Developing a Phototactic Electrostatic Insect Trap Targeting Whiteflies, Leafminers, and Thrips in Greenhouses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
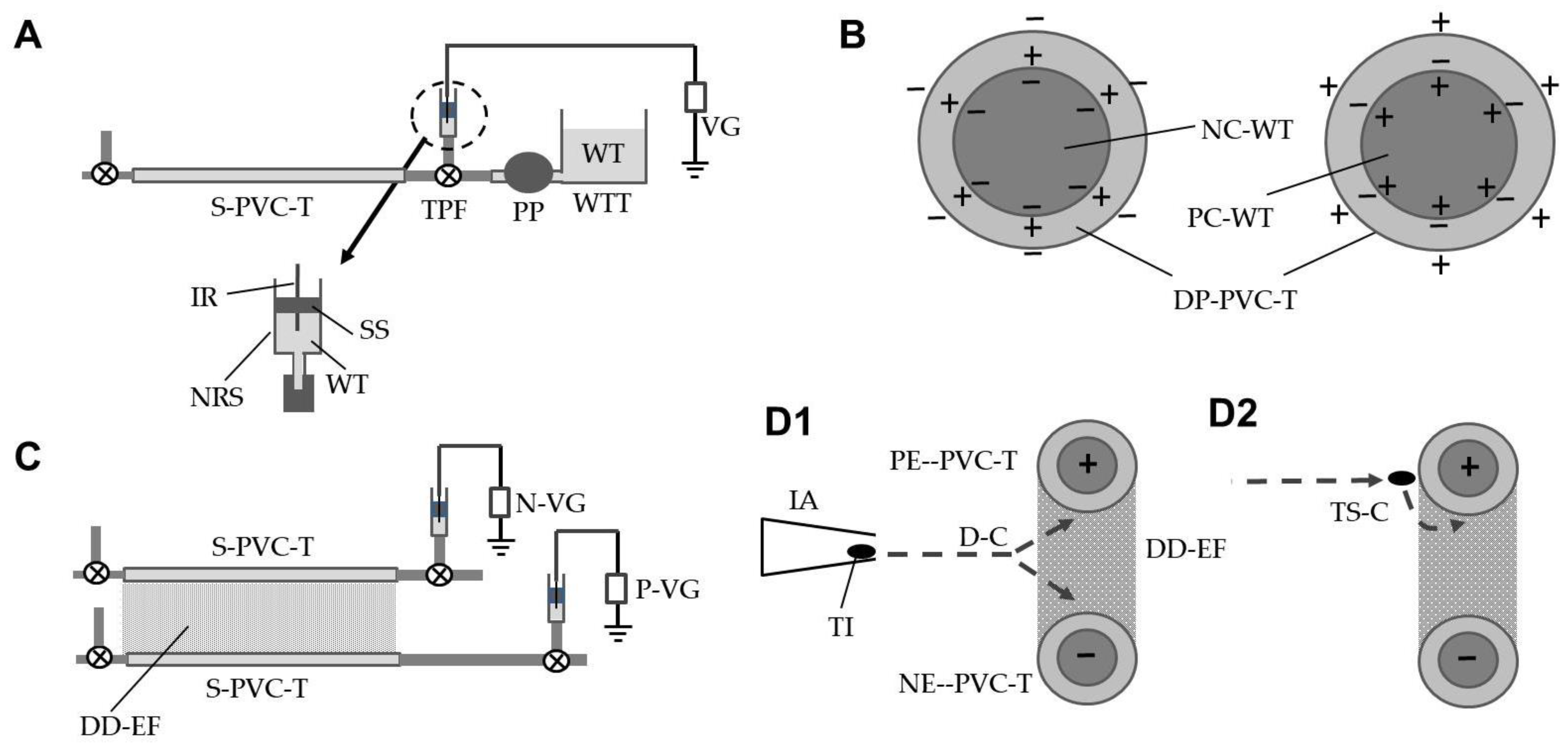
2.2. Electrification of S-PVC Tubes and Formation of the DD-Field for Insect Capture
2.3. Construction of the DD-EFP
2.4. Assay of the Ability of Colored DD-EFP to Attract Insects
2.5. Simplification of DD-EFP Insect-Capturing System for Practical Use
2.6. Statistical Analysis
3. Results and Discussion
3.1. Electrification of an Insulator Tube by Charged Colored Water
3.2. Construction of the DD-EFP for Capturing Insects
3.3. Addition of Insect-Attracting Ability to the DD-EFP
3.4. Feasibility of the DD-EFP for Practical Use
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Navot, N.; Pichersky, E.; Zeiden, M.; Zamir, D.; Czosnek, H. Tomato yellow leaf curl virus, a Bemisia tabaci -transmitted geminivirus with a single genomic component. Virology 1991, 185, 151–161. [Google Scholar] [CrossRef]
- Riley, D.G.; Srinivasan, R. Integrated management of tomato yellow leaf curl virus and its whitefly vector in tomato. J. Econ. Entomol. 2019, 112, 1526–1540. [Google Scholar] [CrossRef]
- Houle, J.L.; Kennedy, G.G. Tomato spotted wilt virus can infect resistant tomato when western flower thrips inoculate blossoms. Plant Dis. 2017, 101, 1666–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Guo, J.-F.; Reitz, S.R.; Lei, Z.-R.; Wu, S.-Y. A global invasion by the thrip, Frankliniella occidentalis: Current virus vector status and its management. Insect Sci. 2020, 27, 626–645. [Google Scholar] [CrossRef] [PubMed]
- Rendina, N.; Nuzzaci, M.; Scopa, A.; Cuypers, A.; Sofo, A. Chitosan-elicited defense responses in cucumber mosaic virus (CMV)-infected tomato plants. J. Plant Physiol. 2019, 234–235, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, D.R.; Menzies, J.G. Fungus gnats vector Fusarium oxysporum f. sp. radicis-lycopersici. Ann. Appl. Biol. 1993, 123, 539–544. [Google Scholar] [CrossRef]
- El-Hamalawi, Z.A. Attraction, acquisition, retention and spatiotemporal distribution of soilborne plant pathogenic fungi by shore flies. Ann. Appl. Biol. 2008, 152, 169–177. [Google Scholar] [CrossRef]
- Matsuda, Y.; Nonomura, T.; Kakutani, K.; Takikawa, Y.; Kimbara, J.; Kasaishi, Y.; Kusakari, S.; Toyoda, H. A newly devised electric field screen for avoidance and capture of cigarette beetles and vinegar flies. Crop Prot. 2011, 30, 155–162. [Google Scholar] [CrossRef]
- Nonomura, T.; Matsuda, Y.; Kakutani, K.; Kimbara, J.; Osamura, K.; Kusakari, S.; Toyoda, H. An electric field strongly deters whiteflies from entering window-open greenhouses in an electrostatic insect exclusion strategy. Eur. J. Plant Pathol. 2012, 134, 661–670. [Google Scholar] [CrossRef]
- Toyoda, H.; Kusakari, S.; Matsuda, Y.; Kakutani, K.; Xu, L.; Nonomura, T.; Takikawa, Y. Practical implementation of single-charged dipolar electric field screen. In An Illustrated Manual of Electric Field Screens: Their Structures and Functions; Toyoda, H., Ed.; RAEFSS Publishing Department: Nara, Japan, 2019; pp. 41–49. [Google Scholar]
