Nine Mitochondrial Genomes of the Pyraloidea and Their Phylogenetic Implications (Lepidoptera)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples, DNA Extraction and Mitogenome Sequencing
2.2. Mitogenome Assembly and Annotation
2.3. Multiple Alignment
2.4. Nucleotide Composition, Substitution Saturation and Heterogeneity of Sequence Divergence
2.5. Phylogenetic Analyses
3. Results and Discussion
3.1. General Features of the Sequenced Mitogenomes
3.2. Tests of Substitution Saturation and Heterogeneity of Sequence Divergence
3.3. Phylogenetic Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munroe, E.; Solis, M.A. Pyraloidea. Lepidoptera, Moths and Butterflies, Volume 1: Evolution, Systematics, and Biogeography. In Handbook of Zoology; Kristensen, N., Ed.; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 1999; Volume IV, pp. 233–256. ISBN 3-11-015704-7. [Google Scholar]
- Van Nieukerken, E.J.; Kaila, L.; Kitching, I.J.; Kristensen, N.P.; Lees, D.C.; Minet, J.; Mitter, C.; Mutanen, M.; Regier, J.C.; Simonsen, T.J.; et al. Order Lepidoptera Linnaeus, 1758. Zootaxa 2011, 3148, 212–221. [Google Scholar] [CrossRef]
- Nuss, M.; Landry, B.; Mally, R.; Vegliante, F.; Tränkner, A.; Bauer, F.; Hayden, J.; Segerer, A.; Schouten, R.; Li, H.; et al. 2003–2021. Global Information System on Pyraloidea. Available online: http://www.pyraloidea.org (accessed on 6 March 2021).
- Léger, T.; Mally, R.; Neinhuis, C.; Nuss, M. Refining the phylogeny of Crambidae with complete sampling of subfamilies (Lepidoptera, Pyraloidea). Zool. Scr. 2021, 50, 84–99. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, K. Advances in molecular systematics of Pyraloidea (Lepidoptera). J. Environ. Entomol. 2017, 39, 254–262. [Google Scholar]
- Kuznetzov, V.I.; Stekolnikov, A.A. Classification and phylogenetic relationships of the families and subfamilies of the Pyraloidea (Lepidoptera) of the palearctic fauna with regard to functional morphology of the male genitalia. Tr. Inst. Zool. Leningr. 1979, 82, 43–74. [Google Scholar]
- Yoshiyasu, Y. A systematic study of the Nymphulinae and the Musotiminae of Japan (Lepidoptera: Pyralidae). Sci. Rep. Kyoto Prefect. Univ. Agric. 1985, 37, 1–162. [Google Scholar]
- Solis, M.A.; Maes, K.V.N. Preliminary phylogenetic analysis of the subfamilies of Crambidae (Pyraloidea Lepidoptera). Belg. J. Entomol. 2002, 4, 53–95. [Google Scholar]
- Solis, M. Phylogenetic studies and modern classification of the Pyraloidea (Lepidoptera). Rev. Colomb. Entomol. 2007, 33, 1–8. [Google Scholar]
- Regier, J.; Mitter, C.; Solis, M.; Hayden, J.; Landry, B.; Nuss, M.; Simonsen, T.; Yen, S.-H.; Zwick, A.; Cummings, M. A molecular phylogeny for the pyraloid moths (Lepidoptera: Pyraloidea) and its implications for higher-level classification. Syst. Entomol. 2012, 37, 635–656. [Google Scholar] [CrossRef]
- Zhu, W.; Yan, J.; Song, J.; You, P. The first mitochondrial genomes for Pyralinae (Pyralidae) and Glaphyriinae (Crambidae), with phylogenetic implications of Pyraloidea. PLoS ONE 2018, 13, e0194672. [Google Scholar]
- Léger, T.; Landry, B.; Nuss, M. Phylogeny, character evolution and tribal classification in Crambinae and Scopariinae. Syst. Entomol. 2019, 44, 757–776. [Google Scholar] [CrossRef]
- Mally, R.; Hayden, J.E.; Neinhuis, C.; Jordal, B.H.; Nuss, M. The phylogenetic systematics of Spilomelinae and Pyraustinae (Lepidoptera: Pyraloidea: Crambidae) inferred from DNA and morphology. Arthropod Syst. Phylogeny 2019, 77, 141–204. [Google Scholar]
- Qi, M.; Zhao, H.; Yu, F.; Zhang, A.; Li, H. The first mitogenomes of the subfamily Odontiinae (Lepidoptera, Crambidae) and phylogenetic analysis of Pyraloidea. Insects 2021, 12, 486. [Google Scholar] [CrossRef]
