Relationships between the Pathogen Erysiphe alphitoides, the Phytophagous Mite Schizotetranychus garmani (Acari: Tetranychidae) and the Predatory Mite Euseius finlandicus (Acari: Phytoseiidae) in Oak
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
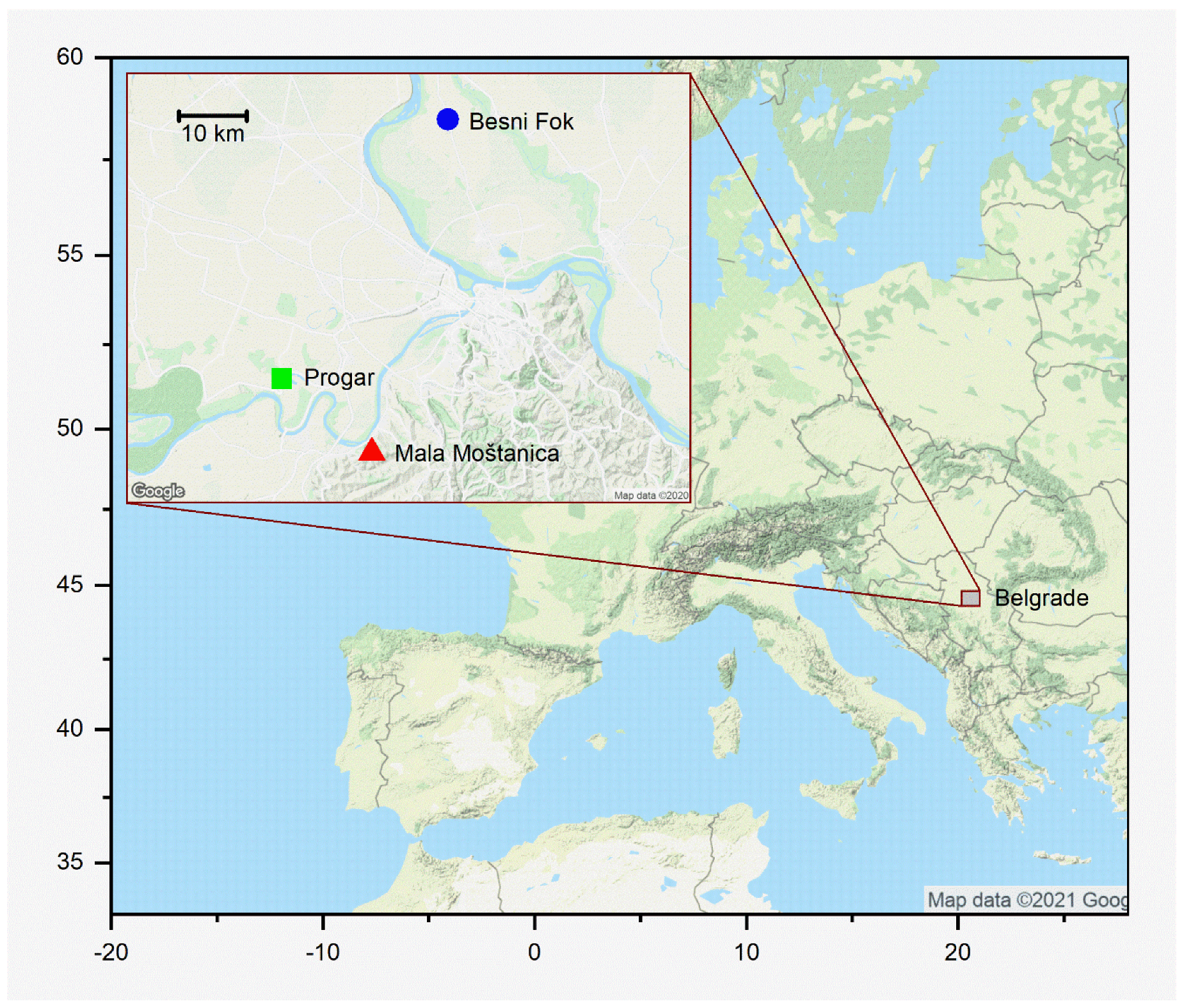
2.1. Study System
2.2. Experimental Procedure
(percentage of leaves in group C)
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milanovic, S.; Jankovic-Tomanic, M.; Kostic, I.; Kostic, M.; Morina, F.; Zivanovic, B.; Lazarevic, J. Behavioural and physiological plasticity of gypsy moth larvae to host plant switching. Entomol. Exp. Appl. 2016, 158, 152–162. [Google Scholar] [CrossRef]
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elvira-Recuenco, M.; Cacciola, S.O.; Sanz-Ros, A.V.; Garbelotto, M.; Aguayo, J.; Solla, A.; Mullett, M.; Drenkhan, T.; Oskay, F.; Aday Kaya, A.G.; et al. Potential interactions between invasive Fusarium circinatum and other pine pathogens in Europe. Forests 2020, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Schuldt, A.; Hönig, L.; Li, Y.; Fichtner, A.; Härdtle, W.; von Oheimb, G.; Welk, E.; Bruelheide, H. Herbivore and pathogen effects on tree growth are additive, but mediated by tree diversity and plant traits. Ecol. Evol. 2017, 7, 7462–7474. [Google Scholar] [CrossRef] [PubMed]
- Price, P.W.; Bouton, C.E.; Gross, P.; McPheron, B.A.; Thompson, J.N.; Weis, A.E. Interactions among three trophic levels: Influence of plants on interactions between insect herbivores and natural enemies. Annu. Rev. Ecol. Syst. 1980, 11, 41–65. [Google Scholar] [CrossRef] [Green Version]
- Tack, A.J.M.; Dicke, M. Plant pathogens structure arthropod communities across multiple spatial and temporal scales. Funct. Ecol. 2013, 27, 633–645. [Google Scholar] [CrossRef]
- Fernandez-Conradi, P.; Jactel, H.; Robin, C.; Tack, A.J.M.; Castagneyrol, B. Fungi reduce preference and performance of insect herbivores on challenged plants. Ecology 2018, 99, 300–311. [Google Scholar] [CrossRef]
- Mouttet, R.; Bearez, P.; Thomas, C.; Desneux, N. Phytophagous arthropods and a pathogen sharing a host plant: Evidence for indirect plant-mediated interactions. PLoS ONE 2011, 6, e18840. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, L.J.A.; Ehrlén, J.; Tack, A.J.M. The timing and asymmetry of plant-pathogen-insect interactions. Proc. Biol. Sci. 2020, 287, 20201303. [Google Scholar] [CrossRef] [PubMed]
- Milanović, S.; Lazarević, J.; Karadžić, D.; Milenković, I.; Jankovský, L.; Vuleta, A.; Solla, A. Belowground infections of the invasive Phytophthora plurivora pathogen enhance the suitability of red oak leaves to the generalist herbivore Lymantria Dispar. Ecol. Entomol. 2015, 40, 479–482. [Google Scholar] [CrossRef]