- Yee, W.L. Three-dimensional versus rectangular sticky yellow traps for western cherry fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 2019, 112, 1780–1788. [Google Scholar] [CrossRef]
- Moreau, T.L.; Isman, M.B. Trapping whiteflies? A comparison of greenhouse whitefly (Trialeurodes vaporariorum) responses to trap crops and yellow sticky traps. Pest Manag. Sci. 2011, 67, 408–413. [Google Scholar] [CrossRef]
- Hoelmer, K.M.; Simmons, A.M. Yellow sticky trap catches of parasitoids of Bemisia tabaci (Hemiptera: Aleyrodidae) in vegetable crops and their relationship to in-field populations. Environ. Entomol. 2008, 37, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Bei, Y.; Zhang, J. Are yellow sticky traps an effective method for control of sweet potato whitefly, Bemisia tabaci, in the greenhouse or field? J. Insect Sci. 2012, 12, 113. [Google Scholar] [CrossRef]
- Stukenberg, N.; Pietruska, M.; Waldherr, A.; Meyhöfer, R. Wavelength-specific behavior of the western flower thrips (Frankliniella occidentalis): Evidence for a blue-green chromatic mechanism. Insects 2020, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarpour, H.; Rawi, C.S.M. Evaluation of yellow sticky traps for monitoring the population of thrips (Thysanoptera) in a mango orchard. Environ. Entomol. 2011, 40, 873–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrella, M.P.; Jones, V.P. Yellow traps as monitoring tools for Liriomyza trifolii (Diptera: Agromyzidae) in chrysanthemum greenhouses. J. Econ. Entomol. 1985, 78, 53–56. [Google Scholar] [CrossRef]
- Murata, M.; Yamahama, Y.; Hariyama, T. Synergistic effects of the red light and blue traps on control of Thrips palmi (Thysanoptera: Thripidae). J. Econ. Entomol. 2021, 114, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.D.; Zhao, H.Y.; Fu, B.L.; Han, Y.; Liu, K.; Wu, J.H. Colored sticky traps to selectively survey thrips in cowpea ecosystem. Neotrop Entomol. 2016, 45, 96–101. [Google Scholar] [CrossRef]
- Katai, Y.; Ishikawa, R.; Doi, M.; Masui, S. Efficacy of red LED irradiation for controlling Thrips palmi in greenhouse melon cultivation. Jpn. J. Appl. Entomol. Zool. 2015, 59, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Shibao, M.; Tanaka, H. The effects of eggplants illuminated by red LED (light emitting diode) on the population density of the melon thrips, Thrips palmi (Thysanoptera: Thripidae). Jpn. J. Appl. Entomol. Zool. 2015, 59, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Shimoda, M. Recent advances in the optical control of insect pests using light and color. In Proceedings of the 2018 International Symposium on Proactive Technologies for Enhancement of Integrated Pest Management of Key Crops, Taichung, Taiwan, 3–5 April 2018; pp. 87–98. [Google Scholar]
- Toyoda, H.; Matsuda, Y. Basic concept for constructing an electric field screen. In Electric Field Screen; Principles and Applications; Toyoda, H., Ed.; Nobunkyo Production: Tokyo, Japan, 2015; pp. 3–17. [Google Scholar]