- Curole, J.P.; Kocher, T.D. Mitogenomics: Digging deeper with complete mitochondrial genomes. Trends Ecol. Evol. 1999, 14, 394–398. [Google Scholar] [CrossRef]
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef] [Green Version]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef] [Green Version]
- Timmermans, M.J.T.N.; Lees, D.C.; Simonsen, T.J. Towards a mitogenomic phylogeny of Lepidoptera. Mol. Phylogenet. Evol. 2014, 79, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Leavengood, J.M., Jr.; Chapman, E.G.; Burkhardt, D.; Song, F.; Jiang, P.; Liu, J.; Zhou, X.; Cai, W. Mitochondrial phylogenomics of Hemiptera reveals adaptive innovations driving the diversification of true bugs. Proc. R. Soc. B 2017, 284, 20171223. [Google Scholar] [CrossRef]
- Song, N.; Zhang, H.; Zhao, T. Insights into the phylogeny of Hemiptera from increased mitohenomic taxon sampling. Mol. Phylogenet. Evol. 2019, 137, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.; Breeschoten, T.; Timmermans, M.J.T.N.; Nadein, K.; Xue, H.; Bai, M.; Huang, Y.; Yang, X.; Vogler, A.P. The phylogeny of Galerucinae (Coleoptera: Chrysomelidae) and the performance of mitochondrial genomes in phylogenetic inference compared to nuclear rRNA genes. Cladistics 2017, 33, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Tang, P.; Zhu, J.; Zheng, B.; Wei, S.; Sharkey, M.; Chen, X.; Vogler, A. Mitochondrial phylogenomics of the Hymenoptera. Mol. Phylogenet. Evol. 2019, 131, 8–18. [Google Scholar] [CrossRef]
- Zheng, X.; Cao, L.; Chen, P.; Chen, X.; van Achterberg, K.; Hoffmann, A.A.; Liu, J.; Wei, S. Comparative mitogenomics and phylogenetics of the stinging wasps (Hymenoptera: Aculeata). Mol. Phylogenet. Evol. 2021, 159, 107119. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, W.; Zhan, L.; He, S.; Zhang, X.; Tao, Z. The complete mitochondrial genome of the bean pod borer Maruca testulalis (Lepidoptera: Crambidae: Spilomelinae). Mitochondrial DNA Part A 2016, 27, 740–741. [Google Scholar] [CrossRef]
- Hwang, E.J.; Kim, M.J.; Kim, S.S.; Kim, I. Complete mitochondrial genome of Ostrinia palustralis memnialis Walker, 1859 (Lepidoptera: Crambidae). Mitochondrial DNA B Resour. 2019, 4, 1364–1366. [Google Scholar] [CrossRef]
- Luo, Q.; Zhou, N.; Yang, Z. Complete mitochondrial genome of Ostrinia kasmirica (Lepidoptera: Crambidae). Mitochondrial DNA B Resour. 2021, 6, 2316–2318. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Ratnasingham, S.; Waard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, S96–S99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, M.; Lindgreen, S.; Orlando, L. AdapterRemoval v2: Rapid adapter trimming, identification, and read merging. BMC Res. 2016, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Pan, Q. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. Gigascience 2012, 1, 18. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones Havas, S.; Cheung, M.; Sturrock, S. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech. 1994, 3, 294–299. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Donath, A.; Jühling, F.; AlArab, M.; Bernhart, S.H.; Reinhardt, F.; Stadler, P.F.; Middendorf, M.; Bernt, M. Improved annotation of protein-coding genes boundaries in metazoan mitochondrial genomes. Nucleic Acids Res. 2019, 47, 10543–10552. [Google Scholar] [CrossRef]
- Kong, W.; Yang, J. The mitochondrial genome of Diaphania pyloalis Walker (Lepidoptera: Crambidae). Mitochondrial DNA 2016, 6, 4044–4045. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, M.J.; Ahn, S.J.; Kim, I. Complete mitochondrial genome of the grass moth Glyphodes quadrimaculalis (Lepidoptera: Crambidae). Mitochondrial DNA 2013, 26, 247–249. [Google Scholar] [CrossRef]