- Milanović, S.; Milenković, I.; Dobrosavljević, J.; Popović, M.; Solla, A.; Tomšovský, M.; Jankovský, L. Growth rates of Lymantria dispar larvae and Quercus robur seedlings at elevated CO2 concentration and Phytophthora plurivora infection. Forests 2020, 11, 1059. [Google Scholar] [CrossRef]
- Biere, A.; Tack, A.J.M. Evolutionary adaptation in three-way interactions between plants, microbes and arthropods. Funct. Ecol. 2013, 27, 646–660. [Google Scholar] [CrossRef] [Green Version]
- Tack, A.; Gripenberg, S.; Roslin, T. Cross-kingdom interactions matter: Fungal-mediated interactions structure an insect community on oak. Ecol. Lett. 2012, 15, 177–185. [Google Scholar] [CrossRef]
- Castagneyrol, B.; Fernandez-Conradi, P.; Rasmussen, P.U.; Robin, C.; Tack, A.J.M. Belowground–Aboveground Interactions Between Pathogens and Herbivores BT–Aboveground–Belowground Community Ecology; Ohgushi, T., Wurst, S., Johnson, S.N., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 135–174. ISBN 978-3-319-91614-9. [Google Scholar]
- Hatcher, P.E. Three-way interactions between plant pathogenic fungi, herbivorous insects and their host plants. Biol. Rev. 1995, 70, 639–694. [Google Scholar] [CrossRef]
- Eberl, F.; Fernandez de Bobadilla, M.; Reichelt, M.; Hammerbacher, A.; Gershenzon, J.; Unsicker, S.B. Herbivory meets fungivory: Insect herbivores feed on plant pathogenic fungi for their own benefit. Ecol. Lett. 2020, 23, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- van Nouhuys, S.; Laine, A.-L. Population dynamics and sex ratio of a parasitoid altered by fungal-infected diet of host butterfly. Proc. Biol. Sci. 2008, 275, 787–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcobado, T.; Miranda-Torres, J.J.; Martín-García, J.; Jung, T.; Solla, A. Early survival of Quercus ilex subspecies from different populations after infections and co-infections by multiple Phytophthora species. Plant Pathol. 2017, 66, 792–804. [Google Scholar] [CrossRef]
- Biere, A.; Elzinga, J.A.; Honders, S.C.; Harvey, J.A. A plant pathogen reduces the enemy-free space of an insect herbivore on a shared host plant. Proc. Biol. Sci. 2002, 269, 2197–2204. [Google Scholar] [CrossRef] [Green Version]
- Clavijo McCormick, A.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef]
- Stout, M.; Thaler, J.; Thomma, B.; Stout, M.J.; Thaler, J.S.; Thomma, B.P.H.J. Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu. Rev. Entomol. 2006, 51, 663–689. [Google Scholar] [CrossRef]
