Source Regions of the First Immigration of Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) Invading Australia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Surveillance Trapping and Possible Immigrating Date
2.2. Meteorological Data and Modeling
2.3. Trajectory Analysis
2.4. Synoptic Weather Condition Analysis
3. Results
3.1. Surveillance Trapping and Inferred Migration Dates of Fall Armyworm
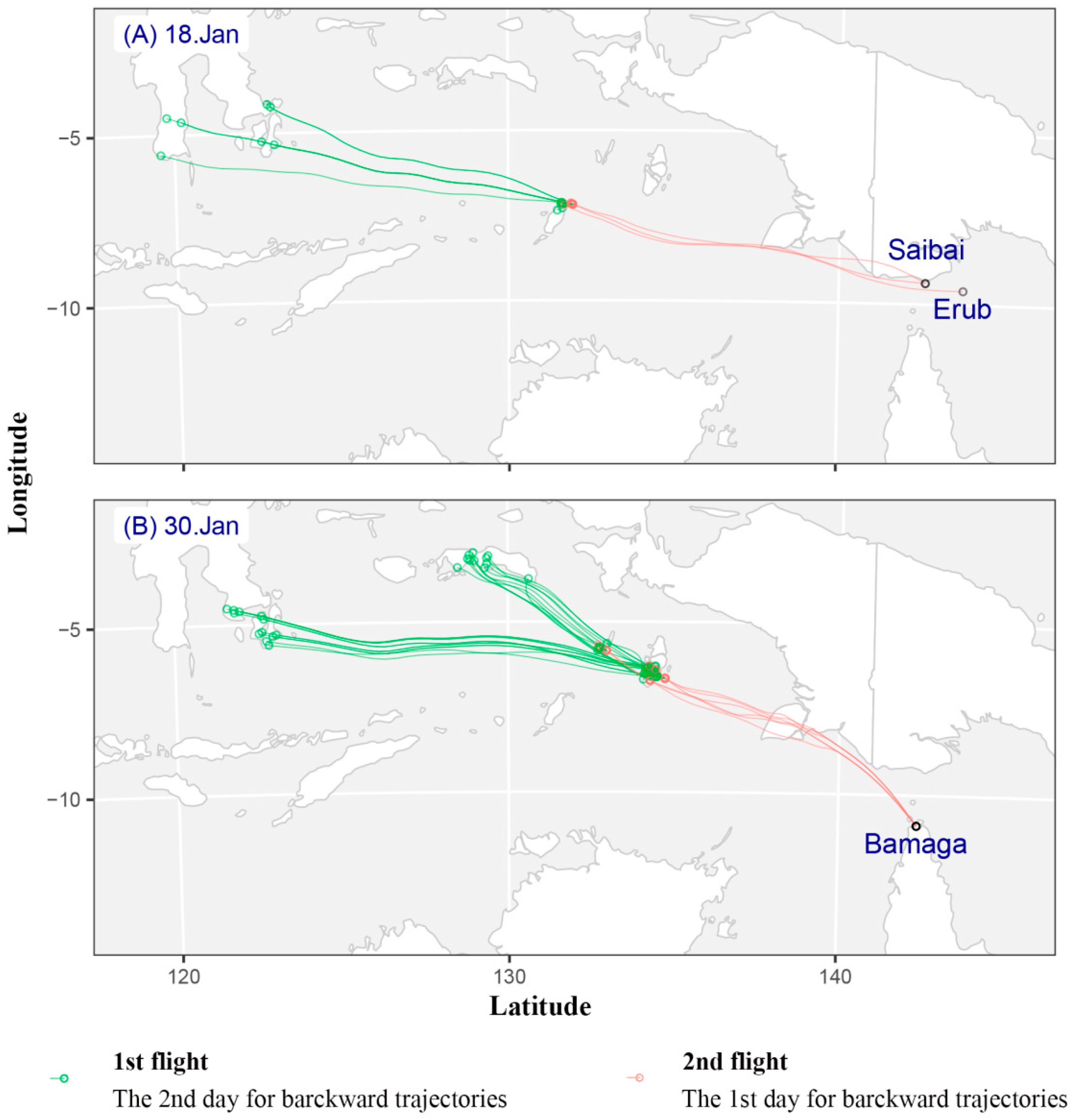
3.2. Source Region of Fall Armyworm Identified by Backward Trajectories
3.2.1. Backward Trajectories Analysis in Saibai and Erub Islands
3.2.2. Backward Trajectories Analysis in Bamaga
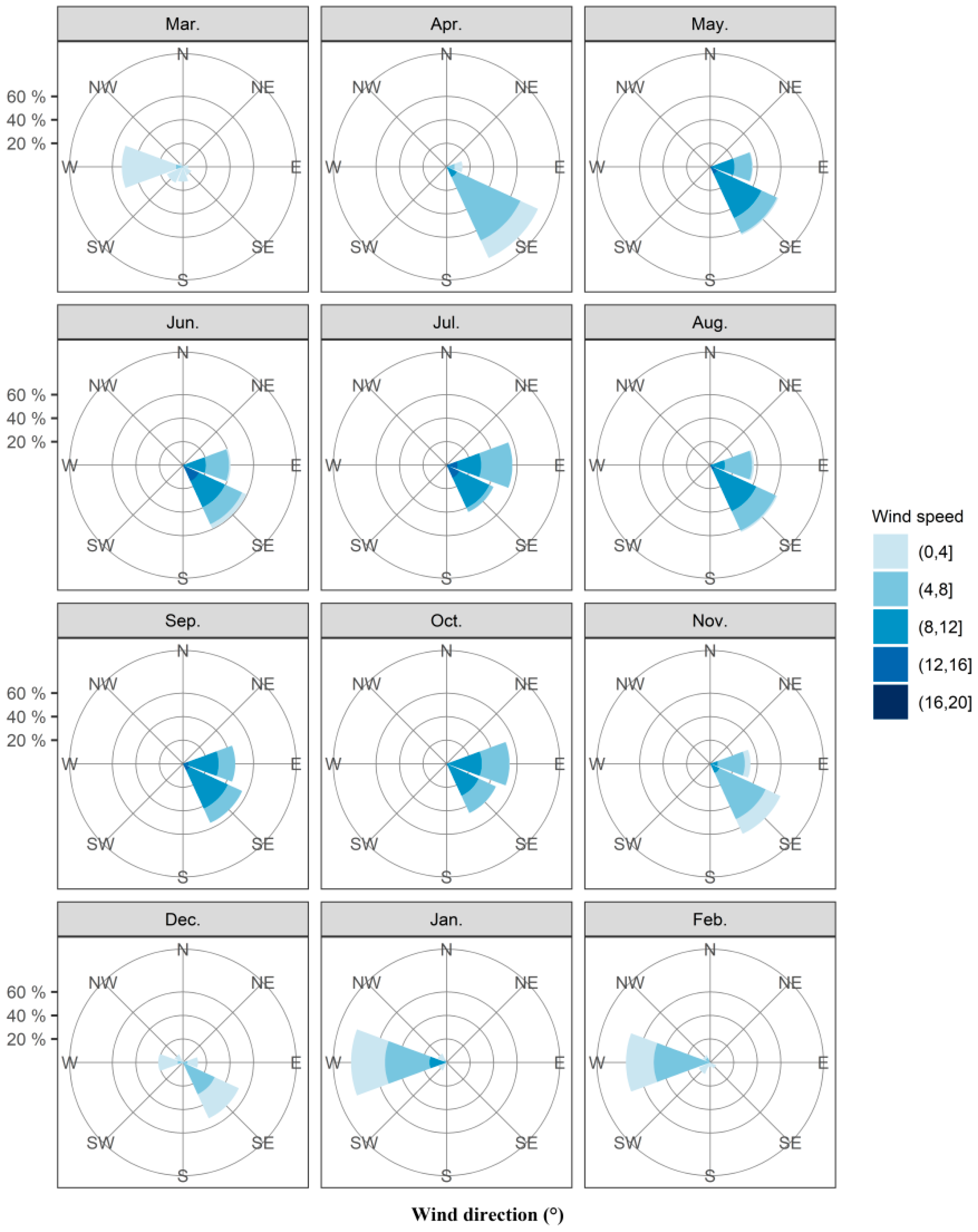
3.3. Synoptic Weather during Fall Armyworm Migration Period
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luginbill, P. The Fall Armyworm; USDA Tech. Bull. U.S. Government Printing Office: Washington, DC, USA, 1928; Volume 34, pp. 1–92. [Google Scholar]
- Sparks, A.N. A review of the biology of the fall Armyworm. Fla. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Johnson, S.J. Migration and the life history strategy of the fall armyworm, Spodoptera frugiperda in the western hemisphere. Int. J. Trop. Insect. Sci. 1987, 8, 543–549. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall armyworm: Impacts and implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef] [Green Version]
- FAO. Fall Armyworm Keeps Spreading and Becomes More Destructive. Available online: http://www.fao.org/news/story/en/item/1142085/icode/ (accessed on 28 June 2018).
- Early, R.; González-Moreno, P.; Murphy, S.T.; Day, R. Forecasting the global extent of invasion of the cereal pest Spodoptera frugiperda, the fall armyworm. NeoBiota 2018, 40, 25–50. [Google Scholar] [CrossRef] [Green Version]
- Stokstad, E. New crop pest takes Africa at lightning speed. Science 2017, 356, 473–474. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Goergen, G.; Tounou, K.A.; Agboka, K.; Koffi, D.; Meagher, R.L. Analysis of strain distribution, migratory potential, and invasion history of fall armyworm populations in northern Sub-Saharan Africa. Sci. Rep. 2018, 8, 3710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldmann, F.; Rieckmann, U.; Winter, S. The spread of the fall armyworm Spodoptera frugiperda in Africa—What should be done next? J. Plant Dis. Protect. 2019, 126, 97–101. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [Green Version]