- Yang, M.; Song, L.; Mao, J.; Shi, Y.; Wu, C.; Zhang, Y.; Wei, L.; Xiao, F.; Liu, M. Complete mitochondrial genome of the soybean leaffolder, Omiodes indicata (Lepidoptera: Pyraloidea: Crambidae), and phylogenetic analysis for Pyraloidea. Int. J. Biol. Macromol. 2018, 115, 53–60. [Google Scholar] [CrossRef]
- Que, S.; Yu, A.; Tu, Y.; Xiong, C.; Liu, X. Complete mitochondrial genome and phylogenetic analysis of Diaphania perspectalis. Mitochondrial DNA B Resour. 2019, 4, 933–934. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Chen, Z.; Ma, L.; Huang, G.; Wang, X.; Gu, X. The complete mitogenome of Parapoynx crisonalis (Walker, 1859) (Lepidoptera: Crambidae), with phylogenetic relationships amongst three acentropine larval forms. Aquat. Insects 2017, 38, 79–91. [Google Scholar] [CrossRef]
- Yang, M.; Shi, S.; Dai, P.; Song, L.; Liu, X. Complete mitochondrial genome of Palpita hypohomalia (Lepidoptera: Pyraloidea: Crambidae) and its phylogenetic implications. Eur. J. Entomol. 2018, 115, 708–717. [Google Scholar] [CrossRef]
- Wu, Q.; Gong, Y.; Shi, B.; Gu, Y.; Wei, S. The complete mitochondrial genome of the yellow peach moth Dichocrocis punctiferalis (Lepidoptera: Pyralidae). Mitochondrial DNA 2013, 24, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, P.; You, P. The complete mitochondrial genome of Tyspanodes hypsalis (Lepidoptera: Crambidae). Mitochondrial DNA 2014, 27, 1821–1822. [Google Scholar]
- Zhao, J.; Sun, Y.; Tan, Y.; Xiao, B.; Bai, L. Complete mitochondrial genome of cotton leaf roller Haritalodes derogata (Lepidoptera: Crambidae). Mitochondrial DNA 2016, 27, 2833–2834. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zou, Y.; Zhang, L.; Ma, W.; Zhang, X.; Yue, B. The complete mitochondrial genome of the beet webworm, Spoladea recurvalis (Lepidoptera: Crambidae) and its phylogenetic implications. PLoS ONE 2015, 10, e0129355. [Google Scholar]
- Wan, X.; Kim, M.J.; Kim, I. Description of new mitochondrial genomes (Spodoptera litura, Noctuoidea and Cnaphalocrocis medinalis, Pyraloidea) and phylogenetic reconstruction of Lepidoptera with the comment on optimization schemes. Mol. Biol. Rep. 2013, 40, 6333–6349. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.; Wang, G. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Tan, M.; Meng, G.; Yang, S.; Sun, X.; Liu, S.; Song, W.; Li, Y.; Wu, Q.; Zhang, A.; et al. Multiplex sequencing of pooled mitochondrial genomes-a crucial step toward biodiversity analysis using mito-metagenomics. Nucleic Acids Res. 2014, 42, e166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, N.; Dong, Y.; Qiao, P.; Yang, Z. Complete mitogenomic structure and phylogenetic implications of the Genus Ostrinia (Lepidoptera: Crambidae). Insects 2020, 11, 232. [Google Scholar] [CrossRef] [Green Version]
- Fisher, K.E.; Bradbury, S.P.; Coates, B.S. Prediction of mitochondrial genome-wide variation through sequencing of mitochondrion-enriched extracts. Sci. Rep. 2020, 10, 19123. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Ren, Y.; Lu, Z.; Song, Y.; Liu, L.; Liu, S.; Yu, Y.; Men, X. Characterization of the complete mitochondrial genome of Asia Corn Borer, Ostrinia furnacalis (Lepidoptera: Crambidae). Mitochondrial DNA B Resour. 2020, 5, 936–937. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.F.; Zhang, X.; Peng, M.; Bian, H.; Chen, M.; Liu, Y.; Jiang, X.; Qin, L. Complete mitochondrial genome of the meadow moth, Loxostege sticticalis (Lepidoptera: Pyraloidea: Crambidae), compared to other Pyraloidea moths. J. Asia-Pac. Entomol. 2016, 19, 697–706. [Google Scholar] [CrossRef]
- Ye, F.; Shi, Y.; Xing, L.; Yu, H.; You, P. The complete mitochondrial genome of Paracymoriza prodigalis (Leech, 1889) (Lepidoptera), with a preliminary phylogenetic analysis of Pyraloidea. Aquat. Insects 2013, 35, 71–88. [Google Scholar] [CrossRef]
