Volatiles Induced from Hypolepis punctata (Dennstaedtiaceae) by Herbivores Attract Sclomina erinacea (Hemiptera: Reduviidae): Clear Evidence of Indirect Defense in Fern
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Survey of Visitation
2.2. Collecting and Analyzing Volatile Organic Compounds from Hypolepis punctata
2.3. Electroantennogram Recordings
2.4. Y-Olfactometer Experiment
2.5. Data Analysis
3. Results
3.1. The Visitation Observation
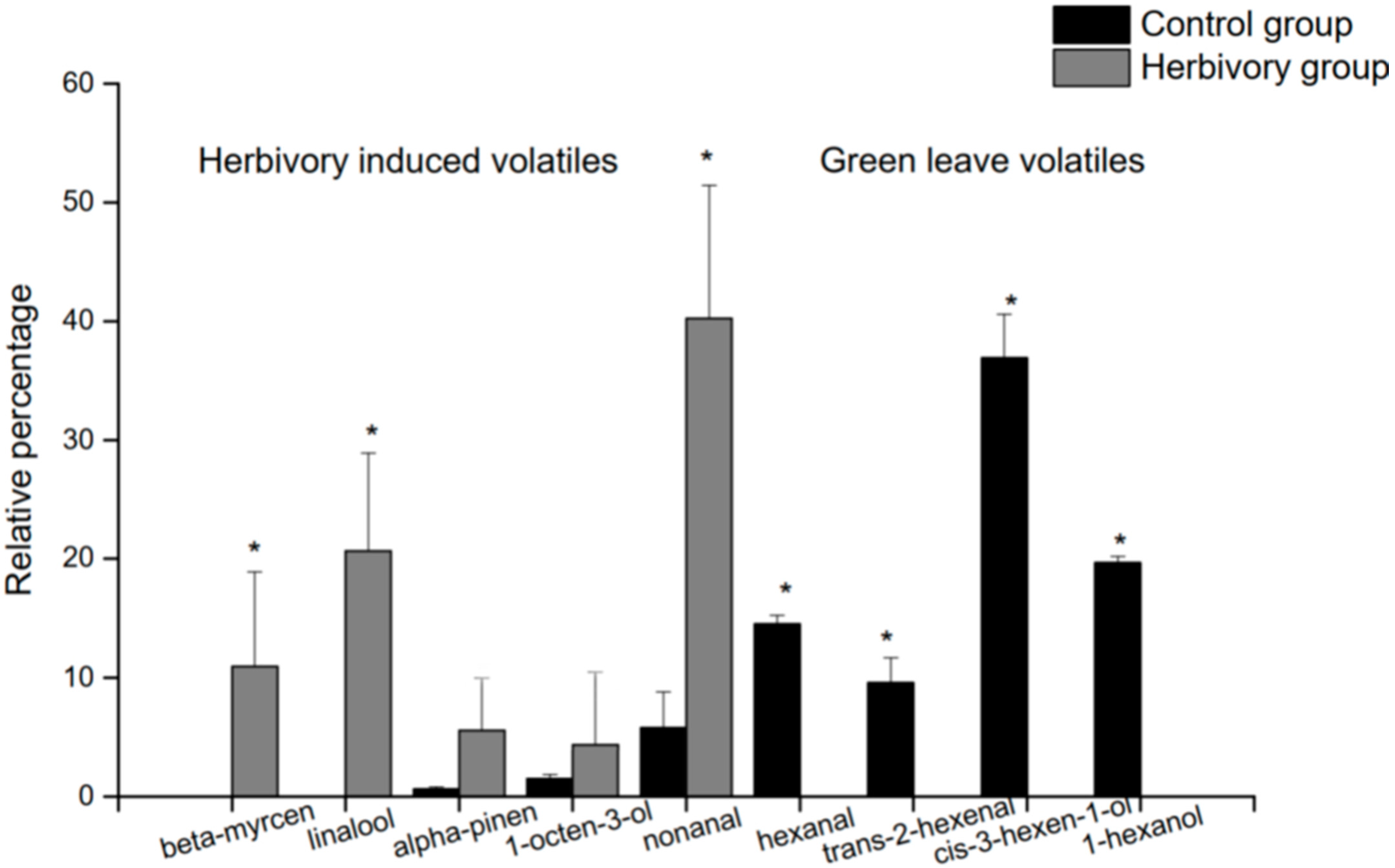
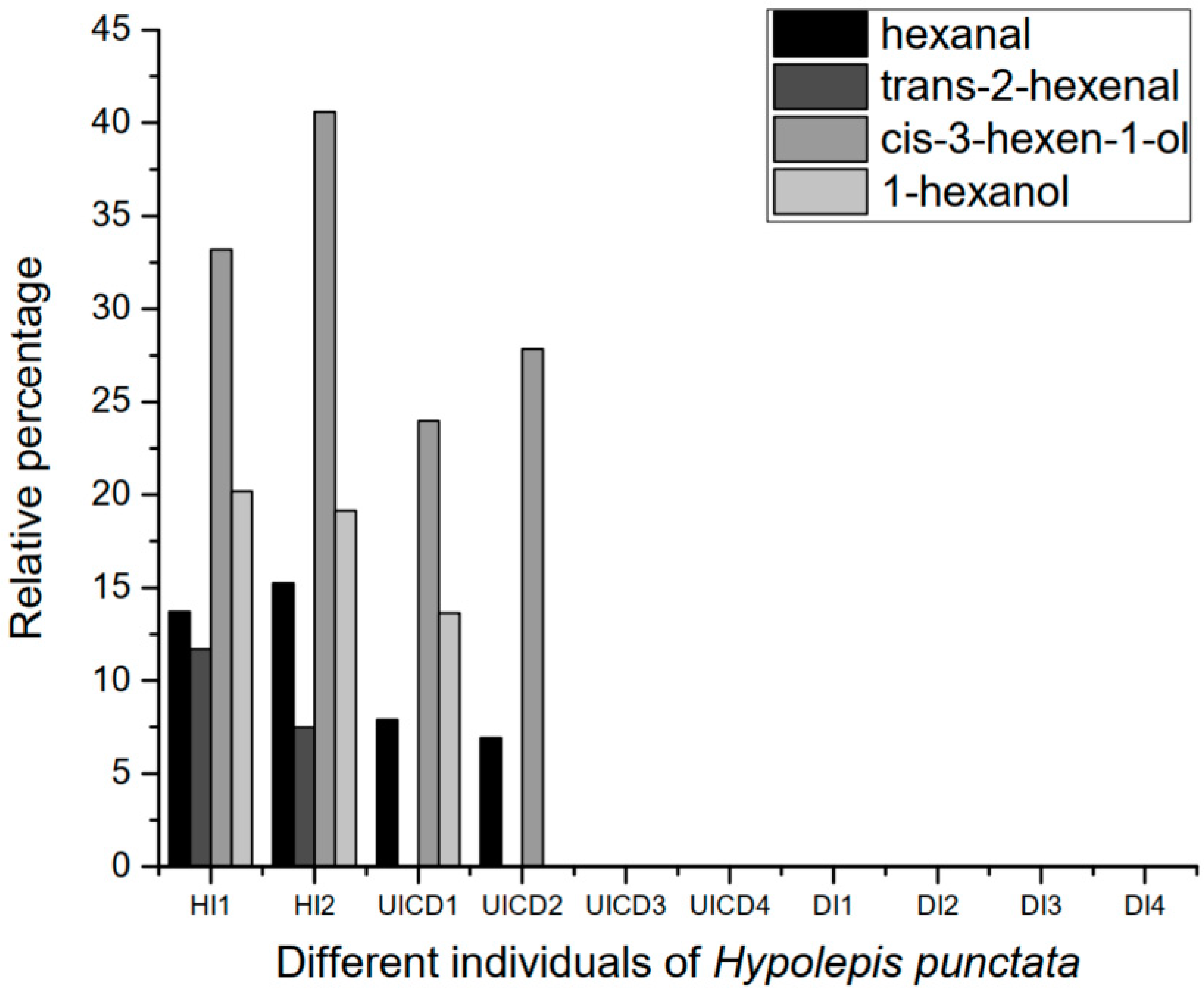
3.2. VOCs of Herbivore Damaged and Undamaged Hypolepis punctata
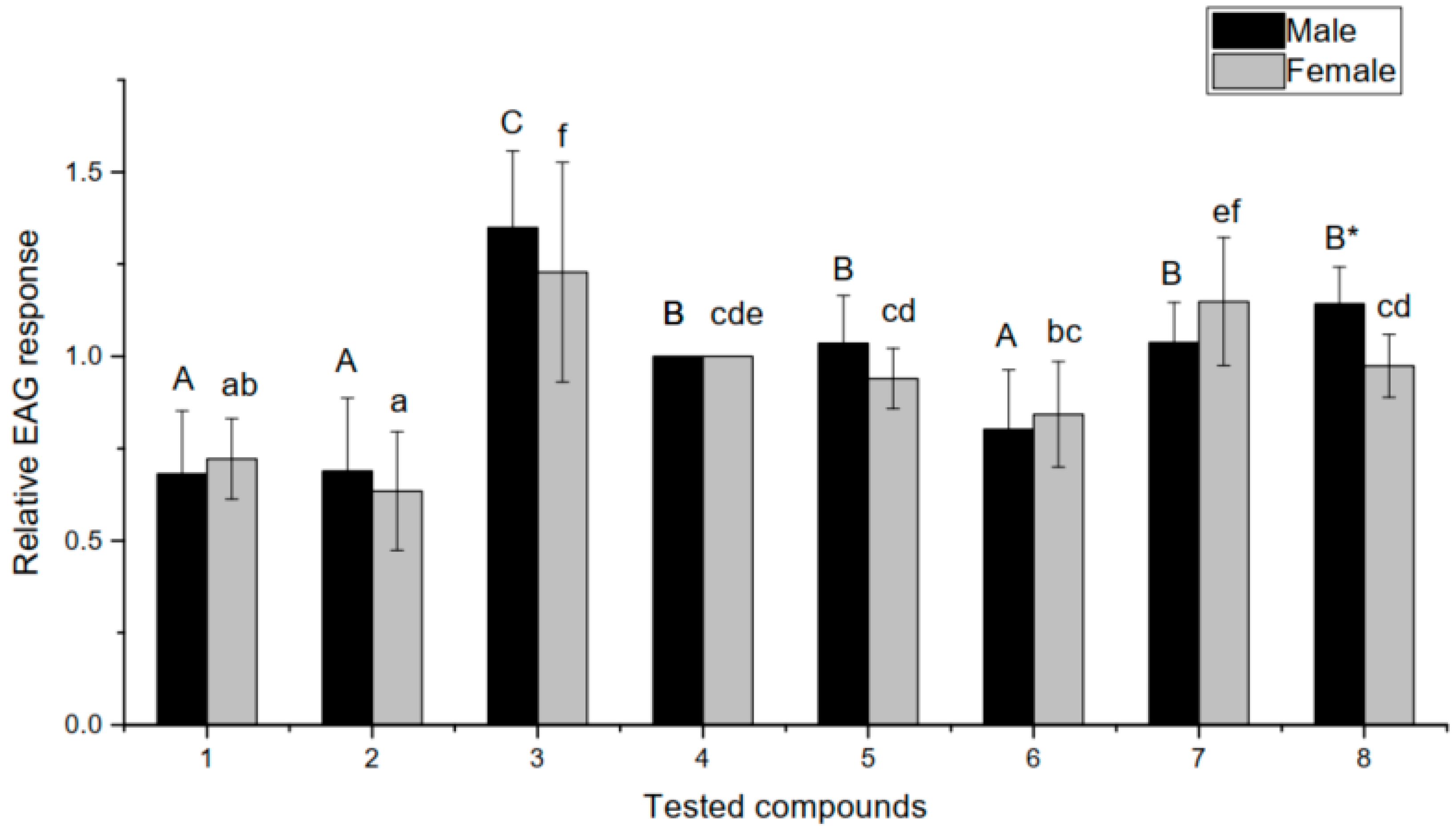
3.3. Electroantennogram Recordings
3.4. Y-Olfactometer Experiment
4. Discussion
4.1. Volatiles Induced from H. Punctata
4.2. Electrophysiological Experiments Using Volatile Standards
4.3. Behavioral Experiments Using Volatile Standards
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VOCs | Volatile organic compounds |
| EAG | electroantennogram |
| GLVs | green leaf volatiles |
References
- Schoonhoven, L.M.; van Loon, J.J.; Dicke, M. Insect-Plant Biology; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, I.; Halitschke, R.; Kessler, A.; Sardanelli, S.; Denno, R.F. Constitutive and induced defenses to herbivory in above- and belowground plant tissues. Ecology 2008, 89, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duke, S.O.; Canel, C.; Rimando, A.M.; Tellez, M.R.; Duke, M.V.; Paul, R.N. Current and potential exploitation of plant glandular trichome productivity. Adv. Bot. Res. Inc. Adv. Plant Pathol. 2000, 31, 121–151. [Google Scholar]
- Heil, M. Indirect defence via tritrophic interactions. New Phytol. 2007, 178, 41–61. [Google Scholar] [CrossRef]
- Dicke, M.; van Beek, T.A.; Posthumus, M.A.; Ben-Dom, N.; van Bokhoven, H.; de Groot, A. Isolation and identification of volatile kairomone that affects acarine predatorprey interactions Involvement of host plant in its production. J. Chem. Ecol. 1990, 16, 381–396. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, C.M.; Lewis, W.J.; Pare, P.W.; Alborn, H.T.; Tumlinson, J.H. Herbivore-infested plants selectively attract parasitoids. Nature 1998, 393, 570–573. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in Nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef]