- Cock, M.J.W.; Beseh, P.K.; Buddie, A.G.; Cafá, G.; Crozier, J. Molecular methods to detect Spodoptera frugiperda in Ghana, and implications for monitoring the spread of invasive species in developing countries. Sci. Rep. 2017, 7, 4103. [Google Scholar] [CrossRef]
- Kalleshwaraswamy, C.M.; Asokan, R.; Swamy, H.M.; Maruthi, M.S.; Pavithra, H.B.; Hegbe, K.; Navi, S.; Prabhu, S.T.; Goergen, G.E. First report of the fall armyworm, Spodoptera frugiperda (J E Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest Manag. Hortic. Ecosyst. 2018, 24, 23–29. [Google Scholar]
- Guo, J.F.; Zhao, J.Z.; He, K.L.; Zhang, F.; Wang, Z.Y. Potential invasion of the crop-devastating insect pest fall armyworm Spodoptera frugiperda to China. Plant. Prot. 2018, 44, 1–10. [Google Scholar]
- Wu, Q.L.; Jiang, Y.Y.; Hu, G.; Wu, K.M. Analysis on spring and summer migration routes of fall armyworm (Spodoptera frugiperda) from tropical and southern subtropical zones of China. Plant Prot. 2019, 45, 1–9. [Google Scholar]
- Sartiami, D.; Harahap, I.S.; Kusumah, Y.M.; Anwar, R. First record of fall armyworm (Spodoptera frugiperda) in Indonesia and its occurence in three provinces. In IOP Conference Series Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 468, p. 012021. [Google Scholar]
- Maino, J.L.; Schouten, R.; Overton, K.; Day, R.; Reynolds, O.L. Regional and seasonal activity predictions for fall armyworm in Australia. Curr. Res. Insect Sci. 2021, 1, 100010. [Google Scholar] [CrossRef]
- Kelso, J.K.; Milne, G.J. A spatial simulation model for the dispersal of the bluetongue vector Culicoides brevitarsis in Australia. PLoS ONE 2013, 9, e104646. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.L.; Hu, G.; Westbrook, J.K.; Sword, G.A.; Zhai, B.P. An advanced numerical trajectory model tracks a corn earworm moth migration event in Texas, USA. Insect 2018, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Lu, M.H.; Tuan, H.A.; Liu, W.C.; Xie, M.C.; McInerney, C.E.; Zhai, B.P. Population dynamics of rice planthoppers, Nilaparvata lugens and Sogatella furcifera (Hemiptera, Delphacidae) in