- Biere, A.; Goverse, A. Plant-mediated systemic interactions between pathogens, parasitic nematodes, and herbivores above- and belowground. Annu. Rev. Phytopathol. 2016, 54, 499–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Fernández, M.; Naves, P.; Musolin, D.L.; Selikhovkin, A.V.; Cleary, M.; Chira, D.; Paraschiv, M.; Gordon, T.; Solla, A.; Papazova-Anakieva, I.; et al. Pine pitch canker and insects: Regional risks, environmental regulation, and practical management options. Forests 2019, 10, 649. [Google Scholar] [CrossRef] [Green Version]
- Franco, F.P.; Moura, D.S.; Vivanco, J.M.; Silva-Filho, M.C. Plant–insect–pathogen interactions: A naturally complex ménage à trois. Curr. Opin. Microbiol. 2017, 37, 54–60. [Google Scholar] [CrossRef]
- Slinn, H.L.; Barbour, M.A.; Crawford, K.M.; Rodriguez-Cabal, M.A.; Crutsinger, G.M. Genetic variation in resistance to leaf fungus indirectly affects spider density. Ecology 2017, 98, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Eberl, F.; Uhe, C.; Unsicker, S.B. Friend or foe? The role of leaf-inhabiting fungal pathogens and endophytes in tree-insect interactions. Fungal Ecol. 2019, 38, 104–112. [Google Scholar] [CrossRef]
- Gallardo, A.; Morcuende, D.; Solla, A.; Moreno, G.; Pulido, F.; Quesada, A. Regulation by biotic stress of tannins biosynthesis in Quercus ilex: Crosstalk between defoliation and Phytophthora cinnamomi infection. Physiol. Plant. 2019, 165, 319–329. [Google Scholar] [CrossRef]
- Moran, P.J. Plant-mediated interactions between insects and a fungal plant pathogen and the role of plant chemical responses to infection. Oecologia 1998, 115, 523–530. [Google Scholar] [CrossRef]
- Thaler, J.S.; Agrawal, A.A.; Halitschke, R. Salicylate-mediated interactions between pathogens and herbivores. Ecology 2010, 91, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Lombardero, M.J.; Solla, A.; Ayres, M.P. Pine defenses against the pitch canker disease are modulated by a native insect newly associated with the invasive fungus. For. Ecol. Manag. 2019, 437, 253–262. [Google Scholar] [CrossRef]
- Gamliel-Atinsky, E.; Freeman, S.; Maymon, M.; Belausov, E.; Ochoa, R.; Bauchan, G.; Skoracka, A.; Pena, J.; Palevsky, E. The role of eriophyoids in fungal pathogen epidemiology, mere association or true interaction? Exp. Appl. Acarol. 2009, 51, 191–204. [Google Scholar] [CrossRef]
- Leroux, S.J.; Loreau, M. Theoretical perspectives on bottom-up and top-down interactions across ecosystems. In Trophic Ecology: Bottom-Up and Top-Down Interactions across Aquatic and Terrestrial Systems; La Pierre, K.J., Hanley, T.C., Eds.; Ecological Reviews; Cambridge University Press: Cambridge, UK, 2015; pp. 3–28. ISBN 9781107077324. [Google Scholar]
