Respiratory Strategies in Relation to Ecology and Behaviour in Three Diurnal Namib Desert Tenebrionid Beetles
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Beetles
2.2. Respirometry
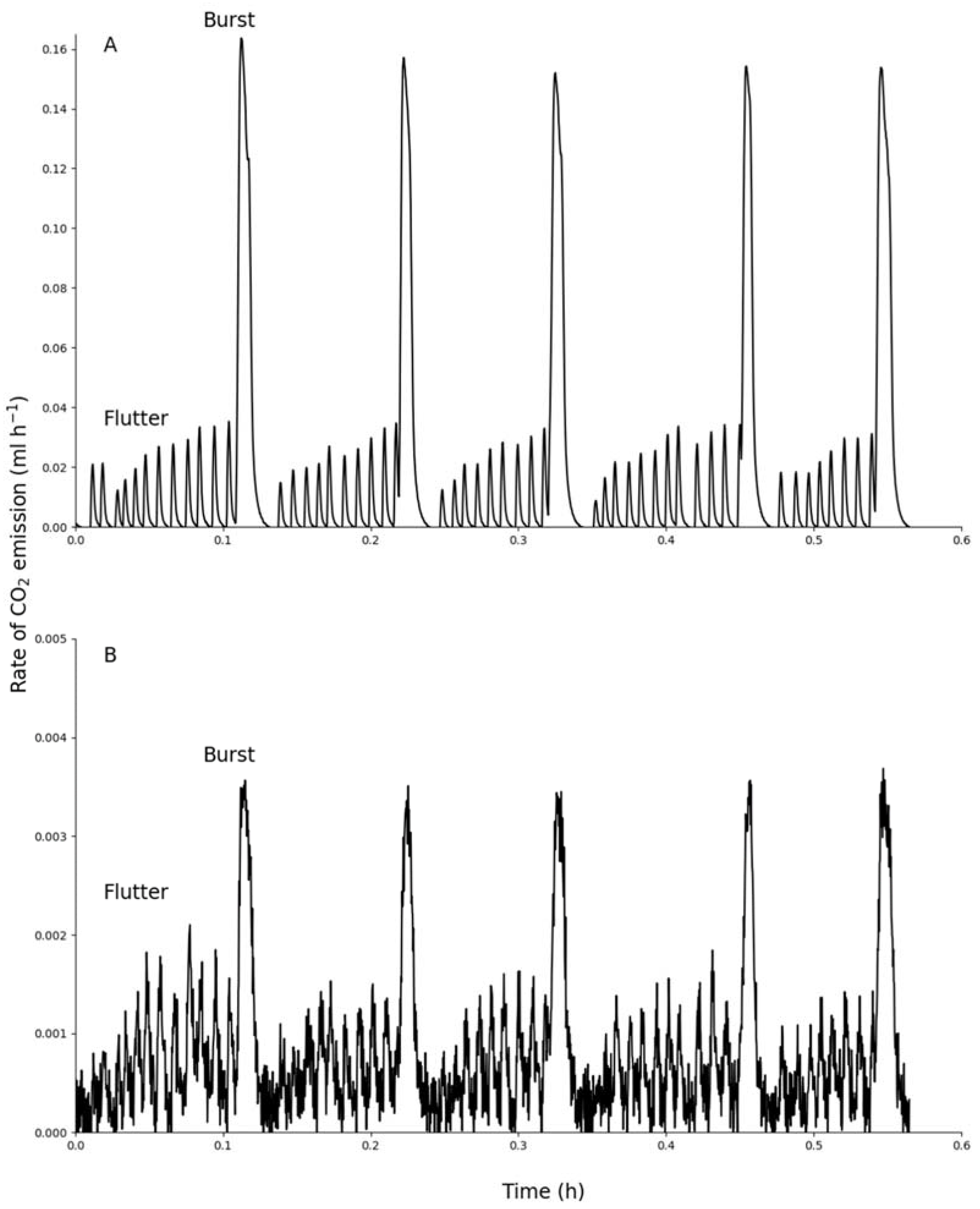
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holm, E.; Edney, E.B. Daily activity of Namib desert arthropods in relation to climate. Ecology 1972, 54, 45–56. [Google Scholar] [CrossRef]
- Henschel, J.R.; Mtuleni, V.; Pallett, J.; Seely, M.K. The surface-dwelling arthropod fauna of Gobabeb with a description of the long-term pitfall trapping project. J. Namib. Sci. Soc. 2003, 51, 65–92. [Google Scholar]
- Louw, S. Periodicity in and ecological equivalence between the ground-living Tenebrionidae (Coleoptera) in the southern Namib and Kalahari ecosystems with notes on phylogenetic relationships. Navors. Nas. Mus. Bloemfontein 1986, 5, 301–324. [Google Scholar]
- Koch, C. Some aspects of abundant life in the vegetationless sand of the Namib desert dunes: Positive psammotropism in tenebrionid beetles. J. SWA Sci. Soc. 1961, 15, 8–34. [Google Scholar]
- Draney, M.L. The subelytral cavity of desert tenebrionids. Fla. Entomol. 1993, 76, 539–549. [Google Scholar] [CrossRef]
- Gorb, S.N. Frictional surfaces of the elytra-to-body arresting mechanism in tenebrionid beetles (Coleoptera: Tenebrionidae): Design of co-opted fields of microtrichia and cuticle ultrastructure. Int. J. Insect Morphol. Embryol. 1998, 27, 205–225. [Google Scholar] [CrossRef]
- Cloudsley-Thompson, J.L. Thermal and water relations of desert beetles. Naturwissenschaften 2001, 88, 447–460. [Google Scholar] [CrossRef]
- Duncan, F.D. The role of the subelytral cavity in respiration in a tenebrionid beetle, Onymacris multistriata (Tenebrionidae: Adesmiini). J. Insect Physiol. 2003, 49, 339–346. [Google Scholar] [CrossRef]
- Duncan, F.D.; Byrne, M.J. The role of the mesothoracic spiracles in respiration in flighted and flightless dung beetles. J. Exp. Biol. 2005, 208, 907–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, F.D.; Dickman, C.R. Respiratory strategies of tenebrionid beetles in arid Australia: Does physiology beget nocturnality? Physiol. Entomol. 2009, 34, 52–60. [Google Scholar] [CrossRef]
- Louw, G.N. Physiological studies on the Namib fauna: A brief critique. In Namib Ecology: 25 Years of Namib Research; Seely, M.K., Ed.; Transvaal Museum Monograph: Pretoria, South Africa, 1990; Volume 7, pp. 203–207. [Google Scholar]
- Matthews, E.G. Origins of Australian arid-zone tenebrionid beetles. Invertebr. Taxon. 2000, 14, 941–951. [Google Scholar] [CrossRef]
- Buckley, R.C. Parallel dunefield ecosystems: Southern Kalahari and central Australia. J. Arid Environ. 1981, 4, 287–298. [Google Scholar] [CrossRef]
- Holm, E.; Scholtz, C.H.; Louw, S.M.; Penrith, M.-L. Notes on the coleopterous fauna of the Kalahari. Koedoe Suppl. 1984, 1984, 153–165. [Google Scholar] [CrossRef]