- Ye, F.; You, P. The complete mitochondrial genome of Paracymoriza distinctalis (Lepidoptera: Crambidae). Mitochondrial DNA 2014, 27, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, M.J.; Kim, S.S.; Kim, I. Complete mitochondrial genome of an aquatic moth, Elophila interruptalis (Lepidoptera: Crambidae). Mitochondrial DNA 2014, 25, 275–277. [Google Scholar] [CrossRef]
- Cao, S.; Du, Y. Characterization of the complete mitochondrial genome of Chilo auricilius and comparison with three other rice stem borers. Gene 2014, 548, 270–276. [Google Scholar] [CrossRef]
- Chai, H.; Du, Y.; Zhai, B. Characterization of the complete mitochondrial genomes of Cnaphalocrocis medinalis and Chilo suppressalis (Lepidoptera: Pyralidae). Int. J. Biol. Sci. 2012, 8, 561–579. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhang, X.; Fan, Z.; Yue, B.; Huang, F.; King, E.; Ran, J. Structural characteristics and phylogenetic analysis of the mitochondrial genome of the sugarcane borer, Diatraea saccharalis (Lepidoptera: Crambidae). DNA Cell Biol. 2011, 30, 3–8. [Google Scholar] [CrossRef]
- Song, J.; You, P. The complete mitochondrial genome of Pseudargyria interruptella (Lepidoptera: Crambidae). Mitochondrial DNA 2016, 27, 3899–3900. [Google Scholar] [CrossRef]
- Dong, W.; Feng, X.; Huang, G.; Jiang, G. Characterization of the mitochondrial genome of the cabbage webworm, Hellula undalis (Lepidoptera: Pyralidae). Mitochondrial DNA 2014, 27, 931–932. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, J.; Yang, J.; Fan, R. Complete mitochondrial genome of Dioryctria yiai (Lepidoptera: Pyralidae). Mitochondrial DNA B Resour. 2020, 5, 1062–1064. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Almaden, J.; Balchan, N.; Bennici-Clendinnen, E.; Bhasin, J.; Brown, C.; Carlson, H.; Chavda, A. The complete mitochondrial genome of the lesser aspen webworm moth Meroptera pravella (Insecta: Lepidoptera: Pyralidae). Mitochondrial DNA B Resour. 2017, 2, 344–346. [Google Scholar]
- Liu, Q.Y.; Jiang, X.H.; Hou, X.H.; Yang, H.; Chen, W.L. The mitochondrial genome of Ephestia elutella (Insecta: Lepidoptera: Pyralidae). Mitochondrial DNA B Resour. 2018, 3, 189–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traut, W.; Vogel, H.; Glöckner, G.; Hartmann, E.; Heckel, D.G. High-throughput sequencing of a single chromosome: A moth W chromosome. Chromosome Res. 2013, 21, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.N.; Chai, X.Y.; Bian, D.D.; Zhou, C.L.; Tang, B.P. The complete mitochondrial genome of Plodia interpunctella (Lepidoptera: Pyralidae) and comparison with other Pyraloidea insects. Genome 2015, 59, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Shen, Q. The complete mitochondrial genome of the navel orangeworm Amyelois transitella (Insecta: Lepidoptera: Pyralidae). Mitochondrial DNA 2015, 27, 4561–4562. [Google Scholar] [CrossRef]
- Yang, M.L.; Feng, S.Q.; Cao, Y.; Han, X.; Xiong, R.C.; Li, Z.H. The complete mitochondrial genome of the pear pyralid moth, Euzophera pyriella Yang. Mitochondrial DNA B Resour. 2017, 2, 275–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, X.; Li, H.X.; Yang, M.F. The complete mitochondrial genome sequences of two insect-tea producers in Pyralidae (Lepidoptera) from South China: Pyralis farinalis and Orthopygia glaucinalis. Mitochondrial DNA B Resour. 2019, 4, 3850–3851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.P.; Li, J.; Zhao, J.L.; Su, T.J.; Luo, A.R.; Fan, R.J.; Chen, M.C.; Wu, C.S.; Zhu, C.D. The complete mitochondrial genome of the rice moth, Corcyra cephalonica. Insect Sci. 2012, 12, 72. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.; Yu, H.; Xu, J.; Li, Y.H. Next-generation sequencing yields the complete mitogenome of the stored nut moth, Paralipsa gularis Zeller (Lepidoptera: Pyralidae). Mitochondrial DNA B Resour. 2021, 6, 2626–2627. [Google Scholar] [CrossRef]