- Nonomura, T.; Matsuda, Y.; Kakutani, K.; Takikawa, Y.; Kimbara, J.; Osamura, K.; Kusakari, S.; Toyoda, H. Prevention of whitefly entry from a greenhouse entrance by furnishing an airflow-oriented pre-entrance room guarded with electric field screens. J. Agric. Sci. 2014, 6, 172–184. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, Y.; Kakutani, K.; Nonomura, T.; Kimbara, J.; Kusakari, S.; Osamura, K.; Toyoda, H. An oppositely charged insect exclusion screen with gap-free multiple electric fields. J. Appl. Phys. 2012, 112, 116103. [Google Scholar] [CrossRef] [Green Version]
- Invasive Species Compendium. Detailed Coverage of Invasive Species Threatening Livelihoods and the Environment Worldwide. Available online: https://www.cabi.org/isc/datasheet/8925 (accessed on 16 October 2021).
- Jones, E.; Childers, R. Electric charge and electric field. In Physics, 3rd ed.; McGraw-Hill Education (Asia), Ed.; McGraw-Hill: Boston, MD, USA, 2002; pp. 495–525. [Google Scholar]
- Halliday, D.; Resnick, R.; Walker, J. Electric discharge and electric fields. In Fundamentals of Physics; Johnson, S., Ford, E., Eds.; John Wiley & Sons: New York, NY, USA, 2005; pp. 561–604. [Google Scholar]
- Munsell Color Company. Munsell Hue Circle Poster. Available online: https://munsell.com/color-blog/munsell-hue-circle-poster/ (accessed on 28 June 2021).
- Kakutani, K.; Matsuda, Y.; Nonomura, T.; Takikawa, Y.; Osamura, K.; Toyoda, H. Remote-controlled monitoring of flying pests with an electrostatic insect capturing apparatus carried by an unmanned aerial vehicle. Agriculture 2021, 11, 176. [Google Scholar] [CrossRef]
- Kaiser, K.L. Rate of charge decay. In Electrostatic Discharge; Kaiser, K.L., Ed.; Taylor & Francis: New York, NY, USA, 2006; pp. 214–224. [Google Scholar]
- Wegner, H.E. Electrical charging generators. In McGraw-Hill Encyclopedia of Science and Technology, 9th ed.; Geller, E., Moore, K., Well, J., Blumet, D., Felsenfeld, S., Martin, T., Rappaport, A., Wagner, C., Lai, B., Taylor, R., Eds.; The Lakeside Press: New York, NY, USA, 2002; pp. 42–43. [Google Scholar]
- Toyoda, H.; Kusakari, S.; Matsuda, Y.; Kakutani, K.; Xu, L.; Nonomura, T.; Takikawa, Y. Electric filed screen structures. In An Illustrated Manual of Electric Field Screens: Their Structures and Functions; Toyoda, H., Ed.; RAEFSS Publishing Department: Nara, Japan, 2019; pp. 9–15. [Google Scholar]
- Kusakari, S.; Okada, K.; Shibao, M.; Toyoda, H. High voltage electric fields have potential to create new physical pest control systems. Insects 2020, 11, 447. [Google Scholar] [CrossRef]
- Guo, Z.-G.; Wang, M.-X.; Cui, L.; Ha, B.-Y. Advance in insect phototaxis and the development and application of colored sticky boards. Ying Yong Sheng Tai Xue Bao 2019, 30, 3615–3626. [Google Scholar]
- Helyer, N.; Brown, K.; Cattlin, N.D. Pest profiles. In A Colour Handbook of Biological Control in Plant Protection; Northcott, J., Ed.; Manson Publishing: London, UK, 2004; pp. 21–41. [Google Scholar]