Central Vietnam and its effects on their spring migration to China. Bull. Entomol. Res. 2017, 107, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Y.; Yang, F.; Lu, M.H.; Luo, S.Y.; Zhai, B.P.; Lim, K.S.; McInerney, C.E.; Hu, G. Determining the migration duration of rice leaf folder (Cnaphalocrocis medinalis (Guenée)) moths using a trajectory analytical approach. Sci. Rep. 2017, 7, 39853. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Wang, Y.P.; Wu, M.F.; Gao, B.Y.; Liu, J.; Lee, G.S.; Otuka, A.; Hu, G. High risk of the fall armyworm invading Japan and the Korean Peninsula via overseas migration. J. Appl. Entomol. 2019, 143, 911–920. [Google Scholar] [CrossRef]
- Li, X.J.; Wu, M.F.; Ma, J.; Gao, B.Y.; Wu, Q.L.; Chen, A.D.; Liu, J.; Jiang, Y.Y.; Zhai, B.P.; Early, R.; et al. Prediction of migratory routes of the invasive fall armyworm in eastern China using a trajectory analytical approach. Pest Manag. Sci. 2019, 76, 454–463. [Google Scholar] [CrossRef]
- Lu, F.; Zhai, B.P.; Hu, G. Trajectory analysis methods for insect migration research. Chin. J. Appl. Entomol. 2013, 50, 853–862. [Google Scholar]
- Qi, G.J.; Lv, L.H.; Lan, R.Q.; Xie, J.H.; Zhang, W.Q. Tracking the source regions of Cnaphalocrocis medinalis in the rice growing region of northern Guangdong Province. Chin. J. Appl. Entomol. 2013, 50, 601–607. [Google Scholar]
- Hu, G.; Wu, Q.L.; Wu, X.W.; Jiang, Y.Y.; Zeng, J.; Zhai, B.P. Outbreak mechanism of second generation armyworms in northeastern China: A case study in 1978. Chin. J. Appl. Entomol. 2014, 51, 943–957. [Google Scholar]
- Wu, Q.L.; Jiang, Y.Y.; Wu, K.M. Analysis of migration routes of fall armyworm, Spodoptera frugiperda (J. E. Smith) from Myanmar to China. Plant Prot. 2019, 45, 1–9. [Google Scholar]
- Qi, G.J.; Ma, J.; Hu, G.; Yu, Y.H.; Chen, A.D.; Gao, Y.; Lv, L.H. Analysis of migratory routes and atmospheric features of newly invaded the fall armyworm, Spodoptera frugiperda (J. E. Smith) in Guangdong province. J. Environ. Entomol. 2019, 41, 487–496. [Google Scholar]
- Wolf, W.W.; Westbrook, J.K.; Raulston, J.; Pair, S.D.; Hobbs, S.E. Recent airborne radar observations of migrant pests in the United States. Philos. Trans. R. Soc. 1990, 328, 619–630. [Google Scholar]
