Life History of Oxybelus variegatus Wesmael, 1852 (Hymenoptera: Crabronidae) with a Description of the Mature Larva
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
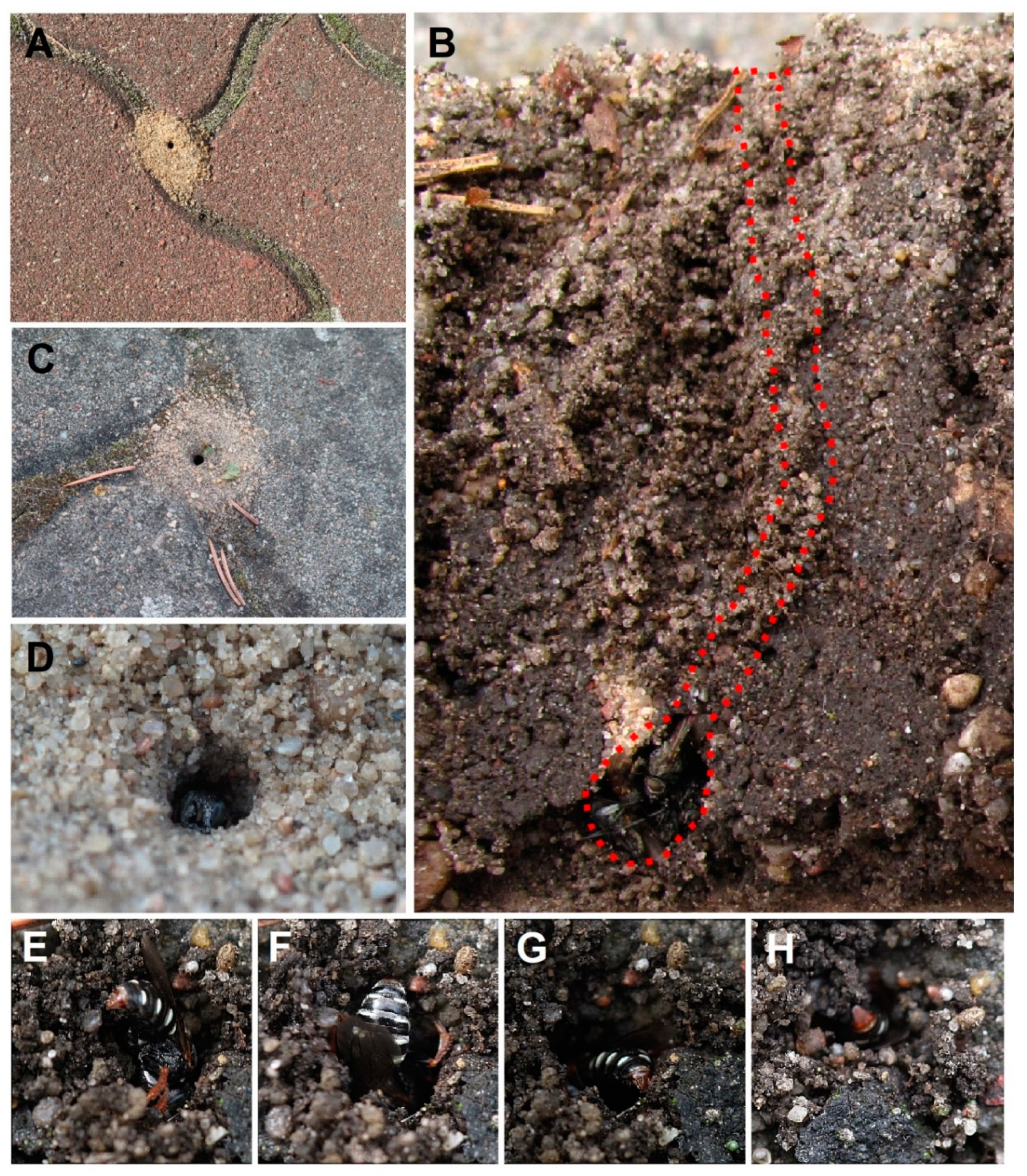
3.1. Environmental Preferences and Nesting Behaviour
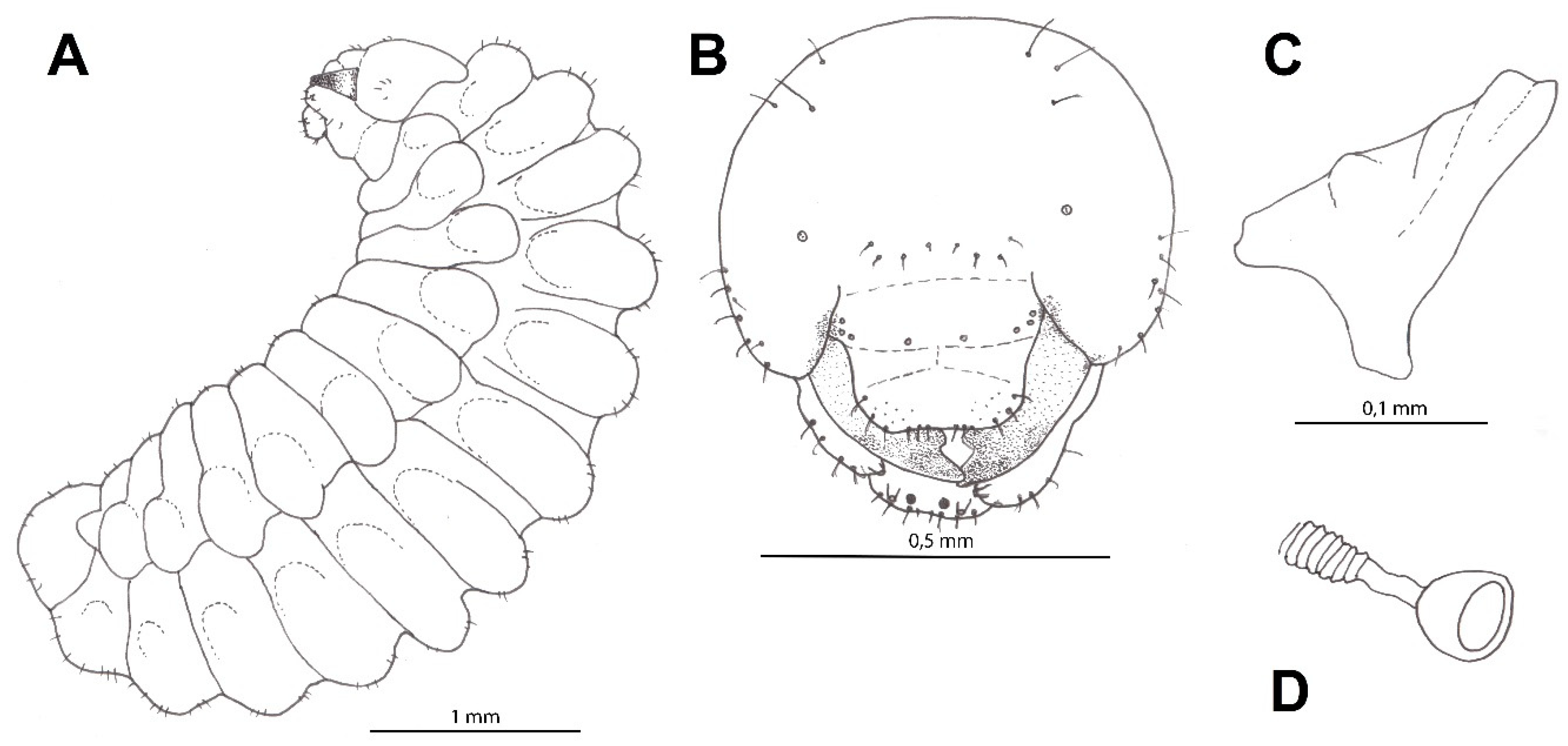
3.2. Description of the Mature Larva
3.2.1. Material Examined
3.2.2. Diagnosis
3.2.3. Description
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pulawski, W. Catalog of Sphecidae. California Academy of Sciences, San Francisco. Available online: http://research.calacademy.org/sites/research.calacademy.org/files/Departments/ent/sphecidae/Genera_and_species_pdf/Oxybe-lus.pdf (accessed on 7 May 2020).
- Barbier, Y. Fauna Europaea: Family Crabronidae. Fauna Europea Version 1.0. Available online: http://www.faunaeur.org/full_results.php?id=11303 (accessed on 2 November 2020).
