Natural Occurrence of Entomopathogenic Fungi as Endophytes of Sugarcane (Saccharum officinarum) and in Soil of Sugarcane Fields
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
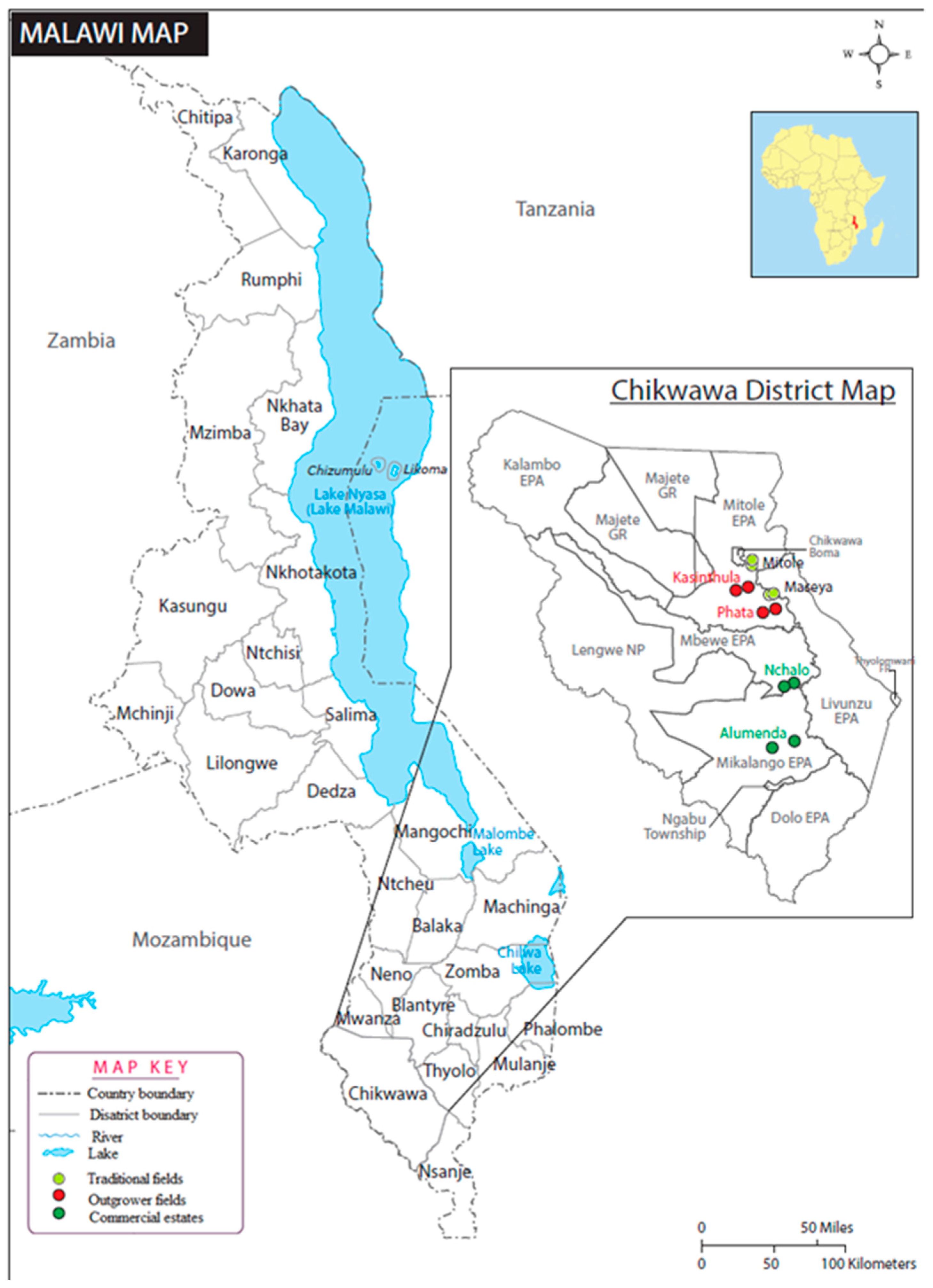
2.1. Description of Sugarcane Production, Location, and Sampling of Plants and Soil
2.2. Isolation of Fungi
2.2.1. Isolation of Endophytic Fungi from Plant Samples
2.2.2. Isolation of Fungi from Soil Samples
2.3. Morphological Identification of Fungi
2.4. Molecular Identification of Fungi to Species Level
2.4.1. DNA Extraction, PCR Amplification, and Sequence Analysis
2.4.2. Phylogenetic Analysis
2.5. Data Analysis
3. Results
3.1. Morphological Identification and Frequency of Occurrence
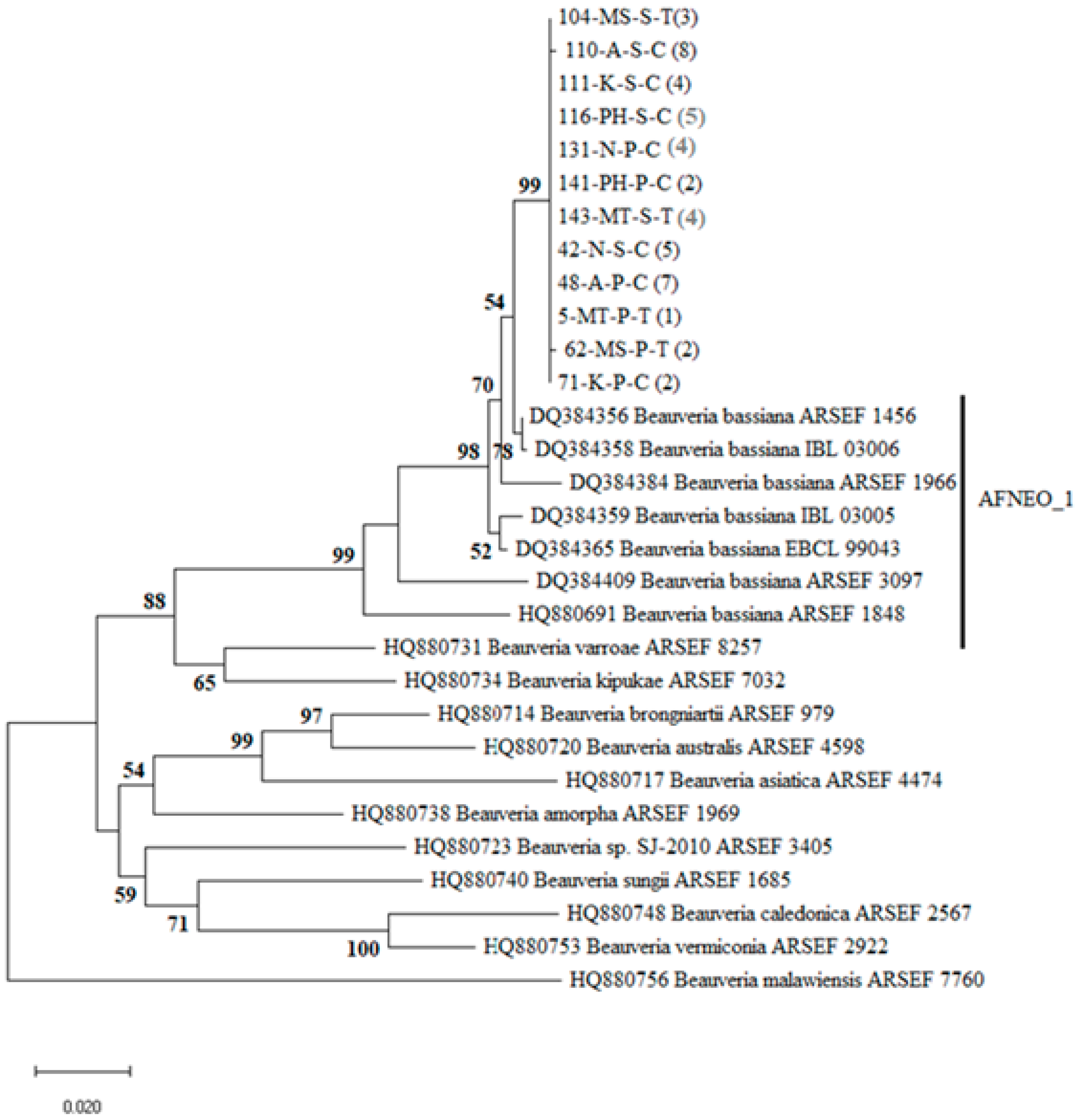
3.2. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vega, F.E.; Posada, F.; Aime, M.C.; Pava-Ripoll, M.; Infante, F.; Rehner, S.A. Entomopathogenic fungal endophytes. Biol. Control 2008, 46, 72–82. [Google Scholar] [CrossRef]
- Gurulingappa, P.; Sword, G.A.; Murdoch, G.; McGee, P.A. Colonization of crop plants by entomopathogenic fungi and their effects on two insect pests when in planta. Biological 2010, 55, 34–41. [Google Scholar]
- Reay, S.D.; Brownbridge, M.; Gicquel, B.; Cummings, N.J.; Nelson, T.L. Isolation and characterization of endophytic Beauveria spp. (Ascomycota: Hypocreales) from Pinus radiata in New Zealand forests. Biol. Control 2010, 54, 52–60. [Google Scholar] [CrossRef]
- Fisher, J.J.; Rehner, S.A.; Bruck, D.J. Diversity of rhizosphere associated entomopathogenic fungi of perennial herbs, shrubs and coniferous trees. J. Invertebr. Pathol. 2011, 106, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Clifton, E.H.; Jaronski, S.T.; Hodgson, E.W.; Gassmann, A.J. Abundance of soil-borne entomopathogenic fungi in organic and conventional fields in the Midwestern USA with an emphasis on the effect of herbicides and fungicides on fungal persistence. PLoS ONE 2015, 10, e0133613. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A.; Solter, L.F. Initial Handling and Diagnosis of Diseased Invertebrates. In Manual of the Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Academic Press: London, UK, 2012; pp. 1–13. [Google Scholar]
- Onwley, B.H.; Gwinn, K.D.; Vega, F.E. Endophytic entomopathogenic fungi with activity against plant pathogens: Ecology and evolution. BioControl 2010, 55, 113–128. [Google Scholar]
- Pell, J.; Eilenberg, J.; Hajek, A.E.; Steinkraus, D.C. Biology, ecology and pest management potential of Entomophthorales. In Fungi as Biocontrol Agents: Progress, Problems and Potential; Butt, T.M., Jackson, C.W., Magan, N., Eds.; CABI: Wallingford, CT, USA, 2001; pp. 71–153. [Google Scholar]
- Akello, J.; Dubois, T.; Gold, C.S.; Coyne, D.; Nakavuma, J.; Paparu, P. Beauveria bassiana (Balsamo) Vuillemin as an endophyte in tissue culture banana (Musa spp.). J. Invertebr. Pathol. 2008, 96, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Meyling, N.V.; Eilenberg, J. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biol. Control 2007, 43, 145–155. [Google Scholar] [CrossRef]
- Posada, F.; Aime, M.C.; Peterson, S.W.; Rehner, S.A.; Vega, F.E. Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Mycol. Res. 2007, 111, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Cory, J.S.; Ericsson, J.D. Fungal entomopathogens in a tritrophic context. In The Ecology of Fungal Entomopathogens; Roy, H.E., Vega, F.E., Chandler, D., Goettel, M.S., Chandler, D., Pell, J.K., Wajnberg, E., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 75–88. [Google Scholar]
- Bruck, D.J. Entomopathogenic fungi in the rhizosphere. Biocontrol 2010, 55, 103–112. [Google Scholar] [CrossRef]