- Chessin, M.; Zipf, A.E. Alarm systems in higher plants. Bot. Rev. 1990, 56, 193–235. [Google Scholar] [CrossRef]
- Karban, R.; Yang, L.; Edwards, K.F. Volatile communication between plants that affects herbivory: A meta-analysis. Ecol. Lett. 2013, 17, 44–52. [Google Scholar] [CrossRef]
- Skoczek, A.; Piesik, D.; Wenda-Piesik, A.; Buszewski, B.; Bocianowski, J.; Wawrzyniak, M. Volatile organic compounds released by maize following herbivory or insect extract application and communication between plants. J. Appl. Èntomol. 2016, 141, 630–643. [Google Scholar] [CrossRef]
- Weinhold, A.; Baldwin, I.T. Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. Proc. Natl. Acad. Sci. USA 2011, 108, 7855–7859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.-Q.; Zhang, Z.-X.; Li, Y.-Z.; Li, Y.-X.; Xu, H.-H. Anti-Insect activity of the methanol extracts of fern and gymnosperm. Agric. Sci. China 2010, 9, 249–256. [Google Scholar] [CrossRef]
- Markham, K.; Chalk, T.; Stewart, C.N. Evaluation of fern and moss protein-based defenses against phytophagous insects. Int. J. Plant Sci. 2006, 167, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Imbiscuso, G.; Trotta, A.; Maffei, M.; Bossi, S. Herbivory induces a ROS burst and the release of volatile organic compounds in the fern Pteris vittata L. J. Plant Interact. 2009, 4, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Koptur, S.; Rico-Gray, V.; Palacios-Rios, M. Ant protection of the nectaried fern Polypodium plebeium in central Mexico. Am. J. Bot. 1998, 85, 736–739. [Google Scholar] [CrossRef]
- McAuslane, H.J.; Metcalf, R.L.; Metcalf, E.R. Plant kairomones in insect ecology and control. Fla. Èntomol. 1992, 75, 613. [Google Scholar] [CrossRef]