- Matthews, E.G. Classification, relationships and distribution of the genera Heleini (Coleoptera: Tenebrionidae). Invertebr. Taxon. 1993, 7, 1025–1095. [Google Scholar] [CrossRef]
- Lighton, J.R.B. Ventilation in Namib desert tenebrionid beetles: Mass scaling and evidence of a novel quantized flutter-phase. J. Exp. Biol. 1991, 159, 249–268. [Google Scholar] [CrossRef]
- Forster, T.D.; Hetz, S.K. Spiracle activity in moth pupae—The role of oxygen and carbon dioxide revisited. J. Insect Physiol. 2010, 56, 492–501. [Google Scholar] [CrossRef]
- Lighton, J.R.B.; Fukushi, T.; Wehner, R. Ventilation in Cataglyphis bicolor: Regulation of carbon dioxide release from the thoracic and abdominal spiracles. J. Insect Physiol. 1993, 39, 687–699. [Google Scholar] [CrossRef]
- Kestler, P. Respiration and respiratory water loss. In Environmental Physiology and Biochemistry of Insects; Hoffman, K.H., Ed.; Springer: Berlin, Germany, 1985; pp. 137–186. [Google Scholar]
- Lighton, J.R.B. Discontinuous gas exchange in insects. Annu. Rev. Entomol. 1996, 41, 309–324. [Google Scholar] [CrossRef]
- Quinlan, M.C.; Gibbs, A.G. Discontinuous gas exchange in insects. Respir. Physiol. Neurobiol. 2006, 154, 18–29. [Google Scholar] [CrossRef]
- White, C.R.; Blackburn, T.M.; Terblanche, J.S.; Marais, E.; Gibernau, M.; Chown, S.L. Evolutionary responses of discontinuous gas exchange in insects. Proc. Natl. Acad. Sci. USA 2007, 104, 8357–8361. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.F.; Woods, H.A.; Roberts, S.P. Ecological and Environmental Physiology of Insects; Oxford University Press: Oxford, UK, 2012; pp. 1–378. [Google Scholar]
- Duncan, F.D.; Byrne, M.J. Respiratory airflow in a wingless dung beetle. J. Exp. Biol. 2002, 205, 2489–2497. [Google Scholar] [CrossRef]
- Wharton, R.A.; Seely, M. Species composition of and biological notes on Tenebrionidae of the lower Kuiseb River and adjacent gravel plain. Madoqua 1982, 13, 5–25. [Google Scholar]
- Lease, H.M.; Goelst, K.; Seely, M.K.; Mitchell, D. Evidence of temperature-independent metabolic rates in diurnal Namib Desert tenebrionid beetles. Physiol. Entomol. 2014, 39, 254–262. [Google Scholar] [CrossRef]
- Edney, E.B. Body temperature of tenebrionid beetles in the Namib desert of southern Africa. J. Exp. Biol. 1971, 55, 253–272. [Google Scholar] [CrossRef]
- Edney, E.B. Some aspects of water balance in tenebrionid beetles and a thysanuran from the Namib desert of southern Africa. Physiol. Zool. 1971, 44, 61–76. [Google Scholar] [CrossRef]
- Wharton, R.A. Colouration and diurnal activity patterns in some Namib Desert Zophosini (Coleoptera: Tenebrionidae). J. Arid Environ. 1980, 3, 309–317. [Google Scholar] [CrossRef]
- Henschel, J.R. Long-Term Population Dynamics of Namib Desert Tenebrionid Beetles Reveal Complex Relationships to Pulse-Reserve Conditions. Insects 2021, 12, 804. [Google Scholar] [CrossRef]
- Holm, E.; Scholtz, C.H. Structure and pattern of the Namib Desert dune ecosystem at Gobabeb. Madoqua 1980, 12, 3–39. [Google Scholar]
