangbo Kong. 2021. "Comparative Analysis of Eight Mitogenomes of Bark Beetles and Their Phylogenetic Implications" Insects 12, no. 10: 949. https://doi.org/10.3390/insects12100949
APA StyleDu, H., Fang, J., Shi, X., Zhang, S., Liu, F., Yu, C., Zhang, Z., & Kong, X. (2021). Comparative Analysis of Eight Mitogenomes of Bark Beetles and Their Phylogenetic Implications. Insects, 12(10), 949. https://doi.org/10.3390/insects12100949