- Zhao, P.; Yuan, J.L. Theinsect list and faunal analysis of Harpactorinae in Guizhou province. Guizhou Agric. Sci. 2011, 37, 99–102. [Google Scholar]
- Wu, Z.Y.; Raven, P.H.; Hong, D.Y. Flora of China; Lycopodiaceae through Polypodiaceae; Science Press: Beijing, China, 2013; Volume 2–3. [Google Scholar]
- Paré, P.W.; Tumlinson, J.H. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999, 121, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, Y.; Nishizawa, M.; Sakan, T. Studies on the sesquiterpenoids of Hypolepis punctata mett.—I: Isolation and structure determination of hypacrone, a new seco-illudoid. Tetrahedron 1977, 33, 2509–2511. [Google Scholar] [CrossRef]
- Du, Y.; Poppy, G.M.; Powell, W.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J. Chem. Ecol. 1998, 24, 1355–1368. [Google Scholar] [CrossRef]
- Ishkawa, M.; Shuto, Y.; Watanabe, H. β-Myrcene, a potent attractant component of pine wood for the pine wood nematode, Bursaphelenchus xylophilus. Agric. Biol. Chem. 1986, 50, 1863–1866. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, L.; Lindelöw, Å. Attraction of scolytids and associated beetles by different absolute amounts and proportions of α-pinene and ethanol. J. Chem. Ecol. 1989, 15, 807–817. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Wu, K.; Gao, X.W.; Guo, Y.Y. Field-testing of synthetic herbivore-induced plant volatiles as attractants for beneficial insects. Environ. Èntomol. 2008, 37, 1410–1415. [Google Scholar] [CrossRef]
- Takken, W.; Kline, D. Carbon dioxide and 1-octen-3-ol as mosquito attractants. J. Am. Mosq. Control Assoc. 1989, 5, 311–316. [Google Scholar] [PubMed]
- Zhu, J.; Cossé, A.A.; Obrycki, J.J.; Boo, K.S.; Baker, T.C. Olfactory reactions of the twelve-spotted lady beetle, Coleomegilla maculata and the green lacewing, Chrysoperla carnea to semiochemicals released from their prey and host plant: Electroantennogram and behavioral responses. J. Chem. Ecol. 1999, 25, 1163–1177. [Google Scholar] [CrossRef]