- Louw, G.N.; Seely, M.K. Ecology of Desert Organisms; Longman Group Limited: Basildon, UK, 1982; pp. 1–194. [Google Scholar]
- Lighton, J.R.B. Measuring Metabolic Rates. A Manual for Scientists; Oxford University Press: New York, NY, USA, 2008; pp. 1–288. [Google Scholar]
- Inder, I.M.; Duncan, F.D. Gas exchange pattern transitions in the workers of the harvester termite. J. Insect Physiol. 2015, 75, 47–53. [Google Scholar] [CrossRef]
- Duncan, F.D.; Krasnov, B.; McMaster, M. Metabolic rate and respiratory gas exchange patterns in tenebrionid beetles from the Negev Highlands, Israel. J. Exp. Biol. 2002, 205, 791–798. [Google Scholar] [CrossRef]
- Bartholomew, G.A.; Lighton, J.R.B.; Louw, G.N. Energetics of locomotion and patterns of respiration in tenebrionid beetles from the Namib Desert. J. Comp. Physiol. B 1985, 155, 155–162. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Bartholomew, G.A.; Seely, M.K. Ecological correlates of locomotion speed, morphometrics and body temperature in three Namib Desert tenebrionid beetles. S. Afr. J. Zool. 1984, 19, 131–134. [Google Scholar]
- Roberts, C.S. The Surface and Subsurface Environments and the Physiological and Behavioural Ecology of Dune-Living Beetles in the Namib Desert. Master’s Thesis, University of the Witwatersrand, Johannesburg, South Africa, 1991. [Google Scholar]
- Nicolson, S.W. Water relations of the Namib Desert tenebrionid beetles. In Namib Ecology 25 Years of Namib Research; Seely, M.K., Ed.; Transvaal Museum Monograph: Pretoria, South Africa, 1990; Volume 7, pp. 173–178. [Google Scholar]
- Schimpf, N.G.; Matthews, P.G.D.; Wilson, R.S.; White, C.R. Cockroaches breathe discontinuously to reduce respiratory water loss. J. Exp. Biol. 2009, 212, 2773–2780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, D.; Henschel, J.R.; Hetem, R.S.; Wassenaar, T.D.; Strauss, W.M.; Hanrahan, S.A.; Seely, M.K. Fog and fauna of the Namib Desert: Past and future. Ecosphere 2020, 11, e02996. [Google Scholar] [CrossRef]
- McClain, E.; Seely, M.K.; Hadley, N.F.; Gray, V. Wax blooms in tenebrionid beetles of the Namib Desert: Correlations with environment. Ecology 1985, 66, 112–118. [Google Scholar] [CrossRef]
- Nicolson, S.W. Water balance and osmoregulation in Onymacris plana, a tenebrionid beetle from the Namib Desert. J. Insect Physiol. 1980, 26, 315–320. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Louw, G.N.; Edney, E.B. Use of a ventilated capsule and tritiated water to measure evaporative water losses in a tenebrionid beetle. J. Exp. Biol. 1984, 481, 477–481. [Google Scholar] [CrossRef]
- Zachariassen, K.E. Routes of transpiratory water loss in a dry-habitat tenebrionid beetle. J. Exp. Biol. 1991, 157, 425–437. [Google Scholar] [CrossRef]
- Rasa, O.A.E. Aggregation in a desert tenebrionid beetle: A cost/benefit analysis. Ethology 1997, 103, 466–487. [Google Scholar] [CrossRef]
