NO Synthesis in Immune-Challenged Locust Hemocytes and Potential Signaling to the CNS
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing of Locusta Migratoria
2.2. Bacterial Growth and Infection
2.3. Inhibition of Nitric Oxide Synthase Activity
2.4. Injection of Diverse Immune Stumuli
2.5. Dissection of First Instar Abdomen
2.6. NADPH Diaphorase Activity Staining
2.7. RVFV Immunofluorescence
2.8. Extraction of Adult Circulating Hemocytes
2.9. Citrulline Immuofluorescence
2.10. Evaluation of Citrulline-Positive Hemocytes and Abdomens
2.11. Pre-Adsorption Control for Anti-Citrulline Antibody
2.12. Embryo Dissection
2.13. cGMP Immuostaining and Assessment
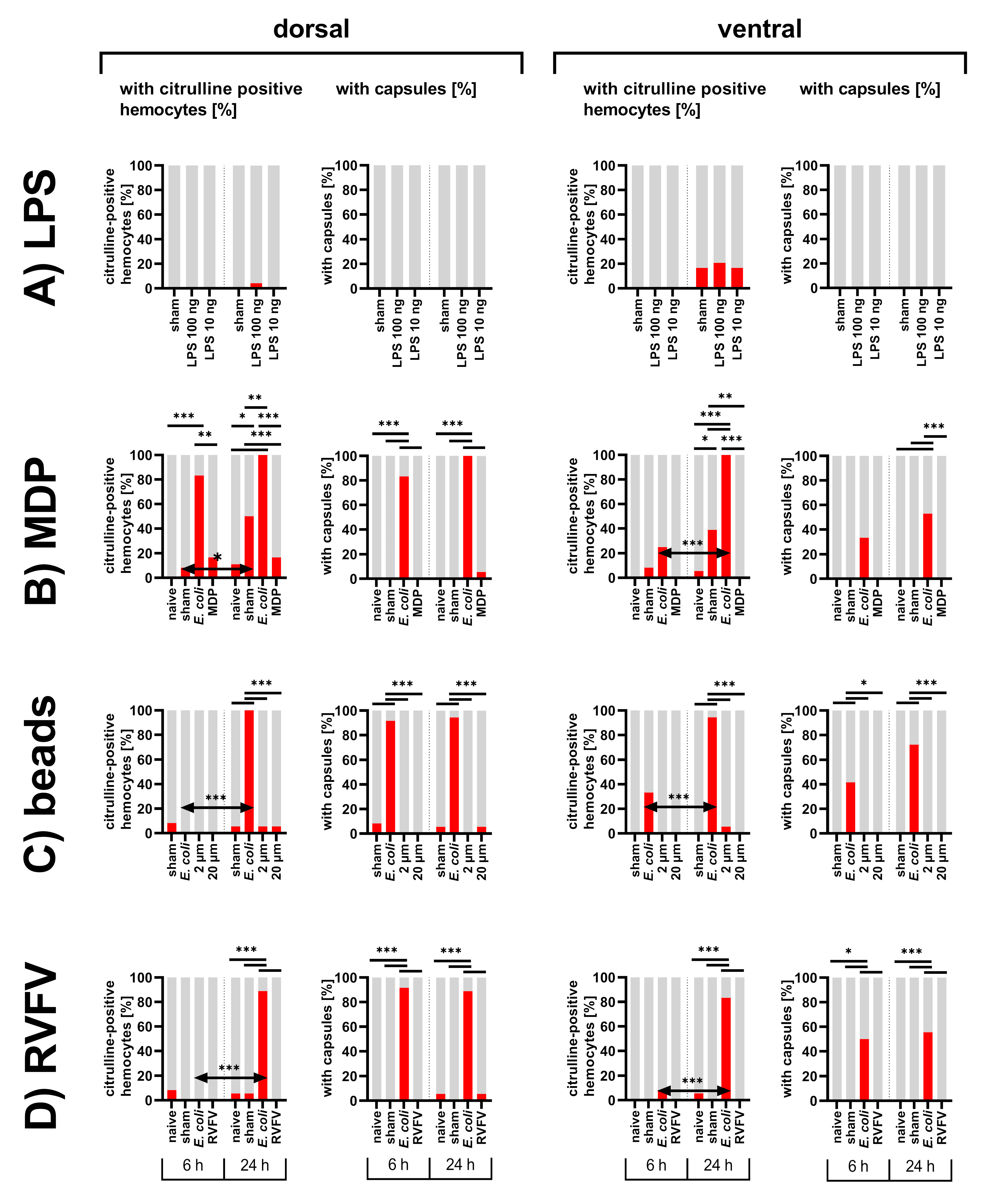
3. Results
3.1. Abdomens of First Instar Locusts
3.1.1. Inhibition of NOS
3.1.2. Bacterial Immune Challenge
3.1.3. Diverse Immune Stimuli
3.1.4. Distribution of Particles in the Abdomen of First Instar Locusts
3.2. Hemocyte Primary Culture of Adult Locusts
3.3. cGMP-Positive Axons in Locust Embryos
4. Discussion
4.1. Localization of Nitric Oxide Synthesis in Response to an Immune Stimulus
4.2. Responses to Different Immune Stimuli
4.3. NO/cGMP Signaling between Immune and Nervous System
4.4. Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edwards, J.S.; Swales, L.S.; Bate, M. The differentiation between neuroglia and connective tissue sheath in insect ganglia revisited: The neural lamella and perineurial sheath cells are absent in a mesodermless mutant of Drosophila. J. Comp. Neurol. 1993, 333, 301–308. [Google Scholar] [CrossRef] [PubMed]
- League, G.P.; Hillyer, J.F. Functional integration of the circulatory, immune, and respiratory systems in mosquito larvae: Pathogen killing in the hemocyte-rich tracheal tufts. BMC Biol. 2016, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- King, J.G.; Hillyer, J.F. Infection-Induced Interaction between the Mosquito Circulatory and Immune Systems. PLoS Pathog. 2012, 8, e1003058. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Hillyer, J.F. The immune and circulatory systems are functionally integrated across insect evolution. Sci. Adv. 2020, 6, eabb3164. [Google Scholar] [CrossRef]
- Hillyer, J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016, 58, 102–118. [Google Scholar] [CrossRef]
- Schmidt, O.; Theopold, U.; Strand, M. Innate immunity and its evasion and suppression by hymenopteran endoparasitoids. BioEssays 2001, 23, 344–351. [Google Scholar] [CrossRef]
- Müller, U.; Vogel, P.; Alber, G.; Schaub, G.A. The innate immune system of mammals and insects. Contrib. Microbiol. 2008, 15, 21–44. [Google Scholar] [CrossRef]
- Leulier, F.; Parquet, C.; Pili-Floury, S.; Ryu, J.-H.; Caroff, M.; Lee, W.-J.; Mengin-Lecreulx, D.; Lemaitre, B. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nat. Immunol. 2003, 4, 478–484. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, M.; Liu, X.; Xia, H.; Chen, K. Peptidoglycan recognition proteins in insect immunity. Mol. Immunol. 2019, 106, 69–76. [Google Scholar] [CrossRef]
- Tsakas, S.; Marmaras, V.J. Insect immunity and its signalling: An overview. Invertebr. Surviv. J. 2010, 7, 228–238. [Google Scholar]
- Lemaitre, B.; Reichhart, J.M.; Hoffmann, J.A. Drosophila host defense: Differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc. Natl. Acad. Sci. USA 1997, 94, 14614–14619. [Google Scholar] [CrossRef] [PubMed]