- Klingen, I.; Eilenberg, J.; Meadow, R. Effects of farming system, field margins and bait insect on the occurrence of entomopathogenic fungi in soils. Agric. Ecosyst. Environ. 2002, 91, 191–198. [Google Scholar] [CrossRef]
- Klingen, I.; Haukeland, S. The soil as a reservoir for natural enemies of pest insects and mites with emphasis on fungi and nematodes. In An Ecological and Societal Approach to Biological Control; Eilenberg, J., Hokkanen, H.M.T., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 145–211. [Google Scholar]
- Quesada-Moraga, E.; Navas-Cortés, J.A.; Maranhao, E.A.A.; Ortiz-Urquiza, A.; Santiago-Alvarez, C. Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol. Res. 2007, 111, 947–966. [Google Scholar] [CrossRef] [PubMed]
- Ramos, Y.; Portal, O.; Lysøe, E.; Meyling, N.V.; Klingen, I. Diversity and abundance of Beauveria bassiana in soils, stink bugs and plant tissues of common bean from organic and conventional fields. J. Invertebr. Pathol. 2017, 150, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Ganley, R.J.; Newcombe, G. Fungal endophytes in seeds and needles of Pinus monticola. Mycol. Res. 2006, 110, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Posada, F.; Vega, F.E. Establishment of the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales) as an endophyte in cocoa seedlings (Theobroma cacao). Mycologia 2005, 97, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Cherry, A.J.; Banito, A.; Djegui, D.; Lomer, C. Suppression of the stem borer Sesamia calamistis (Lepidoptera: Noctuidae) in maize following seed dressing, topical application and stem injection with African isolates of Beauveria bassiana. Int. J. Pest Manag. 2004, 50, 67–73. [Google Scholar] [CrossRef]
- Bing, L.A.; Lewis, L.C. Occurrence of entomopathogen Beauveria bassiana (Balsamo) Vuillemin in different tillage regimes in Zea mays L. and virulence towards Ostrinia nubilalis (Hubner). Agric. Ecosyst. Environ. 1993, 45, 147–156. [Google Scholar] [CrossRef]