- Broughton, S.; Harrison, J. Evaluation of monitoring methods for thrips and the effect of trap colour and semiochemicals on sticky trap capture of thrips (Thysanoptera) and beneficial insects (Syrphidae, Hemerobiidae) in deciduous fruit trees in Western Australia. Crop Protect. 2012, 42, 156–163. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Chu, C.-C.; Fitzgerald, G.; Natwick, E.T.; Henneberry, T.J. Trap evaluations for thrips (Thysanoptera: Thripidae) and hoverflies (Diptera: Syrphidae). Environ. Entomol. 2004, 33, 1416–1420. [Google Scholar] [CrossRef] [Green Version]







| DD-EFP Used | Test Insect | Negative and Positive Voltage (kV) Applied to S-PVC-Tubes | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.2 | 0.4 | 0.6 | 0.8 | 1 | 1.2 | 2 | ||
| Non-colored | Whiteflies | 0 | 21.2 ± 7.5 a | 68.5 ± 2.5 a | 100 a | 100 a | 100 | 100 |
| Western flower thrips | 0 | 9.5 ± 0.8 b | 38.7 ± 1.5 b | 82.5 ± 2.5 b | 100 a | 100 | 100 | |
| Vegetable leafminers | 0 | 1.5 ± 0.09 c | 22.8 ± 1.7 c | 64.6 ± 4.3 c | 83.4 ± 1.8 b | 100 | 100 | |
| Yellow-colored | Whiteflies | 0 | 19.9 ± 3.3 a | 61.6 ± 1.8 a | 100 a | 100 a | 100 | 100 |
| Western flower thrips | 0 | 8.8 ± 0.7 b | 30.9 ± 2.1 b | 85.1 ± 1.3 b | 100 a | 100 | 100 | |
| Vegetable leafminers | 0 | 1.9 ± 0.1 c | 20.8 ± 0.7 c | 69.6 ± 2.3 c | 84.5 ± 2.2 b | 100 | 100 | |
| Blue-colored | Whiteflies | 0 | 20.3 ± 4.5 a | 68.5 ± 2.5 a | 100 a | 100 a | 100 | 100 |
| Western flower thrips | 0 | 9.2 ± 0.4 b | 38.7 ± 1.5 b | 83.7 ± 2.2 b | 100 a | 100 | 100 | |
| Vegetable leafminers | 0 | 1.2 ± 0.09 c | 24.1 ± 1.6 c | 66.8 ± 2.3 c | 87.4 ± 2.2 b | 100 | 100 | |
| Red-colored | Whiteflies | 0 | 21.9 ± 7.5 a | 62.8 ± 1.9 a | 100 a | 100 a | 100 | 100 |
| Western flower thrips | 0 | 8.9 ± 1.1 b | 38.7 ± 1.5 b | 86.5 ± 0.5 b | 100 a | 100 | 100 | |
| Vegetable leafminers | 0 | 1.1 ± 0.08 c | 21.9 ± 2.4 c | 62.5 ± 3.9 c | 82.9 ± 1.4 b | 100 | 100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takikawa, Y.; Nonomura, T.; Sonoda, T.; Matsuda, Y. Developing a Phototactic Electrostatic Insect Trap Targeting Whiteflies, Leafminers, and Thrips in Greenhouses. Insects 2021, 12, 960. https://doi.org/10.3390/insects12110960
Takikawa Y, Nonomura T, Sonoda T, Matsuda Y. Developing a Phototactic Electrostatic Insect Trap Targeting Whiteflies, Leafminers, and Thrips in Greenhouses. Insects. 2021; 12(11):960. https://doi.org/10.3390/insects12110960
Chicago/Turabian StyleTakikawa, Yoshihiro, Teruo Nonomura, Takahiro Sonoda, and Yoshinori Matsuda. 2021. "Developing a Phototactic Electrostatic Insect Trap Targeting Whiteflies, Leafminers, and Thrips in Greenhouses" Insects 12, no. 11: 960. https://doi.org/10.3390/insects12110960
APA StyleTakikawa, Y., Nonomura, T., Sonoda, T., & Matsuda, Y. (2021). Developing a Phototactic Electrostatic Insect Trap Targeting Whiteflies, Leafminers, and Thrips in Greenhouses. Insects, 12(11), 960. https://doi.org/10.3390/insects12110960