- Olszewski, P.; Wiśniowski, B.; Ljubomirov, T. Current list of the Polish digger wasps (Hymenoptera: Spheciformes). Spixiana 2021, manuscript in press. [Google Scholar]
- Bohart, R.M.; Menke, A.S. Sphecid Wasps of the World. A Generic Revision; University of California Press: Berkeley, CA, USA, 1976. [Google Scholar]
- Iwata, K. Evolution of Instinct: Comparative Ethology of Hymenoptera; Amerind Publishing Co. Pvt. Ltd.: New Delhi, India, 1976; 539p. [Google Scholar]
- Bohart, R.M.; Schlinger, E.I. California wasps of the genus Oxybelus (Hymenoptera, Sphecidae, Crabroninae). Bull. Calif. Insect Surv. 1957, 4, 103–134. [Google Scholar]
- Grandi, G. Studi di un Entomologo Sugli Imenotteri Superiori. Boll. Ist. Entomol. della Univ. Studi. Bologna. 1961, 25, 1–661. [Google Scholar]
- Kazenas, V.L. Fauna and Biology of Sphecid Wasps (Hymenoptera, Sphecidae) of Kazakhstan and Central Asia; Kazgos INTI: Almaty, Kazakhstan, 2001; 333p. [Google Scholar]
- Peckham, D.J.; Kurczewski, F.E.; Peckham, D.B. Nesting behavior of Nearctic species of Oxybelus (Hymenoptera: Sphecidae). Ann. Entomol. Soc. Am. 1973, 66, 647–661. [Google Scholar] [CrossRef]
- Tsuneki, K. Gleanings on the bionomics of the East-Asiatic non-social wasps (Hymenoptera). I. Some species of Oxybelus (Sphecidae). Etizenia 1969, 38, 1–24. [Google Scholar]
- Asis, J.D.; Tormos, J.; Gayubo, S.F. Descripción de la larva madura de Oxybelus lamellatus Olivier y O. spectabilis Gerstaecker (Hymenoptera, Sphecidae). Misc. Zool. 1997, 20, 59–64. [Google Scholar]
- Grandi, G. Contributi alla conoscenza biologica e morfologica degli imenotteri melliferi e predatori IX. Boll. Lab. Ent. R. Ist. Sup. Agr. Bologna. 1929, 2, 255–290. [Google Scholar]
- Grandi, G. Contributi alla conoscenza degli imenotteri aculeati XXVI. Boll. Ist. Ent. Univ. Bologna. 1954, 20, 81–255. [Google Scholar]
- Maréchal, P. Sur trois hyménopteres se développant dans un cocon en mosaique (Miscophus spurius Dahlb., Oxybelus bipunctatus Ol., Mutilla rufipes F.). Mém. Soc. Entomol. Belg. 1930, 23, 1–23. [Google Scholar]
- Maréchal, P. Sur trois hyménopteres se développant dans un cocon en mosaique (supplément). Mém. Soc. Entomol. Belg. 1930, 23, 163. [Google Scholar]
- Evans, H.E. Studies on the larvae of digger wasps (Hymenoptera, Sphecidae). Part III: Philanthinae, Trypoxyloninae, and Crabroninae. Trans. Amer. Ent. Soc. 1957, 83, 79–117. [Google Scholar]
- Andrietti, F.; Polidori, C.; Casiraghi, M.; Bellati, A.; Passerini, E.; Martinoli, A. Small-scale sympatric digger wasps Oxybelus argentatus and Oxybelus trispinosus segregate activity, hunt for different prey, and diverge in nesting behavior. Ann. Soc. Entomol. Fr. Nouv. Série 2013, 49, 205–221. [Google Scholar] [CrossRef]
- Blösch, M. Die Grabwespen Deutschlands. Sphecidae s. str., Crabronidae. Lebensweise, Verhalten, Verbreitung. In Die Tierwelt Deutschlands. 71. Teil; Blank, S.M., Taeger, A., Eds.; Goecke & Evers: Keltern, Germany, 2000; 480p. [Google Scholar]
- Tormos, J.; Asís, J.D.; Gayubo, S.F.; Portillo, M.; Torres, F. Nesting behavior of Oxybelus lamellatus Olivier (Hymenoptera: Sphecidae). Ann. Entomol. Soc. Am. 2000, 93, 326–332. [Google Scholar] [CrossRef]
- Nicoli Aldini, R. Behavioural observations on three species of Oxybelus (Hymenoptera Sphecidae) nesting in syntopy. Redia 2004, LXXXVII, 253–256. [Google Scholar]
- Bitsch, J.; Leclercq, J. Hyménoptères Sphecidae d’Europe occidentale. 1. Faune de France. 79; Fédération Française des Sociétés de Sciences Naturelles: Paris, France, 1993; 325p. [Google Scholar]
- Švácha, P.; Danilevsky, M.L. Cerambycoid larvae of Europe and Soviet Union. Part I. Acta Univ. Carol. Biol. 1987, 30, 1–176. [Google Scholar]