- Park, Y.J.; Park, C.E.; Hong, S.J.; Jung, B.K.; Ibal, J.C.; Park, G.S.; Shin, J.H. The complete mitochondrial genome sequence of the greater wax moth Galleria mellonella (Insecta, Lepidoptera, Pyralidae): Sequence and phylogenetic analysis comparison based on whole mitogenome. Mitochondrial DNA B Resour. 2017, 2, 714–715. [Google Scholar] [CrossRef] [Green Version]
- Roh, S.J.; Jeon, J.H.; Kim, D.S.; Byun, B.K. The complete mitochondrial genome of unique snout moth, Cathayia obliquella (Pyralidae: Galeriinae). J. Asia-Pac. Biodivers. 2020, 13, 613–624. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, S.W.; Kim, I. Complete mitochondrial genome of the larch hawk moth, Sphinx morio (Lepidoptera: Sphingidae). Mitochondrial DNA 2013, 24, 622–624. [Google Scholar] [CrossRef]
- Li, J.; Hu, K.; Zhao, Y.; Lin, R.; Zhang, Y.; Li, Y.; Huang, Z.; Peng, S.; Geng, X.; Zhang, H.; et al. Complete mitogenome of Parum colligate (Lepidoptera: Sphingidae) and its phylogenetic position within the Sphingidae. Zootaxa 2019, 4652, 126–134. [Google Scholar] [CrossRef]
- Sima, Y.H.; Chen, M.; Yao, R.; Li, Y.P.; Liu, T.; Jin, X.; Wang, L.P.; Su, J.F.; Li, X.S.; Liu, Y.Q. The complete mitochondrial genome of the Ailanthus silkmoth, Samia cynthia cynthia (Lepidoptera: Saturniidae). Gene 2013, 526, 309–317. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, M.J.; Jeong, J.S.; Kim, I. Complete mitochondrial genome of Saturnia jonasii (Lepidoptera: Saturniidae): Genomic comparisons and phylogenetic inference among Bombycoidea. Genomics 2018, 110, 374–382. [Google Scholar] [CrossRef]
- Derks, M.F.L.; Smit, S.; Salis, L.; Schijlen, E.G.W.M.; Bossers, A.; Mateman, C.; Pijl, A.S.; de Ridder, D.; Groenen, M.A.M.; Visser, M.E.; et al. The genome of winter moth Operophtera brumata) provides a genomic perspective on sexual dimorphism and phenology. Genome Biol. 2015, 7, 2321–2332. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Song, L.; Shi, Y.; Li, J.; Zhang, Y.; Song, N. The first mitochondrial genome of the family Epicopeiidae and higher-level phylogeny of Macroheterocera (Lepidoptera: Ditrysia). Int. J. Biol. Macromol. 2019, 136, 123–132. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, B.; Dai, L.; Wang, L.; Qian, C.; Wei, G.; Liu, C. The complete mitochondrial genome of the common cutworm, Spodoptera litura (Lepidoptera: Noctuidade). Mitochondrial DNA 2014, 27, 122–123. [Google Scholar] [CrossRef]
- Chen, L.; Huang, J.; Dai, J.; Guo, Y.; Sun, J.; Hong, X. Intraspecific mitochondrial genome comparison identified CYTB as a high-resolution population marker in a new pest Athetis lepigone. Genomics 2019, 111, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cameron, S.L.; Lees, D.C.; Xue, D.; Han, H. A mitochondrial genome phylogeny of owlet moths (Lepidoptera: Noctuoidea), and examination of the utility of mitochondrial genomes for lepidopteran phylogenetics. Mol. Phylogenet. Evol. 2015, 85, 230–237. [Google Scholar] [CrossRef]
- Yang, M.; Song, L.; Shi, Y.; Yin, Y.; Wang, Y.; Zhang, P.; Chen, J.; Lou, L.; Liu, X. The complete mitochondrial genome of a medicinal insect, Hydrillodes repugnalis (Lepidoptera: Noctuoidea: Erebidae), and related phylogenetic analysis. Int. J. Biol. Macromol. 