- Valanne, S.; Wang, J.-H.; Rämet, M. The Drosophila Toll signaling pathway. J. Immunol. 2011, 186, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Jan, E.; Sarnow, P.; Schneider, D. The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS ONE 2009, 4, e7436. [Google Scholar] [CrossRef] [PubMed]
- Myllymäki, H.; Valanne, S.; Rämet, M. The Drosophila imd signaling pathway. J. Immunol. 2014, 192, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B.; Kromer-Metzger, E.; Michaut, L.; Nicolas, E.; Meister, M.; Georgel, P.; Reichhart, J.M.; Hoffmann, J.A. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc. Natl. Acad. Sci. USA 1995, 92, 9465–9469. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Bischoff, V.; Kellenberger, C.; Hetru, C.; Royet, J.; Roussel, A. Crystal structure of Drosophila PGRP-SD suggests binding to DAP-type but not lysine-type peptidoglycan. Mol. Immunol. 2008, 45, 2521–2530. [Google Scholar] [CrossRef]
- Kaneko, T.; Goldman, W.E.; Mellroth, P.; Steiner, H.; Fukase, K.; Kusumoto, S.; Harley, W.; Fox, A.; Golenbock, D.; Silverman, N. Monomeric and Polymeric Gram-Negative Peptidoglycan but Not Purified LPS Stimulate the Drosophila IMD Pathway. Immunity 2004, 20, 637–649. [Google Scholar] [CrossRef]
- Nappi, A.J.; Vass, E.; Frey, F.; Carton, Y. Nitric oxide involvement in Drosophila immunity. Nitric Oxide 2000, 4, 423–430. [Google Scholar] [CrossRef]
- Carton, Y.; Nappi, A.J. Drosophila cellular immunity against parasitoids. Parasitol. Today 1997, 13, 218–227. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Heryanto, C.; Bassal, T.; Zhang, W.; Tettamanti, G.; Mohamed, A. Haemocyte-mediated immunity in insects: Cells, processes and associated components in the fight against pathogens and parasites. Immunology 2021, 3, 1–32. [Google Scholar] [CrossRef]
- King, J.G.; Hillyer, J.F. Spatial and temporal in vivo analysis of circulating and sessile immune cells in mosquitoes: Hemocyte mitosis following infection. BMC Biol. 2013, 11, 55. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Strand, M.R. Mosquito hemocyte-mediated immune responses. Curr. Opin. Insect Sci. 2014, 3, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.R.; Pech, L.L. Immunological basis for compatibility in parasitoid-host relationships. Annu. Rev. Entomol. 1995, 40, 31–56. [Google Scholar] [CrossRef]
- Dubovskiy, I.M.; Kryukova, N.A.; Glupov, V.V.; Ratcliffe, N.A. Encapsulation and nodulation in insects. Invertebr. Surviv. J. 2016, 13, 229–246. [Google Scholar] [CrossRef]
- Mullen, L.M.; Goldsworthy, G.J. Immune responses of locusts to challenge with the pathogenic fungus Metarhizium or high doses of laminarin. J. Insect Physiol. 2006, 52, 389–398. [Google Scholar] [CrossRef]
- Schmidt, O.; Söderhäll, K.; Theopold, U.; Faye, I. Role of adhesion in arthropod immune recognition. Annu. Rev. Entomol. 2010, 55, 485–504. [Google Scholar] [CrossRef]
- Abokersh, M.; Barakat, E. Identification of haemocytes in the haemolymph of desert locust, Schistocerca gregaria and their activity against Bacillus thuringiensis israelensis. J. Egypt. Acad. Soc. Environ. Dev. D Environ. Stud. 2020, 21, 61–72. [Google Scholar] [CrossRef]
- Schmit, A.R.; Ratcliffe, N.A. The encapsulation of foreign tissue implants in Galleria mellonella larvae. J. Insect Physiol. 1977, 23, 175–184. [Google Scholar] [CrossRef]