- Westbrook, J.K. Noctuid migration in Texas within the nocturnal aeroecological boundary layer. Integr. Comp. Biol. 2007, 48, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Nagoshi, R.N.; Shelby, F.; Meagher, R.L.; Hay-Roe, M.; Khan, A.; Murúa, M.G.; Silvie, P.; Vergara, C.; Westbrook, J.K. Fall armyworm migration across the Lesser Antilles and the potential for genetic exchanges between North and South American populations. PLoS ONE 2017, 12, e0171743. [Google Scholar]
- Tojo, S.; Ryuda, M.; Fukuda, T.; Matsunaga, T.; Choi, D.R.; Otuka, A. Overseas migration of the common cutworm, Spodoptera litura (Lepidoptera: Noctuidae), from May to mid-July in east Asia. Appl. Entomol. Zool. 2013, 48, 131–140. [Google Scholar] [CrossRef]
- Westbrook, J.K.; Nagoshi, R.N.; Meagher, R.L.; Fleischer, S.J.; Jairam, S. Modeling seasonal migration of fall armyworm moths. Int. J. Biometeorol. 2016, 60, 255–267. [Google Scholar] [CrossRef]
- Wu, Q.L.; He, L.M.; Shen, X.J.; Jiang, Y.Y.; Liu, J.; Hu, G.; Wu, K.M. Estimation of the potential infestation area of newly-invaded fall armyworm Spodoptera frugiperda in the Yangtze River Valley of China. Insects 2019, 10, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, V.A.; Farrow, R.A. The influence of atmospheric structure and motions on insect migration. Annu. Rev. Entomol. 2003, 33, 183–210. [Google Scholar] [CrossRef]
- Hu, G.; Lu, F.; Lu, M.H.; Liu, W.C.; Xu, W.G.; Jiang, X.H.; Zhai, B.P. The influence of typhoon Khanun on the return migration of Nilaparvata lugens (Stål) in eastern China. PLoS ONE 2013, 8, e57277. [Google Scholar] [CrossRef] [Green Version]
- Chapman, J.W.; Reynolds, D.R.; Wilson, K. Long-range seasonal migration in insects: Mechanisms, evolutionary drivers and ecological consequences. Ecol. Lett. 2015, 18, 287–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Ma, J.; Wu, M.F.; Qi, G.J.; Liu, J.; Tang, J.; Hu, G. Original area of fall armyworm individuals newly invaded in Zhejiang province. Chin. J. Rice Sci. 2020, 34, 80–87. [Google Scholar]
- Walker, D. Bridge and Barrier: The Natural and Cultural History of Torres Strait; Research School of Pacific Studies, Australian National University: Canberra, Australia, 1972; pp. 1–437. [Google Scholar]