2019, 123, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xin, Z.; Wang, Y.; Zhang, H.; Zhang, D.; Wang, Z.; Zhou, C.; Tang, B.; Liu, Q. The complete mitochondrial genome of Clostera anachoreta (Lepidoptera: Notodontidae) and phylogenetic implications for Noctuoidea species. Genomics 2017, 109, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Ríos, V.; Franco-Sierra, N.D.; Alvarez, J.C.; Saldamando-Benjumea, C.I.; Villanueva-Mejía, D.F. Mitochondrial genome characterization of Tecia solanivora (Lepidoptera: Gelechiidae) and its phylogenetic relationship with other lepidopteran insects. Gene 2016, 581, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Abascal, F.; Zardoya, R.; Telford, M.J. TranslatorX: Multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Res. 2010, 38, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Steenwyk, J.L.; Buida, T.J.; Li, Y.N.; Shen, X.X.; Rokas, A. ClipKIT: A multiple sequence alignment trimming software for accurate phylogenomic inference. PLoS Biol. 2020, 18, e3001007. [Google Scholar] [CrossRef] [PubMed]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef]
- Xia, X.; Xie, Z.; Salemi, M.; Chen, L.; Wang, Y. An index of substitution saturation and its application. Mol. Phylogenet. Evol. 2003, 26, 1–7. [Google Scholar] [CrossRef]
- Xia, X. DAMBE5: A comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2013, 30, 1720–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.; Lemey, P. The Phylogenetic Handbook: A Practical Approach to Phylogenetic Analysis and Hypothesis Testing, 2nd ed.; Cambridge University Press: Cambridge, UK, 2009; pp. 615–630. [Google Scholar]
- Kuck, P.; Meid, S.A.; Gross, C.; Wagele, J.W.; Misof, B. AliGROOVE-visualization of heterogeneous sequence divergence within multiple sequence alignments and detection of inflated branch support. BMC Bioinform. 2014, 15, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [Green Version]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution formolecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lartillot, N.; Philippe, H. A Bayesian mixture model for across-site heterogeneities in the amino-acid replacement process. Mol. Biol. Evol. 2004, 21, 1095–1109. [Google Scholar] [CrossRef]
- Lartillot, N.; Lepage, T.; Blanquart, S. PhyloBayes 3: A Bayesian software package for phylogenetic reconstruction and molecular dating. Bioinformatics 2009, 25, 2286–2288. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Shi, M.; Chen, X.; Sharkey, M.J.; van Achterberg, C.; Ye, G.Y.; He, J.H. New views on strand asymmetry in insect mitochondrial genomes. PLoS ONE 2010, 5, e12708. [Google Scholar] [CrossRef] [Green Version]
- Cameron, S.L.; Whiting, M.F. The complete mitochondrial genome of the tobacco hornworm, Manduca sexta (Insecta: Lepidoptera: Sphingidae), and an examination of mitochondrial gene variability within butterflies and moths. Gene 2008, 40, 112–123. [Google Scholar] [CrossRef]
- Owen, C.L.; Marshall, D.C.; Hill, K.B.R.; Simon, C. The phylogenetic utility of acetyltransferase (ARD1) and glutaminyl tRNA synthetase (QtRNA) for reconstructing Cenozoic relationships as exemplified by the large Australian cicada Pauropsalta generic complex. Mol. Phylogenet. Evol. 2015, 83, 258–277. [Google Scholar] [CrossRef]
- Qi, M.; Sun, Y.; Li, H. Taxonomic review of the genus Orybina Snellen, 1895 (Lepidoptera, Pyralidae, Pyralinae), with description of two new species. Zootaxa 2017, 4303, 545–558. [Google Scholar] [CrossRef]
- Singh, N.; Ranjan, R. A new species of Orybina Snellen, 1895 from India (Lepidoptera, Pyralidae, Pyralinae). Zootaxa 2018, 4392, 595–597. [Google Scholar] [CrossRef]
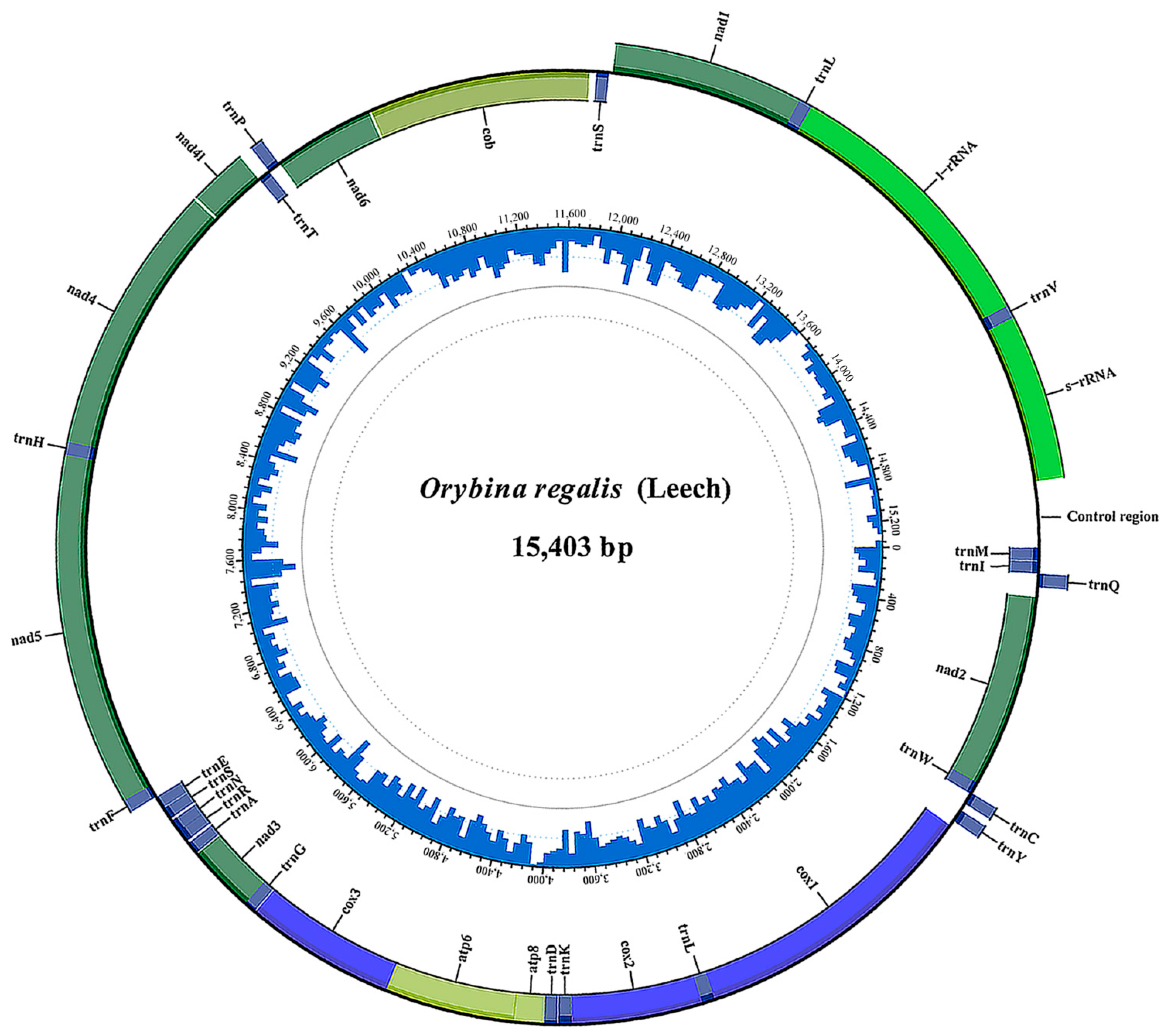
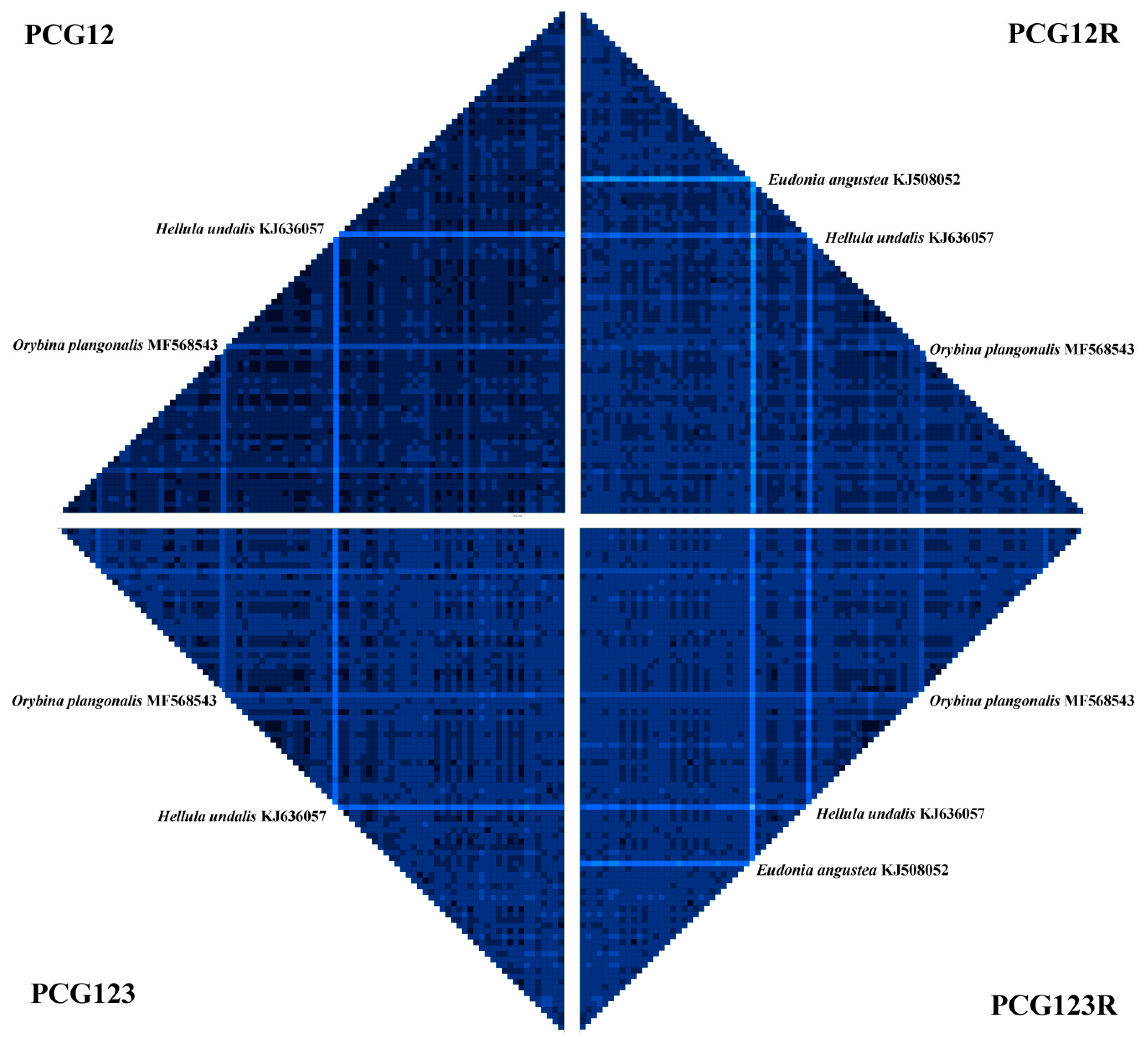

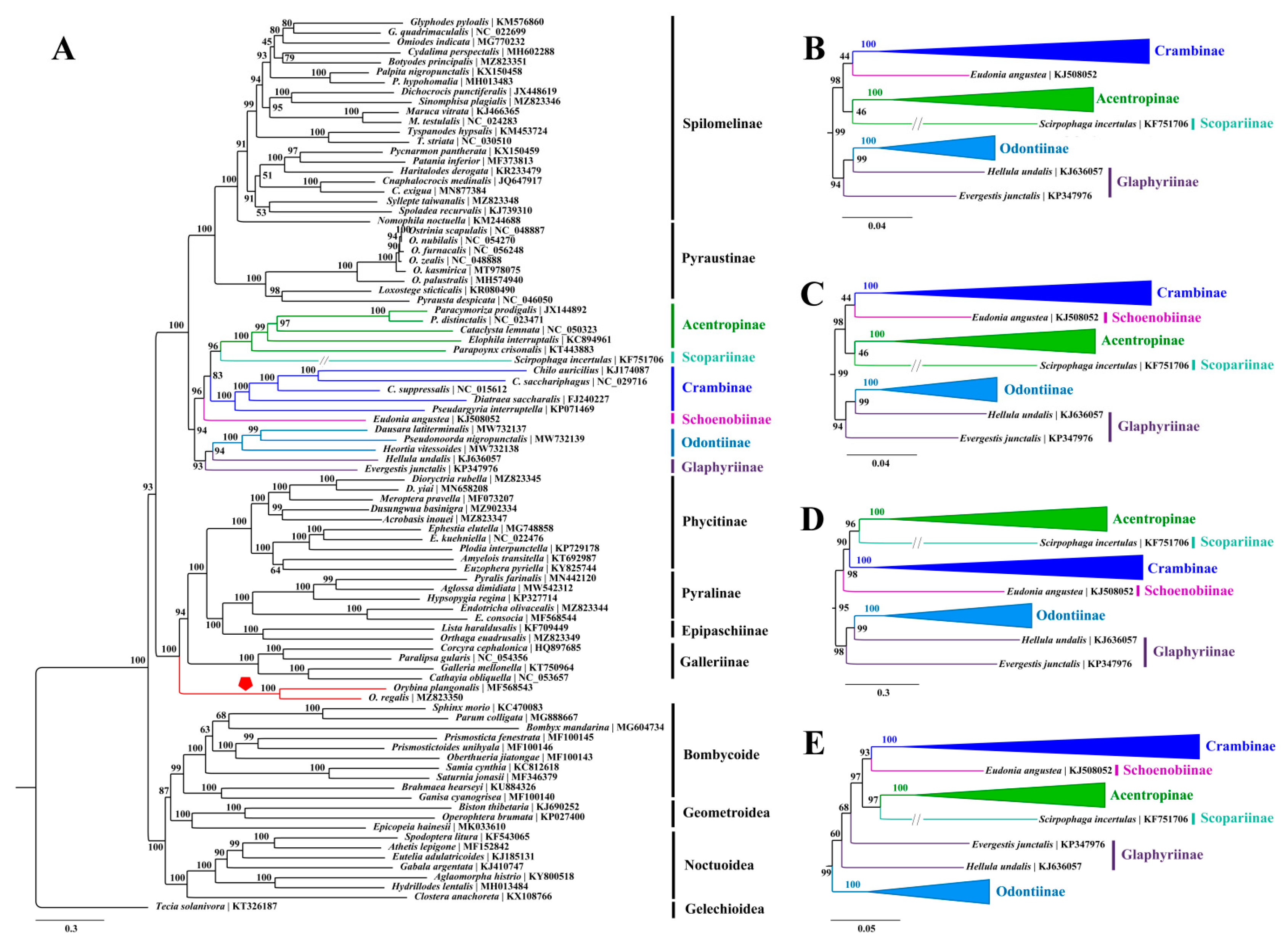
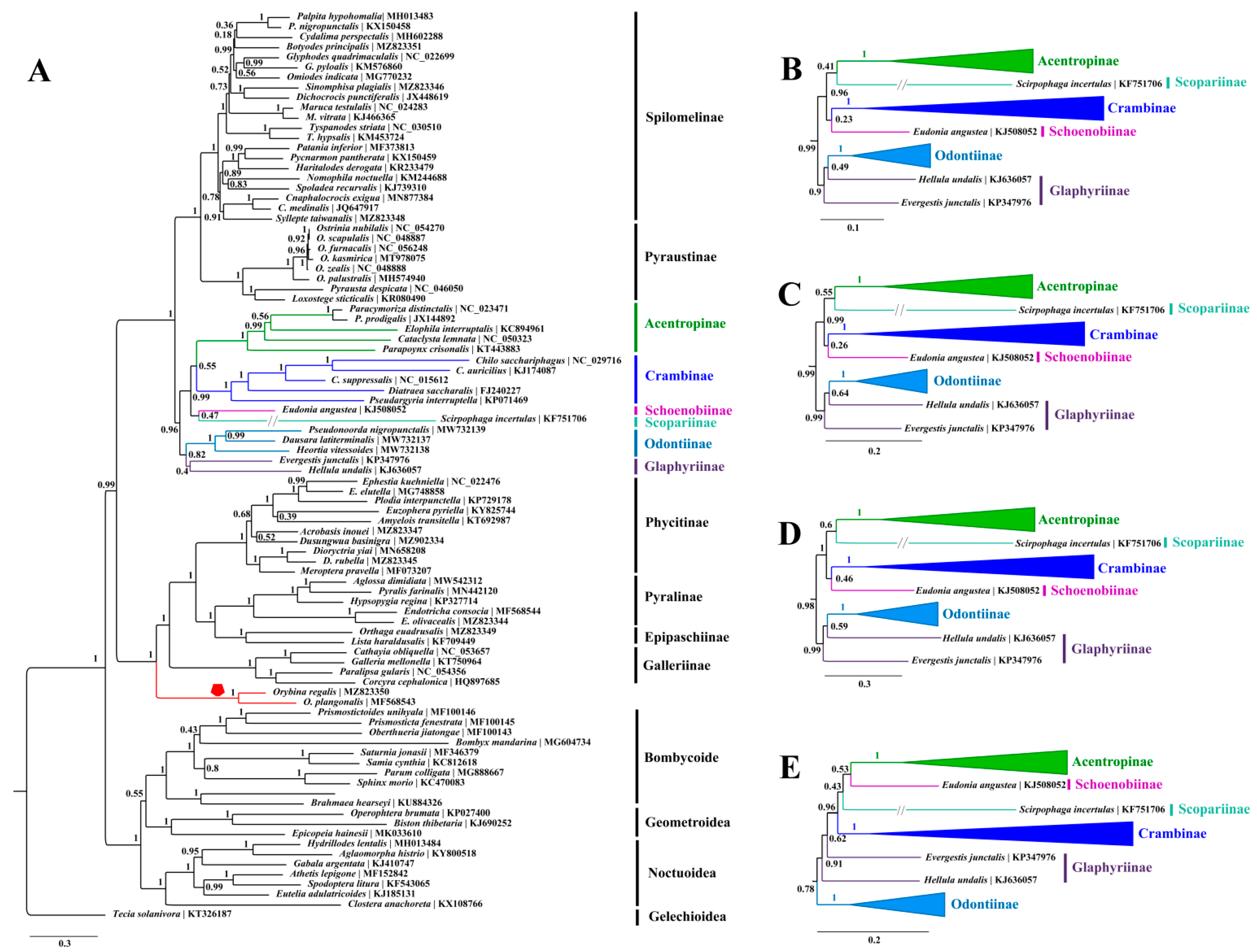
| Superfamily | Family | Taxonomy | Species | GenBank Accession Number | Mitogenome Size (bp) | Reference |
|---|---|---|---|---|---|---|
| Pyraloidea | Crambidae | Spilomelinae | Glyphodes pyloalis | KM576860 | 14,960 | [34] |
| G. quadrimaculalis | NC_022699 | 15,255 | [35] | |||
| Omiodes indicata | MG770232 | 15,367 | [36] | |||
| Cydalima perspectalis | MH602288 | 15,232 | [37] | |||
| Botyodes principalis | MZ823351 | 15,262 | This study | |||