- Goldsworthy, G.; Chandrakant, S.; Opoku-Ware, K. Adipokinetic hormone enhances nodule formation and phenoloxidase activation in adult locusts injected with bacterial lipopolysaccharide. J. Insect Physiol. 2003, 49, 795–803. [Google Scholar] [CrossRef]
- Rivero, A. Nitric oxide: An antiparasitic molecule of invertebrates. Trends Parasitol. 2006, 22, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, G.P.T.; Friedrich, V.L.; Holstein, G.R. L-Citrulline immunostaining identifies nitric oxide production sites within neurons. Neuroscience 2002, 114, 111–122. [Google Scholar] [CrossRef]
- Faraldo, A.C.; Sá-Nunes, A.; Del Bel, E.A.; Faccioli, L.H.; Lello, E. Nitric oxide production in blowfly hemolymph after yeast inoculation. Nitric Oxide 2005, 13, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F.; Estévez-Lao, T.Y. Nitric oxide is an essential component of the hemocyte-mediated mosquito immune response against bacteria. Dev. Comp. Immunol. 2010, 34, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Burgner, D.; Rockett, K.; Kwiatkowski, D. Nitric oxide and infectious diseases. Arch. Dis. Child. 1999, 81, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Estévez-Lao, T.Y.; Sigle, L.T.; Gomez, S.N.; Hillyer, J.F. Nitric oxide produced by periostial hemocytes modulates the bacterial infection-induced reduction of the mosquito heart rate. J. Exp. Biol. 2020, 223, jeb225821. [Google Scholar] [CrossRef]
- Eleftherianos, I.; More, K.; Spivack, S.; Paulin, E.; Khojandi, A.; Shukla, S. Nitric oxide levels regulate the immune response of Drosophila melanogaster reference laboratory strains to bacterial infections. Infect. Immun. 2014, 82, 4169–4181. [Google Scholar] [CrossRef] [PubMed]
- Luckhart, S.; Vodovotz, Y.; Cui, L.; Rosenberg, R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc. Natl. Acad. Sci. USA 1998, 95, 5700–5705. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Ali, M.M.; Dorrah, M.A.; Bassal, T.T.M. Mediation of inducible nitric oxide and immune-reactive lysozymes biosynthesis by eicosanoid and biogenic amines in flesh flies. Int. J. Trop. Insect Sci. 2018, 38, 93–104. [Google Scholar] [CrossRef]
- Dorrah, M.A.; Mohamed, A.A.; Shaurub, E.-S.H. Immunosuppressive effects of the limonoid azadirachtin, insights on a nongenotoxic stress botanical, in flesh flies. Pestic. Biochem. Physiol. 2019, 153, 55–66. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, L.; Yang, C.; Yun, X.; He, Y. The competence of hemocyte immunity in the armyworm Mythimna separata larvae to sublethal hexaflumuron exposure. Pestic. Biochem. Physiol. 2016, 130, 31–38. [Google Scholar] [CrossRef]
- Krishnan, N.; Hyrsl, P.; Simek, V. Nitric oxide production by hemocytes of larva and pharate prepupa of Galleria mellonella in response to bacterial lipopolysaccharide: Cytoprotective or cytotoxic? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 142, 103–110. [Google Scholar] [CrossRef]