- Sands, D.; New, T.R. Conservation status and needs of butterflies (Lepidoptera) on the Torres Strait Islands. J. Insect Conserv. 2008, 12, 325–332. [Google Scholar] [CrossRef]
- Eagles, D.; Deveson, T.; Walker, P.J.; Zalucki, M.P.; Durr, P. Evaluation of longdistance dispersal of Culicoides midges into northern Australia using a migration model. Med. Vet. Entomol. 2012, 26, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Farrow, R.A. Detection of transoceanic migration of insects to a remote island in the Coral Sea, Willis Island. Aust. J. Ecol. 2010, 9, 253–272. [Google Scholar] [CrossRef]
- Johansen, C.A.; Farrow, R.A.; Morrisen, A.; Foley, P.; Ritchie, S.A. Collection of wind-borne haematophagous insects in the Torres Strait, Australia. Med. Vet. Entomol. 2010, 17, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Chapman, H.F.; Hughes, J.M.; Ritchie, S.A.; Kay, B.H. Population structure and dispersal of the freshwater mosquitoes Culex annulirostris and Culex palpalis (Diptera: Culicidae) in Papua New Guinea and Northern Australia. J. Med. Entomol. 2003, 40, 165–169. [Google Scholar] [CrossRef]
- Eagles, D.; Walker, P.J.; Zalucki, M.P.; Durr, P.A. Modelling spatio-temporal patterns of long-distance Culicoides dispersal into northern Australia. Prev. Vet. Med. 2013, 110, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Eagles, D.; Melville, L.; Weir, R.; Davis, S.; Bellis, G.; Zalucki, M.P.; Walker, P.J.; Durr, P.A. Long-distance aerial dispersal modelling of Culicoides biting midges: Case studies of incursions into Australia. BMC Vet. Res. 2014, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Bao, Y.X.; Wang, J.Q.; Zhai, B.P. Case studies on the landing mechanisms of the brown planthoppers Nilaparvata lugens (Stål). Acta Ecol. Sin. 2007, 27, 5068–5075. [Google Scholar]
- Rose, A.H.; Silversides, R.H.; Lindquist, O.H. Migration flight by an aphid, Rhopalosiphum maidis (hemiptera: Aphididae), and a noctuid, Spodoptera frugiperda (Lepidoptera: Noctuidae). Can. Entomol. 1975, 107, 567–576. [Google Scholar] [CrossRef]
- Zhai, B.P.; Zhang, X.X.; Cheng, X.N. Parameterizing the migratory behaviour of insects I. Behavioural analysis. Acta Ecol. Sin. 1997, 1, 9–19. [Google Scholar]


| Trap Name | Location | Latitude | Longitude | Trap Type |
|---|---|---|---|---|