- Pap, P.; Stojnić, S.; Nikolić, N.; Orlović, S.; Marković, M.; Vasić, V.; Stevanov, M. Impact of Microsphaera alphitoides Griff. et Maubl. on leaf physiological parameters in Pedunculate oak (Quercus robur L.) saplings. Baltic For. 2014, 20, 2–9. [Google Scholar]
- Mladenović, K. Species Diversity of Phytophagous and Predatory Mites of Wild Fruit Trees in Forest Ecosystems of Serbia. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2014. [Google Scholar]
- Dobrosavljević, J.; Marković, Č.; Marjanović, M.; Milanović, S. Pedunculate oak leaf miners’ community: Urban vs. rural habitat. Forests 2020, 11, 1300. [Google Scholar] [CrossRef]
- Thomas, F. Recent advances in cause-effect research on oak decline in Europe. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2008, 3, 1–2. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.-L.; Feau, N.; Mougou-Hamdane, A.; Dutech, C. Interspecific and intraspecific diversity in oak powdery mildews in Europe: Coevolution history and adaptation to their hosts. Mycoscience 2011, 52, 165–173. [Google Scholar] [CrossRef]
- Marçais, B.; Kavkova, M.; Desprez-Loustau, M.-L. Phenotypic variation in the phenology of ascospore production between European populations of oak powdery mildew. Ann. For. Sci. 2009, 66, 814. [Google Scholar] [CrossRef] [Green Version]
- Führer, E. Oak Decline in Central Europe: A synopsis of hypotheses. USDA For. Serv. Gen. Tech. Rep. 1998, NE-247, 7–24. [Google Scholar]
- Marçais, B.; Desprez-Loustau, M.-L. European oak powdery mildew: Impact on trees, effects of environmental factors, and potential effects of climate change. Ann. For. Sci. 2014, 71, 633–642. [Google Scholar] [CrossRef] [Green Version]
- Jalas, J.; Suominen, J. Atlas Florae Europaeae: Distribution of Vascular Plants in Europe Vol. 3 Salicaceae to Balanophoraceae; The Committee for Mapping the Flora of Europe & Societas Biologica Fennica Vanam: Helsinki, Finland, 1976; ISBN 951-9108-02-5. [Google Scholar]
- Bolland, H.R.; Gutierrez, J.; Flechtmann, C.H.W. World Catalogue of the Spider Mite Family (Acari:Tetranychidae); Brill: Leiden, The Netherlands; Boston, MA, USA, 1998; ISBN1 9004110879. ISBN2 9789004110878. [Google Scholar]
- Migeon, A.; Nouguier, E.; Dorkeld, F. Spider Mites Web: A Comprehensive Database for the Tetranychidae BT—Trends in Acarology; Sabelis, M.W., Bruin, J., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 557–560. [Google Scholar]