- da Silva, R.; da Silva, S.R.; Lange, A.B. The regulation of cardiac activity by nitric oxide (NO) in the Vietnamese stick insect, Baculum extradentatum. Cell. Signal. 2012, 24, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Foley, E.; O’Farrell, P.H. Nitric oxide contributes to induction of innate immune responses to gram-negative bacteria in Drosophila. Genes Dev. 2003, 17, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.C.; Turangan, R.; Joosse, B.A.; Hillyer, J.F. Adult Mosquitoes Infected with Bacteria Early in Life Have Stronger Antimicrobial Responses and More Hemocytes after Reinfection Later in Life. Insects 2020, 11, 331. [Google Scholar] [CrossRef] [PubMed]
- Moyetta, N.R.; Broll, V.; Perin, A.P.A.; Uberti, A.F.; Coste Grahl, M.V.; Staniscuaski, F.; Carlini, C.R.; Fruttero, L.L. Jaburetox-induced toxic effects on the hemocytes of Rhodnius prolixus (Hemiptera: Reduviidae). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 200, 17–26. [Google Scholar] [CrossRef]
- Holstein, G.R.; Friedrich, V.L.; Martinelli, G.P. Monoclonal L-citrulline immunostaining reveals nitric oxide-producing vestibular neurons. Ann. N. Y. Acad. Sci. 2001, 942, 65–78. [Google Scholar] [CrossRef]
- Stern, M.; Böger, N.; Eickhoff, R.; Lorbeer, C.; Kerssen, U.; Ziegler, M.; Martinelli, G.P.; Holstein, G.R.; Bicker, G. Development of nitrergic neurons in the nervous system of the locust embryo. J. Comp. Neurol. 2010, 518, 1157–1175. [Google Scholar] [CrossRef]
- Müller, U. The nitric oxide system in insects. Prog. Neurobiol. 1997, 51, 363–381. [Google Scholar] [CrossRef]
- Elphick, M.; Rayne, R.; Riveros-Moreno, V.; Moncada, S.; Shea, M. Nitric oxide synthesis in locust olfactory interneurones. J. Exp. Biol. 1995, 198, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Elphick, M.; Williams, L.; Shea, M. New features of the locust optic lobe: Evidence of a role for nitric oxide in insect vision. J. Exp. Biol. 1996, 199, 2395–2407. [Google Scholar] [CrossRef]
- Bicker, G. Sources and targets of nitric oxide signalling in insect nervous systems. Cell Tissue Res. 2001, 303, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Colasanti, M.; Venturini, G. Nitric oxide in invertebrates. Mol. Neurobiol. 1998, 17, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Cayre, M.; Strambi, C.; Strambi, A. Neurogenesis in an adult insect brain and its hormonal control. Nature 1994, 368, 57–59. [Google Scholar] [CrossRef]
- Kuntz, S.; Poeck, B.; Strauss, R. Visual Working Memory Requires Permissive and Instructive NO/cGMP Signaling at Presynapses in the Drosophila Central Brain. Curr. Biol. 2017, 27, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Münch, D.; Ott, S.R.; Pflüger, H.-J. Three-dimensional distribution of NO sources in a primary mechanosensory integration center in the locust and its implications for volume signaling. J. Comp. Neurol. 2010, 518, 2903–2916. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.R.; Burrows, M. NADPH diaphorase histochemistry in the thoracic ganglia of locusts, crickets, and cockroaches: Species differences and the impact of fixation. J. Comp. Neurol. 1999, 410, 387–397. [Google Scholar] [CrossRef]
- Ott, S.R.; Burrows, M.; Elphick, M.R. The Neuroanatomy of Nitric Oxide–Cyclic GMP Signaling in the Locust: Functional Implications for Sensory Systems. Am. Zool. 2001, 41, 321–331. [Google Scholar] [CrossRef][Green Version]
- Shaw, W.R.; Catteruccia, F. Vector biology meets disease control: Using basic research to fight vector-borne diseases. Nat. Microbiol. 2019, 4, 20–34. [Google Scholar] [CrossRef]