- Parsa, S.; Garcia-Lemos, A.M.; Castillo, K.; Ortiz, V.; Lopez-Lavalle, L.A.B.; Braum, J.; Vega, F.E. Fungal endophytes in germinated seeds of the common bean, Phaseolus vulgaris. Fungal Biol. 2016, 120, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Tefera, T.; Pringle, K.L. Mortality and maize leaf consumption of Chilo partellus (Lepidoptera: Pyralidae) larvae treated with Beauveria bassiana and Metarhizium anisopliae. Int. J. Pest Manag. 2004, 50, 29–34. [Google Scholar] [CrossRef]
- Goble, T.A.; Costet, L.; Robene, I.; Nibouche, S.; Rutherford, R.S.; Conlong, D.E.; Hill, M.P. Beauveria brongniartii on white grubs attacking sugarcane in South Africa. J. Invertebr. Pathol. 2012, 111, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Gao, Y.; Zhang, Y.; Wang, E.; Xu, X.; Lei, Z. An entomopathogenic strain of Beauveria bassiana against Frankliniella occidentalis with no detrimental effect on the predatory mite Neoseiulus barkeri: Evidence from laboratory bioassay and scanning electron microscopic observation. PLoS ONE 2014, 9, e84732. [Google Scholar] [CrossRef] [PubMed]
- Kernasa, N.; Uraichuen, S.; Kamata, N. Phylogenetic variation of the green muscadine fungus, Metarhizium anisopliae (Metchnikoff) Sorokin and its virulence to larvae of the sugarcane longhorn stem borer, Dorysthenes buqueti Guerin (Coleoptera: Cerambycidae). Agric. Nat. Resour. 2016, 50, 427–431. [Google Scholar] [CrossRef]
- Ngubane, N.P.; Hatting, J.L.; Truter, M. Entomopathogens associated with African and Mauritian Scarabaeidea affecting sugarcane. Proc. S. Afr. Sugar Technol. Assoc. 2012, 85, 114–117. [Google Scholar]
- Baucum, L.; Rice, R.W.; Muralles, L. Backyard Sugarcane. Sugarcane Handbook. University of Florida. Publication #SS-AGR-253. 2009. Available online: http://edis.ifas.ufl.edu/sc052 (accessed on 17 February 2018).