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Dermauw, W.; Tirry, L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochem. Mol. Biol. 2010, 40, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Jeppson, L.R.; Keifer, H.H.; Baker, E.W. Mites Injurious to Economic Plants; University of California Press: Berkeley, CA, USA, 1975; ISBN 0520023811. [Google Scholar]
- Vacante, V. The Handbook of Mites of Economic Plants: Identification, Bio-Ecology and Control; CABI: Wallingford, UK, 2016; ISBN 9781845939946. [Google Scholar]
- McMurtry, J.A.; Croft, B.A. Life-styles of phytoseiid mites and their roles in biological control. Annu. Rev. Entomol. 1997, 42, 291–321. [Google Scholar] [CrossRef]
- Adar, E.; Inbar, M.; Gal, S.; Issman, L.; Palevsky, E. Plant cell piercing by a predatory mite: Evidence and implications. Exp. Appl. Acarol. 2015, 65, 181–193. [Google Scholar] [CrossRef]
- Krantz, G.W.; Lindquist, E.E. Evolution of phytophagous mites (ACARI). Annu. Rev. Entomol. 1979, 24, 121–158. [Google Scholar] [CrossRef]
- Abdallah, A.A.; Zhang, Z.-Q.; Masters, G.J.; Mcneill, S. Euseius finlandicus (Acari: Phytoseiidae) as a potential biocontrol agent against Tetranychus urticae (Acari: Tetranychidae): Life history and feeding habits on three different types of food. Exp. Appl. Acarol. 2001, 25, 833–847. [Google Scholar] [CrossRef]
- Evans, G.O.; Browning, E. LXXV.—Techniques for the preparation of mites for study. Ann. Mag. Nat. Hist. 1955, 8, 631–635. [Google Scholar] [CrossRef]
- Baker, E.W.; Wharton, G.W. An Introduction to Acarology; The Macmillan Co.: New York, NY, USA; Toronto, CA, Canada, 1952. [Google Scholar]
- Pritchard, A.E.; Arthur, E.; Baker, E.W. A Revision of the Spider Mite Family Tetranychidae; Pacific Coast Entomological Society: San Francisco, CA, USA, 1955; Volume 2. [Google Scholar]
- Baker, E.W.; Tuttle, D.M. A Guide to the Spider Mites (Tetranychidae) of the United States; Indira Pub. House: West Bloomfield, MI, USA, 1994; ISBN1 0930337123. ISBN2 9780930337124. [Google Scholar]
- Flechtmann, C.H.W. Schizotetranychus-like spider mites (Acari, Prostigmata, Tetranychidae)—Revisited, new combinations and a key to groups of Schizotetranychus based on females. Acarologia 2012, 52, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Manson, D.C.M. The spider mite family Tetranychidae in New Zealand. II The genus Tetranychus. Acarologia 1967, 9, 581–597. [Google Scholar]
- Chant, D.A. Phytoseiid Mites (Acarina: Phytoseiidae). Mem. Entomol. Soc. Can. 1959, 91, 5–166. [Google Scholar] [CrossRef]
- Chant, D.A.; McMurtry, J.A. Illustrated Keys and Diagnoses for the Genera and Subgenera of the Phytoseiidae of the World (Acari: Mesostigmata); Indira Pub. House: West Bloomfield, MI, USA, 2007; ISBN1 0930337220. ISBN2 9780930337223. [Google Scholar]