| Palpita nigropunctalis | KX150458 | 15,226 | [38] | |||
| P. hypohomalia | MH013483 | 15,280 | [39] | |||
| Dichocrocis punctiferalis | JX448619 | 15,355 | [40] | |||
| Sinomphisa plagialis | MZ823346 | 15,214 | This study | |||
| Maruca vitrata | KJ466365 | 15,385 | Unpublished | |||
| M. testulalis | NC_024283 | 15,110 | [24] | |||
| Tyspanodes hypsalis | KM453724 | 15,329 | [41] | |||
| T. striata | NC_030510 | 15,255 | [11] | |||
| Pycnarmon pantherata | KX150459 | 15,545 | [38] | |||
| Patania inferior | MF373813 | 15,348 | [38] | |||
| Haritalodes derogata | KR233479 | 15,253 | [42] | |||
| Spoladea recurvalis | KJ739310 | 15,273 | [43] | |||
| Cnaphalocrocis medinalis | JQ647917 | 15,368 | [44] | |||
| C. exigua | MN877384 | 15,262 | [45] | |||
| Syllepte taiwanalis | MZ823348 | 15,264 | This study | |||
| Nomophila noctuella | KM244688 | 15,309 | [46] | |||
| Pyraustinae | Ostrinia scapulalis | NC_048887 | 15,311 | [47] | ||
| O. nubilalis | NC_054270 | 14,838 | [48] | |||
| O. zealis | NC_048888 | 15,208 | [47] | |||
| O. furnacalis | NC_056248 | 15,241 | [49] | |||
| O. kasmirica | MT978075 | 15,214 | [26] | |||
| O. palustralis | MH574940 | 15,246 | [25] | |||
| Loxostege sticticalis | KR080490 | 15,218 | [50] | |||
| Pyrausta despicata | NC_046050 | 15,389 | Unpublished | |||
| Acentropinae | Paracymoriza prodigalis | JX144892 | 15,326 | [51] | ||
| P. distinctalis | NC_023471 | 15,354 | [52] | |||
| Cataclysta lemnata | NC_050323 | 15,333 | Unpublished | |||
| Elophila interruptalis | KC894961 | 15,351 | [53] | |||
| Parapoynx crisonalis | KT443883 | 15,374 | [38] | |||
| Schoenobiinae | Scirpophaga incertulas | KF751706 | 15,220 | [43] | ||
| Crambinae | Chilo auricilius | KJ174087 | 15,367 | [54] | ||
| C. sacchariphagus | NC_029716 | 15,378 | Direct Submission | |||
| C. suppressalis | NC_015612 | 15,395 | [55] | |||
| Diatraea saccharalis | FJ240227 | 15,490 | [56] | |||
| Pseudargyria interruptella | KP071469 | 15,231 | [57] | |||
| Scopariinae | Eudonia angustea | KJ508052 | 15,386 | [18] | ||
| Odontiinae | Dausara latiterminalis | MW732137 | 15,147 | [14] | ||
| Pseudonoorda nigropunctalis | MW732139 | 15,084 | [14] | |||
| Heortia vitessoides | MW732138 | 15,237 | [14] | |||
| Glaphyriinae | Hellula undalis | KJ636057 | 14,678 | [58] | ||
| Evergestis junctalis | KP347976 | 15,438 | [11] | |||
| Pyralidae | Phycitinae | Dioryctria rubella | MZ823345 | 15,422 | This study | |
| D. yiai | MN658208 | 15,430 | [59] | |||
| Meroptera pravella | MF073207 | 15,260 | [60] | |||
| Dusungwua basinigra | MZ902334 | 15,328 | This study | |||
| Acrobasis inouei | MZ823347 | 15,239 | This study | |||
| Ephestia elutella | MG748858 | 15,346 | [61] | |||
| E. kuehniella | NC_022476 | 15,295 | [62] | |||
| Plodia interpuncella | KP729178 | 15,287 | [63] | |||
| Amyelois transitella | KT692987 | 15,205 | [64] | |||
| Euzophera pyriella | KY825744 | 15,184 | [65] | |||
| Pyralinae | Pyralis farinalis | MN442120 | 15,204 | [66] | ||
| Aglossa dimidiata | MW542312 | 15,225 | Unpublished | |||
| Hypsopygia regina | KP327714 | 15,212 | [11] | |||
| Endotricha olivacealis | MZ823344 | 15,239 | This study | |||
| E. consocia | MF568544 | 15,201 | [11] | |||
| Orybina plangonalis | MF568543 | 14,823 | [11] | |||
| O. regalis | MZ823350 | 15,403 | This study | |||
| Epipaschiinae | Lista haraldusalis | KF709449 | 15,213 | [53] | ||
| Orthaga euadrusalis | MZ823349 | 15,268 | This study | |||
| Galleriinae | Corcyra cephalonica | HQ897685 | 15,273 | [67] | ||
| Paralipsa gularis | NC_054356 | 15,280 | [68] | |||
| Galleria mellonella | KT750964 | 15,320 | [69] | |||
| Cathayia obliquella | NC_053657 | 15,408 | [70] | |||
| Bombycoidea | Sphingidae | Sphinginae | Sphinx morio | KC470083 | 15,299 | [71] |
