Aedes albopictus Populations and Larval Habitat Characteristics across the Landscape: Significant Differences Exist between Urban and Rural Land Use Types
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Design
2.2. Water Chemistry and Evaporation
2.3. Temperature and Humidity
2.4. Statistical Analyses
2.4.1. A. albopictus Abundance
2.4.2. Water Chemistry and Evaporation
3. Results
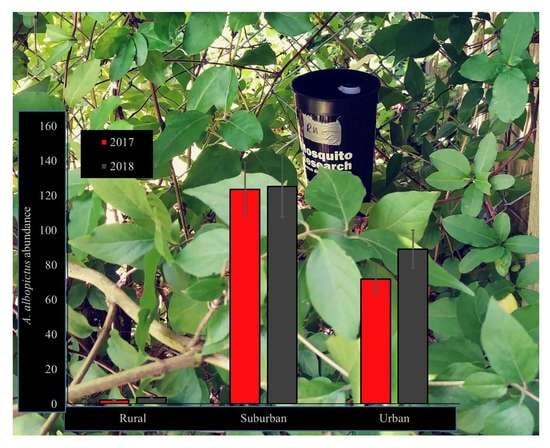
3.1. A. albopictus Abundance
3.2. Water Chemistry and Evaporation
3.3. Temperature and Humidity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowarik, I. Novel urban ecosystems, biodiversity, and conservation. Environ. Pollut. 2011, 159, 1974–1983. [Google Scholar] [CrossRef]
- Choate, B.A.; Hickman, P.L.; Moretti, E.A. Wild bee species abundance and richness across an urban–rural gradient. J. Insect Conserv. 2018, 22, 391–403. [Google Scholar] [CrossRef]
- Wilke, A.B.B.; Chase, C.; Vasquez, C.; Carvajal, A.; Medina, J.; Petrie, W.D.; Beier, J.C. Urbanization creates diverse aquatic habitats for immature mosquitoes in urban areas. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Juliano, S.A.; Lounibos, L.P. Invasions by mosquitoes: The roles of behaviour across the life cycle. In Biological Invasions and Animal Behavior; Weis, J., Sol, D., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 245–265. [Google Scholar]
- Weaver, S.C. Urbanization and geographic expansion of zoonotic arboviral diseases: Mechanisms and potential strategies for prevention. Trends Microbiol. 2013, 21, 360–363. [Google Scholar] [CrossRef] [Green Version]
- Himsworth, C.G.; Parsons, K.L.; Jardine, C.; Patrick, D.M. Rats, cities, people, and pathogens: A systematic review and narrative synthesis of literature regarding the ecology of rat-associated zoonoses in urban centers. Vector-Borne Zoonotic Dis. 2013, 13, 349–359. [Google Scholar] [CrossRef]
- Ferraguti, M.; Martínez-de la Puente, J.; Roiz, D.; Ruiz, S.; Soriguer, R.; Figuerola, J. Effects of landscape anthropization on mosquito community composition and abundance. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Townroe, S.; Callaghan, A. British container breeding mosquitoes: The impact of urbanisation and climate change on community composition and phenology. PLoS ONE 2014, 9, e95325. [Google Scholar] [CrossRef] [Green Version]
- Wahid, B.; Ali, A.; Rafique, S.; Idrees, M. Global expansion of chikungunya virus: Mapping the 64-year history. Int. J. Infect. Dis. 2017, 58, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile virus: Review of the literature. J. Am. Med. Assoc. 2013, 310, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.R.; Tabachnick, W.J. History of domestication and spread of Aedes aegypti—A review. Memórias do Inst. Oswaldo Cruz 2013, 108, 11–17. [Google Scholar] [CrossRef]
- Gangoso, L.; Aragonés, D.; Martínez-de la Puente, J.; Lucientes, J.; Delacour-Estrella, S.; Estrada Peña, R.; Montalvo, T.; Bueno-Marí, R.; Bravo-Barriga, D.; Frontera, E.; et al. Determinants of the current and future distribution of the West Nile virus mosquito vector Culex pipiens in Spain. Environ. Res. 2020, 188, 109837. [Google Scholar] [CrossRef]
- Lounibos, L.P.; Kramer, L.D. Invasiveness of Aedes aegypti and Aedes albopictus and vectorial capacity for chikungunya virus. J. Infect. Dis. 2016, 214, S453–S458. [Google Scholar] [CrossRef] [Green Version]
- Brady, O.J.; Godfray, H.C.J.; Tatem, A.J.; Gething, P.W.; Cohen, J.M.; Ellis McKenzie, F.; Alex Perkins, T.; Reiner, R.C.; Tusting, L.S.; Sinka, M.E.; et al. Vectorial capacity and vector control: Reconsidering sensitivity to parameters for malaria elimination. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 107–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, T.L.; Haque, U.; Monaghan, A.J.; Eisen, L.; Hahn, M.B.; Hayden, M.H.; Savage, H.M.; McAllister, J.; Mutebi, J.P.; Eisen, R.J. Modeling the environmental suitability for Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in the contiguous United States. J. Med. Entomol. 