- Karg, W. Acari (Acarina), Milben Parasitiformes (Anactinochaeta), Cohors Gamasina Leach: Raubmilben; Gustav Fischer Verlag: Jena, Germany, 1993; ISBN1 3334604454. ISBN2 9783334604458. [Google Scholar]
- Tixier, M.S.; Baldassar, A.; Duso, C.; Kreiter, S. Dichotomous Key to Species of Phytoseiidae Mites in European Vine Fields. 2012. Available online: https://www1.montpellier.inra.fr/CBGP/phytoseiidae/sitewebvineyards2/index.htm (accessed on 26 June 2017).
- Takamatsu, S.; Braun, U.; Limkaisang, S.; Kom-un, S.; Sato, Y.; Cunnington, J.H. Phylogeny and taxonomy of the oak powdery mildew Erysiphe alphitoides sensu lato. Mycol. Res. 2007, 111, 809–826. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Cook, R. Taxonomic Manual of the Erysiphales (Powdery Mildews); CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2012; Volume 11, ISBN 978-90-70351-89-2. [Google Scholar]
- Bert, D.; Lasnier, J.-B.; Capdevielle, X.; Dugravot, A.; Desprez-Loustau, M.-L. Powdery mildew decreases the radial growth of oak trees with cumulative and delayed effects over years. PLoS ONE 2016, 11, e0155344. [Google Scholar] [CrossRef]
- Reding, M.E.; Alston, D.G.; Thomson, S.V.; Stark, A.V. Association of powdery mildew and spider mite populations in apple and cherry orchards. Agric. Ecosyst. Environ. 2001, 84, 177–186. [Google Scholar] [CrossRef]
- Roets, F.; Wingfield, M.J.; Wingfield, B.D.; Dreyer, L.L. Mites are the most common vectors of the fungus Gondwanamyces proteae in Protea infructescences. Fungal Biol. 2011, 115, 343–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamliel-Atinsky, E.; Freeman, S.; Sztejnberg, A.; Maymon, M.; Ochoa, R.; Belausov, E.; Palevsky, E. Interaction of the mite Aceria mangiferae with Fusarium mangiferae, the causal agent of mango malformation disease. Phytopathology 2009, 99, 152–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rector, B.G.; Czarnoleski, M.; Skoracka, A.; Lembicz, M. Change in abundance of three phytophagous mite species (Acari: Eriophyidae, Tetranychidae) on quackgrass in the presence of choke disease. Exp. Appl. Acarol. 2016, 70, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzebyta, J.; Karolewski, P.; Zytkowiak, R.; Giertych, M.J.; Werner, A.; Zadworny, M.; Oleksyn, J. Effects of elevated temperature and fluorine pollution on relations between the pedunculate oak (Quercus robur) and oak powdery mildew (Microsphaera alphitoides). Dendrobiology 2005, 53, 27–33. [Google Scholar]