- Erbilgin, N.; Raffa, K.F. Modulation of predator attraction to pheromones of two prey species by stereochemistry of plant volatiles. Oecologia 2001, 127, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Guerin, P.M.; Visser, J.H. Electroantennogram responses of the carrot fly, Psila rosae, to volatile plant components. Physiol. Èntomol. 1980, 5, 111–119. [Google Scholar] [CrossRef]
- Huang, Y.; Hee, S.; Ho, S. Antifeedant and growth inhibitory effects of α-pinene on the stored-product insects, Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Int. Pest Control 1998, 40, 18–20. [Google Scholar]
- Hoballah, M.E.F.; Tamò, C.; Turlings, T.C. Differential attractiveness of induced odors emitted by eight maize varieties for the parasitoid Cotesia marginiventris: Is quality or quantity important? J. Chem. Ecol. 2002, 28, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Piesik, D.; Rochat, D.; Delaney, K.; Marion-Poll, F. Orientation of European corn borer first instar larvae to synthetic green leaf volatiles. J. Appl. Entomol. 2013, 137, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Piesik, D.; Wenda-Piesik, A. Sitophilus granarius responses to blends of five groups of cereal kernels and one group of plant volatiles. J. Stored Prod. Res. 2015, 62, 36–39. [Google Scholar] [CrossRef]

| Compounds | Purity | Source |
|---|---|---|
| Linalool | 97% | AccuStandard® |
| 1-Octen-3-ol | 98% | AccuStandard® |
| Hexanal | 97% | Dr. Ehrenstorfer GmbH |
| trans-2-Hexen-1-al | 98% | Sigma-Aldrich |
| Nonanal | 98% | Dr. Ehrenstorfer GmbH |
| β-Myrcene | 95% | Sigma-Aldrich |
| α-pinene | 98% | Dr. Ehrenstorfer GmbH |
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Total | |
|---|---|---|---|---|---|---|---|---|
| Damaged individuals | 27 | 30 | 26 | 31 | 19 | 18 | 16 | 167 |
| Healthy individuals | 15 | 11 | 9 | 5 | 6 | 7 | 4 | 57 |
| p-Value | >0.05 | 0.015 | 0.017 | <0.001 | 0.027 | >0.05 | 0.018 | <0.001 |