- Seely, M.K.; Henschel, J.R.; Hamilton, W.J.I. Long-term data show behavioural fog collection adaptations determine Namib Desert beetle abundance. S. Afr. J. Sci. 2005, 101, 570–572. [Google Scholar]


| Species | Onymacris Plana | Metriopus Depressus | Zophosis Amabilis | |
|---|---|---|---|---|
| Male | Female | |||
| N | 9 | 6 | 7 | 8 |
| Mass (g) | 0.813 ± 0.19 | 0.893 ± 0.39 | 0.214 ± 0.029 | 0.134 ± 0.04 |
| Total rate of CO2 emission | ||||
| (µL h−1) | 88.41 ± 17.13 | 97.38 ± 47.72 | 31.42 ± 16.23 | 22.26 ± 3.97 |
| (µL h−1g−1) a | 118.72 ± 45.3 | 110.72 ± 37.74 | 146.95 ± 82.53 | 183.56 ± 61.54 |
| Rate of CO2 emission (µL h−1) | ||||
| Mesothoracic spiracles | 10.01 ± 11.04 | 28.62 ± 22.22 | 17.8 ± 13.62 | 18.51 ± 4.19 |
| Elytral case | 78.4 ± 24.76 | 68.76 ± 47.14 | 15.88 ± 7.88 | 4.98 ± 4.21 |
| % total CO2 emitted through | 13.28 ± 15.9 | 33.4 ± 28.8 | 57.4 ± 29.9 | 84.4 ± 18.4 |
| mesothoracic spiracles | ||||
| DGC b frequency (cycles per hour) | 8.94 ± 3.31 | 8.79 ± 4.35 | 9.62 ± 3.95 | 15.93 ± 5.97 |
| Species | Onymacris Plana | Metriopus Depressus | Zophosis Amabilis | |
|---|---|---|---|---|
| Male | Female | |||
| Flutter period: | ||||
| VCO2 (µL g−1) Mesothoracic spiracles | 0.202 ± 0.19 | 0.842 ± 0.69 | 2.265 ± 1.791 | 2.429 ± 1.35 |
| VCO2 (µL g−1) Elytral case | 1.681 ± 0.81 | 1.599 ± 1.0 | 3.901 ± 1.441 | 0.406 ± 0.268 |
| Ratio of Volume CO2 | ||||
| mesothoracic spiracles: elytral case | 0.19 ± 0.23 | 1.9 ± 3.1 | 0.52 ± 0.52 | 9.9 ± 9.7 |
| Duration (s) | 211.9 ± 123 | 274.3 ± 166 | 439.8 ± 93 | 142.8 ± 69 |
| % Flutter period of DGC a | 42.1% | 43.1% | 68.3% | 53.5% |
| Burst period: | ||||
| VCO2 (µL g−1) Mesothoracic spiracles | 1.35 ± 1.65 | 3.95 ± 3.89 | 7.364 ± 4.01 | 7.995 ± 2.52 |
| VCO2 (µL g−1) Elytral case | 10.019 ± 3.42 | 7.143 ± 4.73 | 6.592 ± 3.23 | 1.721 ± 1.53 |
| Ratio of volume CO2 | ||||
| mesothoracic spiracles: elytral case | 0.24 ± 0.38 | 1.91 ± 3.32 | 2.0 ± 1.8 | 18.4 ± 22.9 |
| Duration (s) | 138.9 ± 59 | 202.6 ± 192 | 129.1 ± 20 | 71.1 ± 19 |
| % Burst period of DGC a | 31.3% | 31.4% | 30% | 29.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duncan, F.D. Respiratory Strategies in Relation to Ecology and Behaviour in Three Diurnal Namib Desert Tenebrionid Beetles. Insects 2021, 12, 1036. https://doi.org/10.3390/insects12111036
Duncan FD. Respiratory Strategies in Relation to Ecology and Behaviour in Three Diurnal Namib Desert Tenebrionid Beetles. Insects. 2021; 12(11):1036. https://doi.org/10.3390/insects12111036
Chicago/Turabian StyleDuncan, Frances D. 2021. "Respiratory Strategies in Relation to Ecology and Behaviour in Three Diurnal Namib Desert Tenebrionid Beetles" Insects 12, no. 11: 1036. https://doi.org/10.3390/insects12111036
APA StyleDuncan, F. D. (2021). Respiratory Strategies in Relation to Ecology and Behaviour in Three Diurnal Namib Desert Tenebrionid Beetles. Insects, 12(11), 1036. https://doi.org/10.3390/insects12111036