- Aa, S.M.; Srinivasa, N. Qualitative damage of spider mites on selected medicinal plants and the corresponding biochemical changes. J. Pharmacogn. Phytochem. 2021, 9, 1880–1885. [Google Scholar]
- Zhurov, V.; Navarro, M.; Bruinsma, K.A.; Arbona, V.; Santamaria, M.E.; Cazaux, M.; Wybouw, N.; Osborne, E.J.; Ens, C.; Rioja, C.; et al. Reciprocal responses in the interaction between Arabidopsis and the cell-content-feeding chelicerate herbivore spider mite. Plant Physiol. 2014, 164, 384–399. [Google Scholar] [CrossRef] [Green Version]
- Copolovici, L.; Väärtnõu, F.; Estrada, M.P.; Niinemets, Ü. Oak powdery mildew (Erysiphe alphitoides)-induced volatile emissions scale with the degree of infection in Quercus robur. Tree Physiol. 2014, 34, 1399–1410. [Google Scholar] [CrossRef] [Green Version]
- Shimoda, T. A key volatile infochemical that elicits a strong olfactory response of the predatory mite Neoseiulus californicus, an important natural enemy of the two-spotted spider mite Tetranychus urticae. Exp. Appl. Acarol. 2010, 50, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Rostás, M.; Simon, M.; Hilker, M. Ecological cross-effects of induced plant responses towards herbivores and phytopathogenic fungi. Basic Appl. Ecol. 2003, 41, 43–62. [Google Scholar] [CrossRef] [Green Version]
- Puchalska, E.; Kozak, M. Typhlodromus pyri and Euseius finlandicus (Acari: Phytoseiidae) as potential biocontrol agents against spider mites (Acari: Tetranychidae) inhabiting willows: Laboratory studies on predator development and reproduction on four diets. Exp. Appl. Acarol. 2016, 68, 39–53. [Google Scholar] [CrossRef] [Green Version]


| Model | Dependent Variable | Predictor | Type | Degree of Freedom | F Ratio | p-Value |
|---|---|---|---|---|---|---|
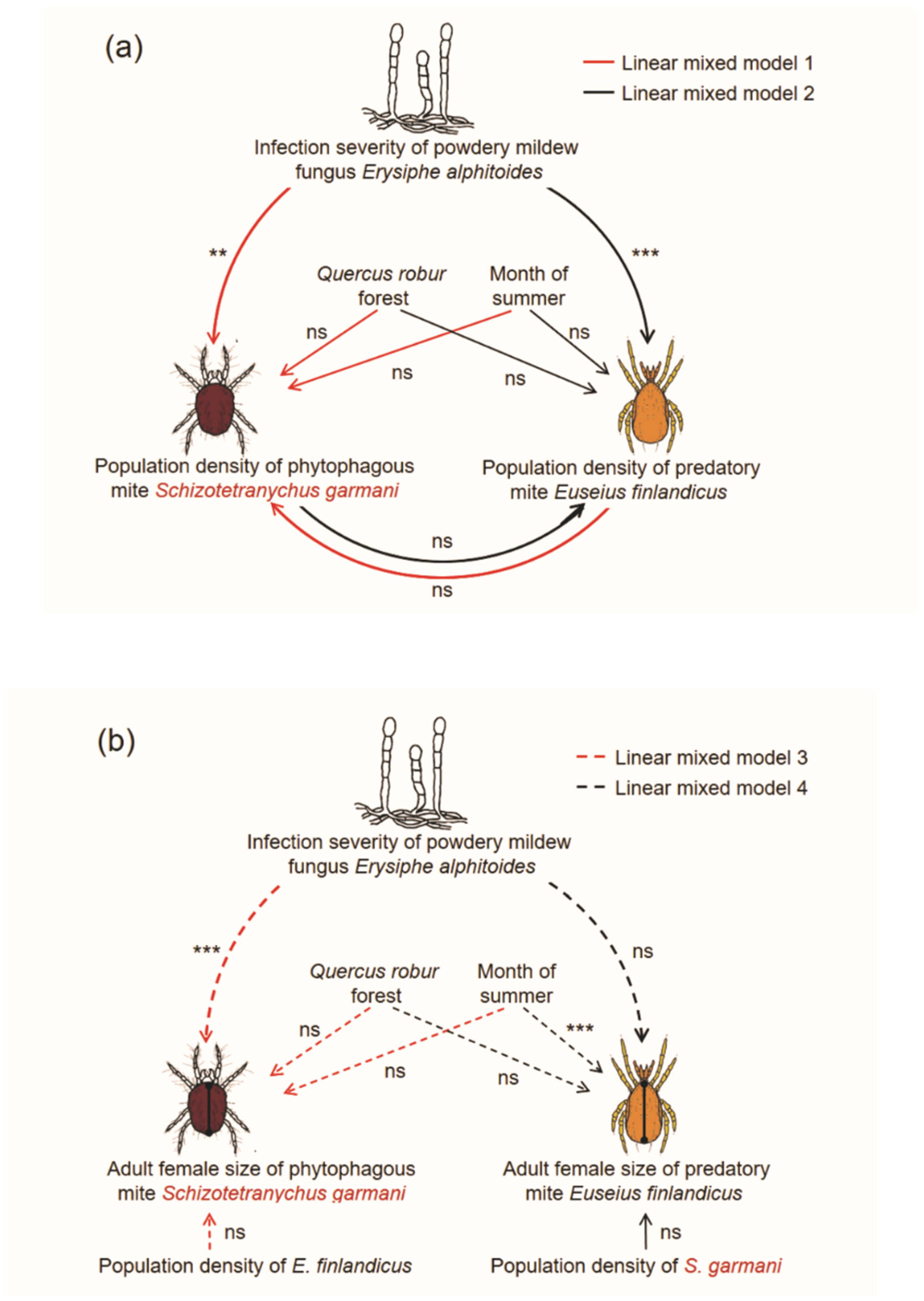
| 1 | Population density of Schizotetranychus garmani | Forest (F) | Random effect | 2 | 0.3 | 0.696 |
| Month of summer (M) | Fixed effect | 2 | 0.2 | 0.750 | ||
| Severity of Erysiphe alphitoides (Ea) | Covariate | 1 | 5.0 | 0.027 | ||
| Population density of E. finlandicus (Ef) | Covariate | 1 | 0.9 | 0.331 | ||
| F × M | Random effect | 4 | 2.0 | 0.104 | ||
| F × Ea | - | 2 | 1.0 | 0.345 | ||
| M × Ea | - | 2 | 6.1 | 0.003 | ||
| Ea × Ef | - | 1 | 0.3 | 0.541 | ||
| 2 | Population density of Euseius finlandicus | Forest (F) | Random effect | 2 | 0.2 | 0.795 |
| Month of summer (M) | Fixed effect | 2 | 1.0 | 0.353 | ||
| Severity of Erysiphe alphitoides (Ea) | Covariate | 1 | 12.4 | <0.001 | ||
| Population density of S. garmani (Sg) | Covariate | 1 | 0.4 | 0.530 | ||
| F × M | Random effect | 4 | 0.9 | 0.451 | ||
| F × Ea | - | 2 | 0.7 | 0.470 | ||