- Donga, T.K.; Eklo, O.M. Environmental load of pesticides used in conventional sugarcane production in Malawi. Crop Prot. 2018, 108, 71–77. [Google Scholar] [CrossRef]
- Orr, A.; Ritchie, J.M. Learning from failure: Smallholder farming systems and IPM in Malawi. Agric. Syst. 2004, 79, 31–54. [Google Scholar] [CrossRef]
- Meyer, J.H.; Heathman, W.Z. Report of Further Outcomes from the Reconnaissance Soil Suitability Survey of Five Estates in the Nchalo Sugarcane Supply Area; Jan Meyer Soil Fertility Consultants: Cape Town, South Africa, 2015; 61p. [Google Scholar]
- Parsa, S.; Ortiz, V.; Vega, F.E. Establishing entomopathogenic fungi as endophytes: Towards endophytic biological control. J. Vis. Exp. 2013, 74, e50360. [Google Scholar] [CrossRef]
- Zimmermann, G. The ‘Galleria bait method’ for detection of entomopathogenic fungi. J. Appl. Entomol. 1986, 102, 213–215. [Google Scholar] [CrossRef]
- Woodring, J.L.; Kaya, H.K. Steinermematid and Heterorhabditid Nematodes: A Handbook of Biology and Techniques Southern Cooperative Series Bulletin; Arkansas Agicultural Experiment Station: Fayetteville, AR, USA, 1988; Volume 331, pp. 1–17. [Google Scholar]
- Humber, R.A. Identification of entomopathogenic fungi. In Manual of the Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 154–182. [Google Scholar]
- Rehner, S.A.; Posada, F.; Buckley, E.P.; Infante, F.; Castillo, A.; Vega, F.E. Phylogenetic origins of African and Neotropical Beauveria bassiana s.l. pathogens of the coffee berry borer, Hypothenemus hampei. J. Invert. Pathol. 2006, 93, 11–21. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Clifton, E.H.; Hajek, A.E.; Jenkins, N.E.; Roush, R.T.; Rost, J.P.; Biddinger, D.J. Applications of Beauveria bassiana (Hypocreales: Cordycipitaceae) to control populations of spotted lanternfly (Hemiptera: Fulgoridae), in semi-natural landscapes and on grapevines. Environ. Entomol. 2020, 49, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Donga, T.K.; Vega, E.; Klingen, I. Establishment of the fungal entomopathogen Beauveria bassiana as an endophyte in sugarcane, Saccharum officinarum. Fungal Ecol. 2018, 35, 70–77. [Google Scholar] [CrossRef]
- Hajek, A.E. Ecology of terrestrial fungal entomopathogens. Adv. Microb. Ecol. 1997, 15, 193–249. [Google Scholar]
- Bruck, D.J.; Lewis, L.C. Carpophilus freemani (Coleoptera: Nitidulidae) as a vector of Beauveria bassiana. J. Invertebr. Pathol. 2002, 80, 188–190. [Google Scholar] [CrossRef]
- Akutse, K.S.; Maniania, N.K.; Fiaboe, K.K.M.; van Den Berg, J.; Ekesi, S. Endophytic colonization of Vicia faba and Phaseolus vulgaris (Fabaceae) by fungal pathogens and their effects on the life history parameters of Liriomyza huidobrensis (Diptera: Agromyzidae). Fungal Ecol. 2013, 6, 293–301. [Google Scholar] [CrossRef]
- Tian, X.L.; Cao, L.X.; Tan, H.M.; Zeng, Q.G.; Jia, Y.Y.; Han, W.Q.; Zhou, S.N. Study on the communities of endophytic fungi and endophytic Actinomycetes from rice and their antipathogenic activities in vitro. World J. Microbiol. Biotechnol. 2004, 20, 303–309. [Google Scholar] [CrossRef]