- Gaburro, J.; Bhatti, A.; Harper, J.; Jeanne, I.; Dearnley, M.; Green, D.; Nahavandi, S.; Paradkar, P.N.; Duchemin, J.-B. Neurotropism and behavioral changes associated with Zika infection in the vector Aedes aegypti. Emerg. Microbes Infect. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Lima-Camara, T.N.; Bruno, R.V.; Luz, P.M.; Castro, M.G.; Lourenço-de-Oliveira, R.; Sorgine, M.H.F.; Peixoto, A.A. Dengue infection increases the locomotor activity of Aedes aegypti females. PLoS ONE 2011, 6, e17690. [Google Scholar] [CrossRef]
- Tallon, A.K.; Lorenzo, M.G.; Moreira, L.A.; Martinez Villegas, L.E.; Hill, S.R.; Ignell, R. Dengue infection modulates locomotion and host seeking in Aedes aegypti. PLoS Negl. Trop. Dis. 2020, 14, e0008531. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.E.; Hopper, J.E.; Stuart, M.A.; West, M.; Drolet, B.S. Blood-Feeding Behavior of Vesicular Stomatitis Virus Infected Culicoides sonorensis (Diptera: Ceratopogonidae). J. Med. Entomol. 2008, 45, 921–926. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.H.; Rowley, W.A.; Platt, K.B. Longevity and spontaneous flight activity of Culex tarsalis (Diptera: Culicidae) infected with western equine encephalomyelitis virus. J. Med. Entomol. 2000, 37, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Gaburro, J.; Paradkar, P.N.; Klein, M.; Bhatti, A.; Nahavandi, S.; Duchemin, J.-B. Dengue virus infection changes Aedes aegypti oviposition olfactory preferences. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cator, L.J.; George, J.; Blanford, S.; Murdock, C.C.; Baker, T.C.; Read, A.F.; Thomas, M.B. ‘Manipulation’ without the parasite: Altered feeding behaviour of mosquitoes is not dependent on infection with malaria parasites. Proc. Biol. Sci. 2013, 280, 20130711. [Google Scholar] [CrossRef]
- Zhang, L.; Lecoq, M.; Latchininsky, A.; Hunter, D. Locust and Grasshopper Management. Annu. Rev. Entomol. 2019, 64, 15–34. [Google Scholar] [CrossRef]
- Wright, N.J.D. A review of the actions of Nitric Oxide in development and neuronal function in major invertebrate model systems. AIMS Neurosci. 2019, 6, 146–174. [Google Scholar] [CrossRef]
- Gillespie, J.P.; Burnett, C.; Charnley, A.K. The immune response of the desert locust Schistocerca gregaria during mycosis of the entomopathogenic fungus, Metarhizium anisopliae var acridum. J. Insect Physiol. 2000, 46, 429–437. [Google Scholar] [CrossRef]
- Duressa, T.F.; Vanlaer, R.; Huybrechts, R. Locust cellular defense against infections: Sites of pathogen clearance and hemocyte proliferation. Dev. Comp. Immunol. 2015, 48, 244–253. [Google Scholar] [CrossRef]
- Brehélin, M.; Hoffmann, J.A. Phagocytosis of inert particles in Locusta migratoria and Galleria mellonella: Study of ultrastructure and clearance. J. Insect Physiol. 1980, 26, 103–111. [Google Scholar] [CrossRef]
- Ratcliffe, N.A.; Brookman, J.L.; Rowley, A.F. Activation of the prophenoloxidase cascade and initiation of nodule formation in locusts by bacterial lipopolysaccharides. Dev. Comp. Immunol. 1991, 15, 33–39. [Google Scholar] [CrossRef]
- Macours, N.; Hens, K.; Francis, C.; de Loof, A.; Huybrechts, R. Molecular evidence for the expression of angiotensin converting enzyme in hemocytes of Locusta migratoria: Stimulation by bacterial lipopolysaccharide challenge. J. Insect Physiol. 2003, 49, 739–746. [Google Scholar] [CrossRef]