| M × Ea | - | 2 | 0.1 | 0.849 | ||
| Ea × Sg | - | 1 | 0.1 | 0.738 | ||
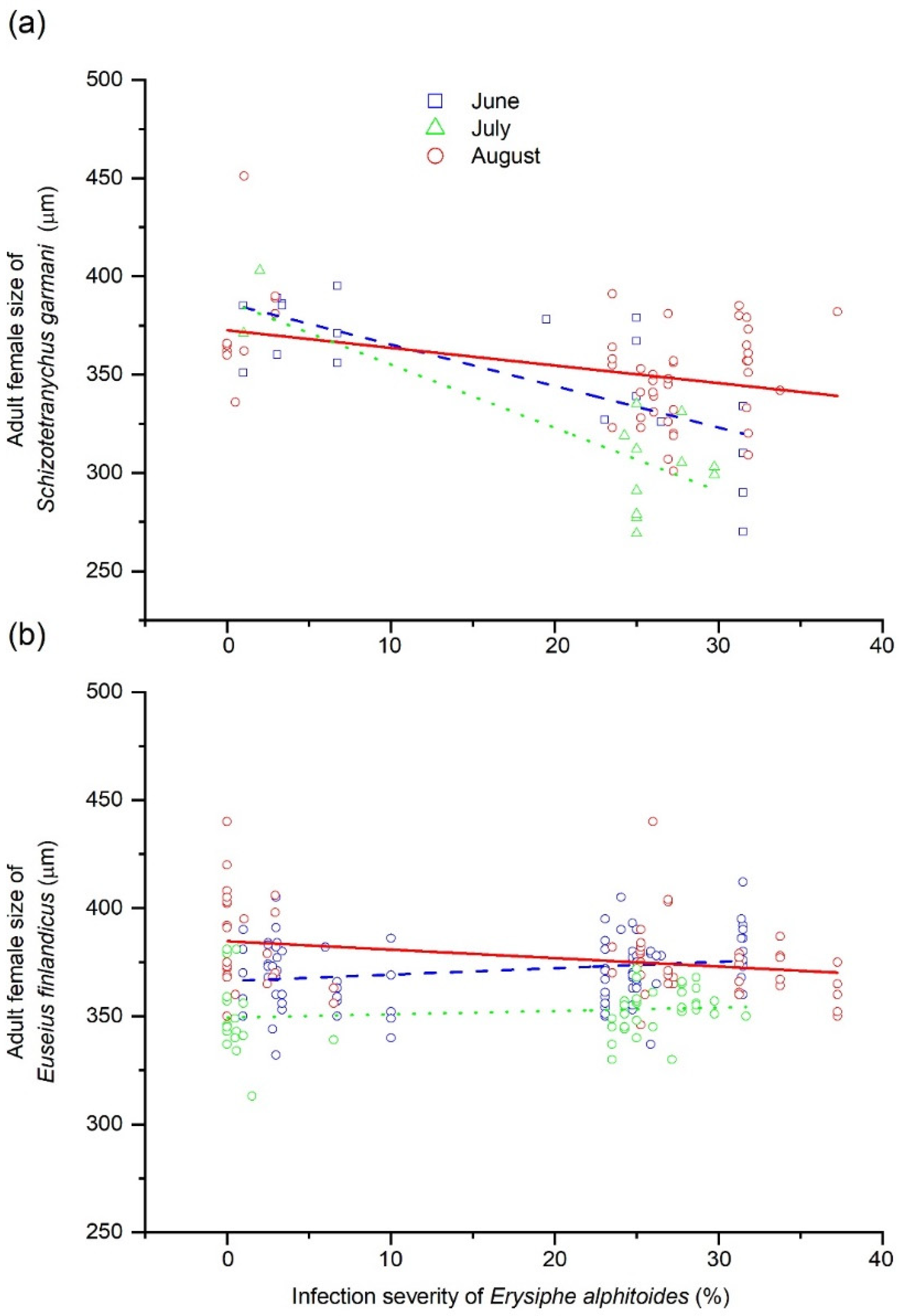
| 3 | Adult female size of Schizotetranychus garmani | Forest (F) | Random effect | 2 | 2.9 | 0.079 |
| Month of summer (M) | Fixed effect | 2 | 0.9 | 0.411 | ||
| Severity of Erysiphe alphitoides (Ea) | Covariate | 1 | 9.3 | 0.006 | ||
| Population density of E. finlandicus (Ef) | Covariate | 1 | 0.0 | 0.958 | ||
| F × M | Random effect | 4 | 1.6 | 0.201 | ||
| F × Ea | - | 2 | 1.6 | 0.214 | ||
| M × Ea | - | 2 | 3.7 | 0.044 | ||
| Ea × Ef | - | 1 | 0.0 | 0.865 | ||
| 4 | Adult female size of Euseius finlandicus | Forest (F) | Random effect | 2 | 0.4 | 0.620 |
| Month of summer (M) | Fixed effect | 2 | 9.6 | <0.001 | ||
| Severity of Erysiphe alphitoides (Ea) | Covariate | 1 | 0.0 | 0.825 | ||
| Population density of S. garmani (Sg) | Covariate | 1 | 0.4 | 0.507 | ||
| F × Ea | Random effect | 4 | 0.6 | 0.627 | ||
| F × Ea | - | 2 | 0.2 | 0.801 | ||
| M × Ea | - | 2 | 1.5 | 0.222 | ||
| Ea × Sg | - | 1 | 0.2 | 0.602 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milanović, S.; Mladenović, K.; Stojnić, B.; Solla, A.; Milenković, I.; Uremović, V.; Tack, A.J.M. Relationships between the Pathogen Erysiphe alphitoides, the Phytophagous Mite Schizotetranychus garmani (Acari: Tetranychidae) and the Predatory Mite Euseius finlandicus (Acari: Phytoseiidae) in Oak. Insects 2021, 12, 981. https://doi.org/10.3390/insects12110981
Milanović S, Mladenović K, Stojnić B, Solla A, Milenković I, Uremović V, Tack AJM. Relationships between the Pathogen Erysiphe alphitoides, the Phytophagous Mite Schizotetranychus garmani (Acari: Tetranychidae) and the Predatory Mite Euseius finlandicus (Acari: Phytoseiidae) in Oak. Insects. 2021; 12(11):981. https://doi.org/10.3390/insects12110981
Chicago/Turabian StyleMilanović, Slobodan, Katarina Mladenović, Bojan Stojnić, Alejandro Solla, Ivan Milenković, Vanja Uremović, and Ayco J. M. Tack. 2021. "Relationships between the Pathogen Erysiphe alphitoides, the Phytophagous Mite Schizotetranychus garmani (Acari: Tetranychidae) and the Predatory Mite Euseius finlandicus (Acari: Phytoseiidae) in Oak" Insects 12, no. 11: 981. https://doi.org/10.3390/insects12110981
APA StyleMilanović, S., Mladenović, K., Stojnić, B., Solla, A., Milenković, I., Uremović, V., & Tack, A. J. M. (2021). Relationships between the Pathogen Erysiphe alphitoides, the Phytophagous Mite Schizotetranychus garmani (Acari: Tetranychidae) and the Predatory Mite Euseius finlandicus (Acari: Phytoseiidae) in Oak. Insects, 12(11), 981. https://doi.org/10.3390/insects12110981