- Iwata, K. Comparative studies on the habits of solitary wasps. Tenthredo Acta Entomol. 1942, 4, 1–142. [Google Scholar]
- Berland, L. Hyménoptères vespiformes, I, Sphegidae, Pompilidae, Scoliidae, Sapygidae, Mutillidae; Paul Lechevalier: Paris, France, 1925; Volume 10, 364p. [Google Scholar]
- Guiglia, D. Gli Oxybelini d’Italia. Ann. Mus. Civ. Storia Nat. Genova 1953, 66, 55–158. [Google Scholar]
- Mingo Perez, E. Los Oxybelini de la penisula iberica (Hymenoptera). Graellsia 1966, XXII, 57–121. [Google Scholar]
- Spofford, M.G.; Kurczewski, F.E. Counter-cleptoparasitic behaviours of species of Sphecidae (Hymenoptera) in response to Miltogrammini larviposition (Diptera, Sarcophagidae). J. Nat. Hist. 1992, 26, 993–1012. [Google Scholar] [CrossRef]
- Spofford, M.G.; Kurczewski, F.E. Comparative larvipositional behaviours and cleptoparasitic frequencies of Nearctic species of Miltogrammini (Diptera: Sarcophagidae). J. Nat. Hist. 1990, 24, 731–755. [Google Scholar] [CrossRef]
- Peckham, D.J.; Hook, A.W. Behavioral observations on Oxybelus in southeastern North America. Ann. Entomol. Soc. Am. 1980, 73, 557–567. [Google Scholar] [CrossRef]
- Ellis, S.; Scatcherd, J. Bean seed fly (Delia platura, Delia florilega) and onion fly (Delia antiqua) incidence in England and an evaluation of chemical and biological control options. Tests Agrochem. Cultiv. 2007, 151, 259–267. [Google Scholar] [CrossRef]
- Gayubo, S.F.; Tormos, J. Nuevas Aportaciones al Conocimiento de la Esfecidofauna Valenciana (II) (Hym., Sphecidae); Serie Hymenoptera, Cuaderno 2; Publica Fun da ción Entomológica “Juan de Torres Sala”: Valencia, Spain, 1986; 35p. [Google Scholar]
- Skibińska, E. Sphecidae Grzebaczowate. In Red List of Threatened Animals in Poland; Głowaciński, Z., Ed.; Instytut Ochrony Przyrody PAN: Kraków, Poland, 2002; pp. 66–68. [Google Scholar]
- Schmid-Egger, C. Rote Liste der Wespen Deutschlands. Hymenoptera Aculeata: Grabwespen (Ampulicidae, Crabronidae, Sphecidae), Wegwespen (Pompilidae), Goldwespen (Chrydididae), Faltenwespen (Vespidae), Spinnenameisen (Mutillidae), Dolchwespen (Scoliidae), Rollwespen (Tiphiidae) und Keulhornwespen (Sapygidae). Ampulex 2010, 1, 5–39. [Google Scholar]
- Heneberg, P.; Bogusch, P.; Řezáč, M. Roadside verges can support spontaneous establishment of steppe-like habitats hosting diverse assemblages of bees and wasps (Hymenoptera: Aculeata) in an intensively cultivated central European landscape. Biodivers. Conserv. 2017, 26, 843–864. [Google Scholar] [CrossRef]
- Heneberg, P.; Bogusch, P.; Řezáč, M. Numerous drift sand “specialists” among bees and wasps (Hymenoptera: Aculeata) nest in wetlands that spontaneously form de novo in arable fields. Ecol. Eng. 2018, 117, 133–139. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewski, P.; Bogusch, P.; Szpila, K. Life History of Oxybelus variegatus Wesmael, 1852 (Hymenoptera: Crabronidae) with a Description of the Mature Larva. Insects 2021, 12, 100. https://doi.org/10.3390/insects12020100
Olszewski P, Bogusch P, Szpila K. Life History of Oxybelus variegatus Wesmael, 1852 (Hymenoptera: Crabronidae) with a Description of the Mature Larva. Insects. 2021; 12(2):100. https://doi.org/10.3390/insects12020100
Chicago/Turabian StyleOlszewski, Piotr, Petr Bogusch, and Krzysztof Szpila. 2021. "Life History of Oxybelus variegatus Wesmael, 1852 (Hymenoptera: Crabronidae) with a Description of the Mature Larva" Insects 12, no. 2: 100. https://doi.org/10.3390/insects12020100
APA StyleOlszewski, P., Bogusch, P., & Szpila, K. (2021). Life History of Oxybelus variegatus Wesmael, 1852 (Hymenoptera: Crabronidae) with a Description of the Mature Larva. Insects, 12(2), 100. https://doi.org/10.3390/insects12020100