| Compounds | Relative Percentages of Each Compound in Hypolepis punctata Individuals (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RT | HI1 | HI 2 | UICD1 | UICD 2 | UICD 3 | UICD 4 | DI1 | DI 2 | DI 3 | DI 4 | |
| hexanal | 3.467 | 13.73 | 15.24 | 7.88 | 6.93 | - | - | - | - | - | - |
| trans-2-hexenal | 4.39 | 11.67 | 7.49 | - | - | - | - | - | - | - | - |
| cis-3-hexen-1-ol | 4.455 | 33.19 | 40.59 | 23.97 | 27.86 | - | - | - | - | - | - |
| 1-hexanol | 4.717 | 20.19 | 19.13 | 13.63 | - | - | - | - | - | - | - |
| alpha-pinene | 5.941 | 0.48 | 0.81 | 1.02 | 1.61 | 14.41 | 3.40 | 5.27 | - | 14.11 | - |
| 2-heptenal | 6.494 | 1.43 | - | - | - | - | - | - | - | - | - |
| benzaldehyde | 6.61 | 1.06 | - | - | - | - | - | - | - | - | - |
| sabinene | 6.779 | 0.38 | 0.71 | - | - | - | - | - | - | - | - |
| 1-octen-3-ol | 6.887 | 1.85 | 1.15 | 5.21 | 6.77 | - | 9.40 | - | - | - | 17.93 |
| β-myrcene | 7.098 | - | - | 4.70 | 5.26 | 19.28 | 6.82 | 8.55 | 2.88 | 24.15 | 8.24 |
| Cis-3-hexen-1-ol, acetate | 7.449 | 1.21 | - | - | - | - | - | - | - | - | - |
| benzeneacetaldehyde | 8.171 | 5.75 | - | - | - | - | - | - | - | - | - |
| sabinene | 8.191 | - | - | - | - | - | - | 4.34 | - | - | - |
| undecane, 3,8-dimethyl-(CAS) | 8.321 | - | - | 3.45 | - | - | - | - | - | - | - |
| acetophenone | 8.608 | 1.66 | 2.41 | - | - | - | - | - | - | - | - |
| (+)-(R)-p-Mentha-1,8-dien-4-ol | 8.882 | - | - | - | - | - | - | - | - | - | 3.50 |
| linalool | 9.127 | - | - | 9.19 | 7.76 | 11.39 | 13.56 | 12.88 | 12.78 | 32.26 | 24.61 |
| nonanal | 9.147 | 2.78 | 8.81 | 20.69 | 22.24 | 54.91 | 66.82 | 55.91 | 45.78 | 29.47 | 29.72 |
| phenylethyl alcohol | 9.466 | 4.63 | - | - | - | - | - | - | - | - | - |
| delta.-(2)-dodecanol | 13.91 | - | - | - | - | - | - | 13.05 | - | - | - |
| longifolene | 14.065 | - | 3.67 | - | - | - | - | - | - | - | - |
| nerylacetone | 14.53 | - | - | 7.67 | 15.05 | - | - | - | - | - | - |
| zingiberene | 15.07 | - | - | 2.59 | 6.53 | - | - | - | 7.13 | - | - |
| alpha.-farnesene | 15.239 | - | - | - | - | - | - | - | - | - | 16.00 |