- Jäckel, S.; Eiden, M.; Dauber, M.; Balkema-Buschmann, A.; Brun, A.; Groschup, M.H. Generation and application of monoclonal antibodies against Rift Valley fever virus nucleocapsid protein NP and glycoproteins Gn and Gc. Arch. Virol. 2014, 159, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Gregor, K.M.; Michaely, L.M.; Gutjahr, B.; Rissmann, M.; Keller, M.; Dornbusch, S.; Naccache, F.; Schön, K.; Jansen, S.; Heitmann, A.; et al. Rift Valley fever virus detection in susceptible hosts with special emphasis in insects. Sci. Rep. 2021, 11, 9822. [Google Scholar] [CrossRef] [PubMed]
- Huxham, I.M.; Lackie, A.M. A simple visual method for assessing the activation and inhibition of phenoloxidase production by isect haemocytes in vitro. J. Immunol. Methods 1986, 94, 271–277. [Google Scholar] [CrossRef]
- Hoskins, S.G.; Homberg, U.; Kingan, T.G.; Christensen, T.A.; Hildebrand, J.G. Immunocytochemistry of GABA in the antennal lobes of the sphinx moth Manduca sexta. Cell Tissue Res. 1986, 244, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Bentley, D.; Keshishian, H.; Shankland, M.; Toroian-Raymond, A. Quantitative staging of embryonic development of the grasshopper, Schistocerca nitens. Development 1979, 54, 47–74. [Google Scholar] [CrossRef]
- Stern, M.; Bicker, G. Nitric oxide regulates axonal regeneration in an insect embryonic CNS. Dev. Neurobiol. 2008, 68, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, I.; Jansen, S.; Fall, G.; Lorenzen, S.; Rudolf, M.; Huber, K.; Heitmann, A.; Schicht, S.; Ndiaye, E.H.; Watson, M.; et al. RNA Interference Restricts Rift Valley Fever Virus in Multiple Insect Systems. mSphere 2017, 2, e00090-17. [Google Scholar] [CrossRef]
- Moy, R.H.; Gold, B.; Molleston, J.M.; Schad, V.; Yanger, K.; Salzano, M.-V.; Yagi, Y.; Fitzgerald, K.A.; Stanger, B.Z.; Soldan, S.S.; et al. Antiviral autophagy restrictsRift Valley fever virus infection and is conserved from flies to mammals. Immunity 2014, 40, 51–65. [Google Scholar] [CrossRef]
- Léger, P.; Lara, E.; Jagla, B.; Sismeiro, O.; Mansuroglu, Z.; Coppée, J.Y.; Bonnefoy, E.; Bouloy, M. Dicer-2- and Piwi-mediated RNA interference in Rift Valley fever virus-infected mosquito cells. J. Virol. 2013, 87, 1631–1648. [Google Scholar] [CrossRef] [PubMed]
- Bicker, G. NO news from insect brains. Trends Neurosci. 1998, 21, 349–355. [Google Scholar] [CrossRef]
- Stern, M.; Bicker, G. Nitric oxide as a regulator of neuronal motility and regeneration in the locust embryo. J. Insect Physiol. 2010, 56, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Nakatsubo, N.; Kikuchi, K.; Kawahara, S.; Kirino, Y.; Nagoshi, H.; Hirata, Y.; Nagano, T. Detection and imaging of nitric oxide with novel fluorescent indicators: Diaminofluoresceins. Anal. Chem. 1998, 70, 2446–2453. [Google Scholar] [CrossRef]
- Semenova, A.D.; Glazachev, Y.I.; Slepneva, I.A.; Glupov, V.V. Quantitative determination of nitric oxide production in haemocytes: Nitrite reduction activity as a potential pathway of NO formation in haemolymph of Galleria mellonella larvae. Nitric Oxide 2014, 37, 46–52. [Google Scholar] [CrossRef]
- Scheiblich, H.; Roloff, F.; Singh, V.; Stangel, M.; Stern, M.; Bicker, G. Nitric oxide/cyclic GMP signaling regulates motility of a microglial cell line and primary microglia in vitro. Brain Res. 2014, 1564, 9–21. [Google Scholar] [CrossRef]