- Ananda, K.; Sridhar, K.R. Diversity of endophytic fungi in the roots of mangrove species on the west coast of India. Can. J. Microbiol. 2002, 48, 871–887.8. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.E.; Simpkins, A.; Aime, M.C.; Posada, F.; Peterson, S.W.; Rehner, S.A.; Infante, F.; Castillo, A.; Arnold, A.E. Fungal endophyte diversity in coffee plants from Colombia, Hawai’i, Mexico and Puerto Rico. Fungal Ecol. 2010, 3, 122–138. [Google Scholar] [CrossRef]
- Gange, A.C.; Koricheva, J.; Currie, A.F.; Jaber, L.R.; Vidal, S. Meta-analysis of the role of entomopathogenic and unspecialized fungal endophytes as plant bodyguards. New Phytol. 2019. [Google Scholar] [CrossRef]
- Vega, F.E. The use of fungal entomopathogens as endophytes in insects: A review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Iwanicki, N.S.; Pereira, A.A.; Botelho, A.B.R.Z.; Rezende, J.M.; de Moral, R.A.; Zucchi, M.I.; Delalibera, I., Jr. Monitoring of the field application of Metarhizium anisopliae in Brazil revealed high molecular diversity of Metarhizium spp in insects, soil and sugarcane roots. Sci. Rep. 2019, 9, 4443. [Google Scholar] [CrossRef] [PubMed]
- Tkaczuk, C.; Król, A.; Majchrowska-Safaryan, A.; Nicewicz, Ł. The occurrence of entomopathogenic fungi in soils from fields cultivated in a conventional and organic system. J. Ecol. Eng. 2014, 15, 137–144. [Google Scholar]
- Goble, T.A.; Dames, J.F.; Hill, M.P.; Moore, S.D. The effects of farming system, habitat type and bait type on the isolation of entomopathogenic fungi from citrus soils in the Eastern Cape province, South Africa. BioControl 2010, 55, 399–412. [Google Scholar] [CrossRef]
- Meyling, N.V.; Thorup-Kristensen, K.; Eilenberg, J. Below-and aboveground abundance and distribution of fungal entomopathogens in experimental conventional and organic cropping systems. Biol. Control 2011, 59, 180–186. [Google Scholar] [CrossRef]
- Oliveira, I.; Pereira, J.A.; Quesada-Moraga, E.; Lino-Neto, T.; Bento, A.; Baptista, P. Effect of soil tillage on natural occurrence of fungal entomopathogens associated to Prays oleae Bern. Sci. Hortic. 2013, 159, 190–196. [Google Scholar] [CrossRef]
- Arnold, A.E.; Herre, E.A. Canopy cover and leaf age affect colonization by tropical fungal endophytes: Ecological pattern and process in Theobroma cacao (Malvaceae). Mycologia 2003, 95, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Bon, H.; Huat, J.; Parrot, L.; Sinzogan, A.; Martin, T.; Malézieux, E.; Vayssières, J. Pesticide risks from fruit and vegetable pest management by small farmers in sub-Saharan Africa. A review. Agron. Sustain. Dev. 2014, 34, 723–736. [Google Scholar] [CrossRef]
- De Snoo, G.R. Unsprayed margins: Effects on environment, biodiversity and agricultural practice. Landsc. Urban Plan. 1999, 46, 151–160. [Google Scholar] [CrossRef]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Inchausti, P. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Meyling, N.V.; Pilz, C.; Keller, S.; Widmer, F.; Enkerli, J. Diversity of Beauveria spp. isolates from pollen beetles Meligethes aeneus in Switzerland. J. Invertebr. Pathol. 2012, 109, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Meyling, N.V.; Lübeck, M.; Buckley, E.P.; Eilenberg, J.; Rehner, S.A. Community composition, host range and genetic structure of the fungal entomopathogen Beauveria in adjoining agricultural and seminatural habitats. Mol. Ecol. 2009, 18, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- López-González, R.C.; Gómez-Cornelio, S.; de la Rosa-García, S.C.; Garrido, E.; Oropeza- Mariano, O.; Heil, M.; Partida-Martínez, L.P. The age of lima bean leaves influences the richness and diversity of the endophytic fungal community, but not the antagonistic effect of endophytes against Colletotrichum lindemuthianum. Fungal Ecol. 2017, 26, 1–10. [Google Scholar] [CrossRef]