| phenol, 3,5-bis (1,1-dimethylethyl)- | 15.341 | - | - | - | - | - | - | - | 31.43 | - | - |
| Compounds | Average Relative Percent in DI Group (%) | Sex | Number of Choices to Experiment Group | Number of Choices to Control (Hexane) Group | p-Value |
|---|---|---|---|---|---|
| linalool | 20.63 | Total | 39 | 15 | * 0.0013 |
| Female | 23 | 7 | * 0.0027 | ||
| Male | 16 | 8 | 0.0992 | ||
| β-myrcene | 10.96 | Total | 30 | 18 | 0.0816 |
| Female | 15 | 12 | 0.5633 | ||
| Male | 15 | 6 | * 0.0459 | ||
| alpha-pinene | 4.85 | Total | 23 | 25 | 0.7728 |
| Female | 14 | 13 | 0.8474 | ||
| Male | 9 | 12 | 0.5120 | ||
| hexanal | 0.00 | Total | 20 | 28 | 0.2471 |
| Female | 12 | 15 | 0.5633 | ||
| Male | 8 | 13 | 0.2729 | ||
| 1-octen-3-ol | 4.48 | Total | 22 | 26 | 0.5635 |
| Female | 8 | 13 | 0.2729 | ||
| Male | 14 | 13 | 0.8474 | ||
| trans-2-hexenal | 0.00 | Total | 33 | 15 | * 0.0085 |
| Female | 18 | 9 | 0.0803 | ||
| Male | 15 | 6 | * 0.0459 | ||
| nonanal | 40.42 | Total | 26 | 22 | 0.5635 |
| Female | 11 | 16 | 0.3345 | ||
| Male | 15 | 6 | * 0.0459 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.; Shang, H.; Zhou, Q.; Wang, Y.; Shen, H.; Yan, Y. Volatiles Induced from Hypolepis punctata (Dennstaedtiaceae) by Herbivores Attract Sclomina erinacea (Hemiptera: Reduviidae): Clear Evidence of Indirect Defense in Fern. Insects 2021, 12, 978. https://doi.org/10.3390/insects12110978
Huang K, Shang H, Zhou Q, Wang Y, Shen H, Yan Y. Volatiles Induced from Hypolepis punctata (Dennstaedtiaceae) by Herbivores Attract Sclomina erinacea (Hemiptera: Reduviidae): Clear Evidence of Indirect Defense in Fern. Insects. 2021; 12(11):978. https://doi.org/10.3390/insects12110978
Chicago/Turabian StyleHuang, Kerui, Hui Shang, Qiong Zhou, Yun Wang, Hui Shen, and Yuehong Yan. 2021. "Volatiles Induced from Hypolepis punctata (Dennstaedtiaceae) by Herbivores Attract Sclomina erinacea (Hemiptera: Reduviidae): Clear Evidence of Indirect Defense in Fern" Insects 12, no. 11: 978. https://doi.org/10.3390/insects12110978