- Dudzic, J.P.; Hanson, M.A.; Iatsenko, I.; Kondo, S.; Lemaitre, B. More Than Black or White: Melanization and Toll Share Regulatory Serine Proteases in Drosophila. Cell Rep. 2019, 27, 1050–1061. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Schmidt, S.L.; Christensen, B.M. The antibacterial innate immune response by the mosquito Aedes aegypti is mediated by hemocytes and independent of Gram type and pathogenicity. Microbes Infect. 2004, 6, 448–459. [Google Scholar] [CrossRef]
- Traub, S.; von Aulock, S.; Hartung, T.; Hermann, C. Invited review: MDP and other muropeptides—Direct and synergistic effects on the immune system. J. Endotoxin Res. 2006, 12, 69–85. [Google Scholar] [CrossRef]
- Velikova, N.; Kavanagh, K.; Wells, J.M. Evaluation of Galleria mellonella larvae for studying the virulence of Streptococcus suis. BMC Microbiol. 2016, 16, 291. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, G.; Dixon, A.; Kavanagh, K. Utilization of Galleria mellonella larvae to characterize the development of Staphylococcus aureus infection. Microbiology 2019, 165, 863–875. [Google Scholar] [CrossRef]
- El Chamy, L.; Leclerc, V.; Caldelari, I.; Reichhart, J.-M. Sensing of ‘danger signals’ and pathogen-associated molecular patterns defines binary signaling pathways ‘upstream’ of Toll. Nat. Immunol. 2008, 9, 1165–1170. [Google Scholar] [CrossRef]
- Chang, P.; Li, W.; Shi, G.; Li, H.; Yang, X.; Xia, Z.; Ren, Y.; Li, Z.; Chen, H.; Bei, W. The VraSR regulatory system contributes to virulence in Streptococcus suis via resistance to innate immune defenses. Virulence 2018, 9, 771–782. [Google Scholar] [CrossRef]
- Kuroda, M.; Kuroda, H.; Oshima, T.; Takeuchi, F.; Mori, H.; Hiramatsu, K. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol. Microbiol. 2003, 49, 807–821. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Buchon, N.; Poidevin, M.; Kwon, H.-M.; Guillou, A.; Sottas, V.; Lee, B.-L.; Lemaitre, B. A single modular serine protease integrates signals from pattern-recognition receptors upstream of the Drosophila Toll pathway. Proc. Natl. Acad. Sci. USA 2009, 106, 12442–12447. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Motoi, Y.; Taniai, K.; Kadono-Okuda, K.; Hiramatsu, M.; Yamakawa, M. Clearance of lipopolysaccharide in hemolymph of the silkworm Bombyx mori: Its role in the termination of cecropin mRNA induction. Insect Biochem. Mol. Biol. 1994, 24, 539–545. [Google Scholar] [CrossRef]
- Kato, Y.; Motoi, Y.; Taniai, K.; Kadono-Okuda, K.; Yamamoto, M.; Higashino, Y.; Shimabukuro, M.; Chowdhury, S.; Xu, J.; Sugiyama, M.; et al. Lipopolysaccharide-lipophorin complex formation in insect hemolymph: A common pathway of lipopolysaccharide detoxification both in insects and in mammals. Insect Biochem. Mol. Biol. 1994, 24, 547–555. [Google Scholar] [CrossRef]
- Kitchens, R.L.; Wolfbauer, G.; Albers, J.J.; Munford, R.S. Plasma lipoproteins promote the release of bacterial lipopolysaccharide from the monocyte cell surface. J. Biol. Chem. 1999, 274, 34116–34122. [Google Scholar] [CrossRef] [PubMed]
- Bidla, G.; Lindgren, M.; Theopold, U.; Dushay, M.S. Hemolymph coagulation and phenoloxidase in Drosophila larvae. Dev. Comp. Immunol. 2005, 29, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Bidla, G.; Dushay, M.S.; Theopold, U. Crystal cell rupture after injury in Drosophila requires the JNK pathway, small GTPases and the TNF homolog Eiger. J. Cell Sci. 2007, 120, 1209–1215. [Google Scholar] [CrossRef]