| FAW-Erub001 | Sewerage treatment plant, Erub Island | −9.590167 | 143.75698 | Unitrap |
| FAW-Erub002 | Northeast of water storage, Erub Island | −9.5915 | 143.77208 | Unitrap |
| FAW-Saibai001 | East of cemetary, Saibai Island | −9.382222 | 142.6075 | Unitrap |
| FAW-Saibai002 | Southern end of airstrip, Saibai Island | −9.381111 | 142.62528 | Unitrap |
| FAW-Bamaga001 | Sagaukaz Street, Bamaga | −10.89583 | 142.38442 | Unitrap |
| FAW-Bamaga002 | Koraba Road, Seisia | −10.84815 | 142.36731 | Unitrap |
| FAW-Badu001 | Blanket Yabu Street, Badu Island | −10.15776 | 142.1682 | Unitrap |
| FAW-Badu002 | Esplanade, Badu Island | −10.16836 | 142.16694 | Unitrap |
| Item | Domain 1 |
|---|---|
| Location | 10° S, 132° E |
| The number of grid points | 130 × 150 |
| Distance between grid points | 30 |
| Layers | 30 |
| Map projection | Mercator |
| Microphysics scheme | WSM6 |
| Longwave radiation scheme | RRTMG |
| Shortwave radiation scheme | RRTMG |
| Surface layer scheme | Monin-Obukhov |
| Land/water surface scheme | Noah |
| Planetary boundary layer scheme | YSU |
| Cumulus parameterization | Tiedtke |
| Forecast time | 72 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, G.-J.; Ma, J.; Wan, J.; Ren, Y.-L.; McKirdy, S.; Hu, G.; Zhang, Z.-F. Source Regions of the First Immigration of Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) Invading Australia. Insects 2021, 12, 1104. https://doi.org/10.3390/insects12121104
Qi G-J, Ma J, Wan J, Ren Y-L, McKirdy S, Hu G, Zhang Z-F. Source Regions of the First Immigration of Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) Invading Australia. Insects. 2021; 12(12):1104. https://doi.org/10.3390/insects12121104
Chicago/Turabian StyleQi, Guo-Jun, Jian Ma, Jing Wan, Yong-Lin Ren, Simon McKirdy, Gao Hu, and Zhen-Fei Zhang. 2021. "Source Regions of the First Immigration of Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) Invading Australia" Insects 12, no. 12: 1104. https://doi.org/10.3390/insects12121104
APA StyleQi, G.-J., Ma, J., Wan, J., Ren, Y.-L., McKirdy, S., Hu, G., & Zhang, Z.-F. (2021). Source Regions of the First Immigration of Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) Invading Australia. Insects, 12(12), 1104. https://doi.org/10.3390/insects12121104