| Smerinthinae | Parum colligata | MG888667 | 15,288 | [72] | ||
| Saturniidae | Saturniinae | Samia cynthia | KC812618 | 15,345 | [73] | |
| Saturnia jonasii | MF346379 | 15,261 | [74] | |||
| Endromidae | Prismosticta fenestrata | MF100145 | 15,772 | Unpublished | ||
| Prismostictoides unihyala | MF100146 | 15,355 | Unpublished | |||
| Bombycidae | Oberthuerinae | Oberthueria jiatongae | MF100143 | 15,673 | Unpublished | |
| Bombycinae | Bombyx mandarina | MG604734 | 15,682 | Unpublished | ||
| Brahmaeidae | Brahmaea hearseyi | KU884326 | 15,442 | Direct Submission | ||
| Eupterotidae | Ganisa cyanogrisea | MF100140 | 15,250 | Unpublished | ||
| Geometroidea | Geometridae | Ennominae | Biston thibetaria | KJ690252 | 15,485 | [38] |
| Larentiinae | Operophtera brumata | KP027400 | 15,748 | [75] | ||
| Epicopeiidae | Epicopeia hainesii | MK033610 | 15,395 | [76] | ||
| Noctuoidea | Noctuidae | Amphipyrinae | Spodoptera litura | KF543065 | 15,374 | [77] |
| Noctuinae | Athetis lepigone | MF152842 | 15,589 | [78] | ||
| Erebidae | Euteliinae | Eutelia adulatricoides | KJ185131 | 15,360 | [79] | |
| Nolidae | Chloephorinae | Gabala argentata | KJ410747 | 15,337 | [79] | |
| Erebidae | Arctiinae | Aglaomorpha histrio | KY800518 | 15,472 | Direct Submission | |
| Herminiinae | Hydrillodes lentalis | MH013484 | 15,570 | [80] | ||
| Notodontidae | Pygaerinae | Clostera anachoreta | KX108766 | 15,456 | [81] | |
| Gelechioidea | Gelechiidae | Gelechiinae | Tecia solanivora | KT326187 | 15,251 | [82] |
| Species | Mitogenome Size (bp) | A% | G% | C% | T% | AT% | AT-Skew | GC-Skew |
|---|---|---|---|---|---|---|---|---|
| Syllepte taiwanalis | 15,264 | 40.5 | 7.5 | 10.8 | 41.2 | 81.7 | −0.00857 | −0.18033 |
| Botyodes principalis | 15,262 | 39.8 | 7.8 | 11.5 | 40.9 | 80.7 | −0.01363 | −0.19171 |
| Sinomphisa plagialis | 15,214 | 40.0 | 7.6 | 11.8 | 40.6 | 80.6 | −0.00744 | −0.21649 |
| Orthaga euadrusalis | 15,268 | 39.3 | 7.9 | 11.9 | 40.9 | 80.2 | −0.01995 | −0.20202 |
| Endotricha olivacealis | 15,239 | 39.0 | 7.6 | 11.7 | 41.7 | 80.7 | −0.03346 | −0.21244 |
| Orybina regalis | 15,403 | 39.8 | 7.6 | 11.4 | 41.2 | 81.0 | −0.01728 | −0.20000 |
| Dioryctria rubella | 15,422 | 39.0 | 7.7 | 12.4 | 40.8 | 79.8 | −0.02256 | −0.23383 |
| Dusungwua basinigra | 15,328 | 39.4 | 7.9 | 12.1 | 40.6 | 80.0 | −0.01500 | −0.21000 |
| Acrobasis inouei | 15,239 | 39.3 | 7.7 | 12.0 | 41.0 | 80.3 | −0.02117 | −0.21827 |
| Data Partitions | NumOTU | Iss | Iss.cSym | PSym | Iss.cAsym | PAsym |
|---|---|---|---|---|---|---|
| PCG1s | 32 | 0.233 | 0.809 | 0.0000 | 0.554 | 0.0000 |
| PCG2s | 32 | 0.145 | 0.809 | 0.0000 | 0.554 | 0.0000 |
| PCG3s | 32 | 0.582 | 0.809 | 0.0000 | 0.554 | 0.0036 |
| rRNAs | 32 | 0.439 | 0.793 | 0.0000 | 0.525 | 0.0004 |
| tRNAs | 32 | 0.279 | 0.775 | 0.0000 | 0.492 | 0.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Qi, M.; Xu, H.; Wu, Z.; Hu, L.; Yang, M.; Li, H. Nine Mitochondrial Genomes of the Pyraloidea and Their Phylogenetic Implications (Lepidoptera). Insects 2021, 12, 1039. https://doi.org/10.3390/insects12111039
Liu X, Qi M, Xu H, Wu Z, Hu L, Yang M, Li H. Nine Mitochondrial Genomes of the Pyraloidea and Their Phylogenetic Implications (Lepidoptera). Insects. 2021; 12(11):1039. https://doi.org/10.3390/insects12111039
Chicago/Turabian StyleLiu, Xiaomeng, Mujie Qi, Haizhen Xu, Zhipeng Wu, Lizong Hu, Mingsheng Yang, and Houhun Li. 2021. "Nine Mitochondrial Genomes of the Pyraloidea and Their Phylogenetic Implications (Lepidoptera)" Insects 12, no. 11: 1039. https://doi.org/10.3390/insects12111039
APA StyleLiu, X., Qi, M., Xu, H., Wu, Z., Hu, L., Yang, M., & Li, H. (2021). Nine Mitochondrial Genomes of the Pyraloidea and Their Phylogenetic Implications (Lepidoptera). Insects, 12(11), 1039. https://doi.org/10.3390/insects12111039