- Bidla, G.; Hauling, T.; Dushay, M.S.; Theopold, U. Activation of insect phenoloxidase after injury: Endogenous versus foreign elicitors. J. Innate Immun. 2009, 1, 301–308. [Google Scholar] [CrossRef]
- Karpac, J.; Younger, A.; Jasper, H. Dynamic coordination of innate immune signaling and insulin signaling regulates systemic responses to localized DNA damage. Dev. Cell 2011, 20, 841–854. [Google Scholar] [CrossRef]
- Hoffmann, J.A. Blood-forming tissues in orthopteran insects: An analogue to vertebrate hemopoietic organs. Experientia 1973, 29, 50–51. [Google Scholar] [CrossRef]
- Hoffmann, J.A.; Porte, A.; Joly, P. Présence d’un tissu hématopoïétique au niveau du diaphragme dorsal de Locusta migratoria (Orthoptère). CR Acad. Sci. 1968, 266, 1882–1883. [Google Scholar]
- Garthwaite, J. Concepts of neural nitric oxide-mediated transmission. Eur. J. Neurosci. 2008, 27, 2783–2802. [Google Scholar] [CrossRef] [PubMed]
- de Vente, J.; Garssen, J.; Tilders, F.; Steinbusch, H.; Schipper, J. Single cell quantitative immunocytochemistry of cyclic GMP in the superior cervical ganglion of the rat. Brain Res. 1987, 411, 120–128. [Google Scholar] [CrossRef]
- Schmachtenberg, O.; Bicker, G. Nitric oxide and cyclic GMP modulate photoreceptor cell responses in the visual system of the locust. J. Exp. Biol. 1999, 202, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Unoki, S.; Aonuma, H.; Mizunami, M. Critical role of nitric oxide-cGMP cascade in the formation of cAMP-dependent long-term memory. Learn. Mem. 2006, 13, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, B.; Kunst, M.; Günther, C.; Ganter, G.K.; Lakes-Harlan, R.; Elsner, N.; Heinrich, R. Nitric oxide/cyclic guanosine monophosphate signaling in the central complex of the grasshopper brain inhibits singing behavior. J. Comp. Neurol. 2005, 488, 129–139. [Google Scholar] [CrossRef]
- Bergmann, G.A.; Bicker, G. Cholinergic calcium responses in cultured antennal lobe neurons of the migratory locust. Sci. Rep. 2021, 11, 10018. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F.; Pass, G. The Insect Circulatory System: Structure, Function, and Evolution. Annu. Rev. Entomol. 2020, 65, 121–143. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergmann, S.; Gerhards, J.-P.; Schmitz, A.; Becker, S.C.; Stern, M. NO Synthesis in Immune-Challenged Locust Hemocytes and Potential Signaling to the CNS. Insects 2021, 12, 951. https://doi.org/10.3390/insects12100951
Bergmann S, Gerhards J-P, Schmitz A, Becker SC, Stern M. NO Synthesis in Immune-Challenged Locust Hemocytes and Potential Signaling to the CNS. Insects. 2021; 12(10):951. https://doi.org/10.3390/insects12100951
Chicago/Turabian StyleBergmann, Stella, Jan-Phillipp Gerhards, Anne Schmitz, Stefanie C. Becker, and Michael Stern. 2021. "NO Synthesis in Immune-Challenged Locust Hemocytes and Potential Signaling to the CNS" Insects 12, no. 10: 951. https://doi.org/10.3390/insects12100951
APA StyleBergmann, S., Gerhards, J.-P., Schmitz, A., Becker, S. C., & Stern, M. (2021). NO Synthesis in Immune-Challenged Locust Hemocytes and Potential Signaling to the CNS. Insects, 12(10), 951. https://doi.org/10.3390/insects12100951