- Sanchez-Azofeifa, A.; Oki, Y.; Fernandes, G.W.; Ball, R.A.; Gamon, J. Relationships between endophyte diversity and leaf optical properties. Trees 2012, 26, 291–299. [Google Scholar] [CrossRef]
- Lin, Y.; Qasim, M.; Hussain, M.; Akutse, K.S.; Avery, P.B.; Dash, C.K.; Wang, L. The herbivore-induced plant volatiles methyl salicylate and menthol positively affect growth and pathogenicity of entomopathogenic fungi. Sci. Rep. 2017, 7, 40494. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hussain, M.; Avery, P.B.; Qasim, M.; Fang, D.; Wang, L. Volatiles from plants induced by multiple aphid attacks promote conidial performance of Lecanicillium lecanii. PLoS ONE 2016, 11, e0151844. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, G.; Ownley, B.H.; Augé, R.M.; Toler, H.; Dee, M.; Vu, A.; Köllner, T.G.; Chen, F. Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect. Symbiosis 2015, 65, 65–74. [Google Scholar] [CrossRef]
- Thompson, S.R.; Brandenburg, R.L. Tunneling responses of mole crickets (Orthoptera: Gryllotalpidae) to the entomopathogenic fungus, Beauveria bassiana. Environ. Entomol. 2005, 34, 140–147. [Google Scholar] [CrossRef]
| Location | Fungal Species Isolated | Total | |||||
|---|---|---|---|---|---|---|---|
| Beauveria spp. | Metarhizium spp. | Isaria spp. | |||||
| Plant | Soil | Plant | Soil | Plant | Soil | ||
| Alumenda | 7 | 8 | 0 | 3 | 3 | 0 | 21 |
| Nchalo | 4 | 5 | 0 | 0 | 3 | 1 | 13 |
| Kasinthula | 2 | 4 | 0 | 0 | 4 | 2 | 12 |
| Phata | 2 | 5 | 0 | 0 | 2 | 2 | 11 |
| Maseya | 2 | 3 | 0 | 1 | 0 | 0 | 6 |
| Mitole | 1 | 4 | 1 | 4 | 3 | 0 | 13 |
| Total | 18 | 29 | 1 | 8 | 15 | 5 | 76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasambala Donga, T.; Meadow, R.; Meyling, N.V.; Klingen, I. Natural Occurrence of Entomopathogenic Fungi as Endophytes of Sugarcane (Saccharum officinarum) and in Soil of Sugarcane Fields. Insects 2021, 12, 160. https://doi.org/10.3390/insects12020160
Kasambala Donga T, Meadow R, Meyling NV, Klingen I. Natural Occurrence of Entomopathogenic Fungi as Endophytes of Sugarcane (Saccharum officinarum) and in Soil of Sugarcane Fields. Insects. 2021; 12(2):160. https://doi.org/10.3390/insects12020160
Chicago/Turabian StyleKasambala Donga, Trust, Richard Meadow, Nicolai V. Meyling, and Ingeborg Klingen. 2021. "Natural Occurrence of Entomopathogenic Fungi as Endophytes of Sugarcane (Saccharum officinarum) and in Soil of Sugarcane Fields" Insects 12, no. 2: 160. https://doi.org/10.3390/insects12020160
APA StyleKasambala Donga, T., Meadow, R., Meyling, N. V., & Klingen, I. (2021). Natural Occurrence of Entomopathogenic Fungi as Endophytes of Sugarcane (Saccharum officinarum) and in Soil of Sugarcane Fields. Insects, 12(2), 160. https://doi.org/10.3390/insects12020160