2017, 54, 1605–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, M.V.; Hintz, C.W.; Jones, L.; Shiau, J.; Solano, N.; Drake, J.M.; Murdock, C.C. Microclimate and larval habitat density predict adult Aedes albopictus abundance in urban areas. Am. J. Trop. Med. Hyg. 2019, 101, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Service, M.W. The daytime distribution of mosquitoes resting in vegetation. J. Med. Entomol. 1971, 8, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Lounibos, L.P.; O’Meara, G.F.; Juliano, S.A.; Nishimura, N.; Escher, R.L.; Reiskind, M.H.; Cutwa, M.; Greene, K. Differential survivorship of invasive mosquito species in south Florida cemeteries: Do site-specific microclimates explain patterns of coexistence and exclusion? Ann. Entomol. Soc. Am. 2010, 103, 757–770. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.E.; Lounibos, L.P.; Marra, P.P.; Kilpatrick, A.M. Rainfall influences Survival of Culex pipiens (Diptera: Culicidae) in a residential neighborhood in the Mid-Atlantic United States. J. Med. Entomol. 2012, 49, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Murdock, C.; Evans, M.; McClanahan, T.; Miazgowicz, K.; Tesla, B. Fine-scale variation in microclimate across and urban landscape changes the capacity of Aedes albopictus to vector arboviruses. PLoS Negl. Trop. Dis. 2017, 11, e0005640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faraji, A.; Egizi, A.; Fonseca, D.M.; Unlu, I.; Crepeau, T.; Healy, S.P.; Gaugler, R. Comparative host feeding patterns of the Asian tiger mosquito, Aedes albopictus, in urban and suburban northeastern USA and implications for disease transmission. PLoS Negl. Trop. Dis. 2014, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valerio, L.; Marini, F.; Bongiorno, G.; Facchinelli, L.; Pombi, M.; Caputo, B.; Maroli, M.; Torre, A. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in urban and rural contexts within Rome, Province Italy. Vector-Borne Zoonotic Dis. 2010, 10, 291–294. [Google Scholar] [CrossRef]
- Stone, C.M.; Jackson, B.T.; Foster, W.A. Effects of plant-community composition on the vectorial capacity and fitness of the malaria mosquito Anopheles gambiae. Am. J. Trop. Med. Hyg. 2012, 87, 727–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, T.J.; Kline, D.L.; Kaufman, P.E. Aedes albopictus (Diptera: Culicidae) oviposition preference as influenced by container size and Buddleja davidii plants. J. Med. Entomol. 2016, 53, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Juliano, S.A.; Westby, K.M.; Ower, G.D. Know your enemy: Effects of a predator on native and invasive container mosquitoes. J. Med. Entomol. 2019, 56, 320–328. [Google Scholar] [CrossRef]
- Fader, J.E. The importance of interspecific interactions on the present range of the invasive mosquito Aedes albopictus (Diptera: Culicidae) and persistence of resident container species in the United States. J. Med. Entomol. 2016, 53, 992–1001. [Google Scholar] [CrossRef] [Green Version]
- Yee, D.A.; Kneitel, J.M.; Juliano, S.A. Environmental correlates of abundances of mosquito species and stages in discarded vehicle tires. J. Med. Entomol. 2010, 47, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Clements, A. The Biology of Mosquitoes. Viral, Arboviral, and Bacterial Pathogens; CABI Publishing: Cambridge, UK, 2011; Volume 3. [Google Scholar]
- Clements, A.N. The Biology of Mosquitoes. Development, Nutrition, and Reproduction; CABI Publishing: Cambridge, UK, 1992; Volume 1. [Google Scholar]
- Gardner, A.M.; Anderson, T.K.; Hamer, G.L.; Johnson, D.E.; Varela, K.E.; Walker, E.D.; Ruiz, M.O. Terrestrial vegetation and aquatic chemistry influence larval mosquito abundance in catch basins, Chicago, USA. Parasit. Vectors 2013, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Müller, G.C.; Xue, R.; De Schlein, Y. Differential attraction of Aedes albopictus in the field to flowers, fruits and honeydew. Acta Trop. 2011, 118, 45–49. [Google Scholar] [CrossRef]
- Davis, A.; Major, R.E.; Taylor, C.E. The association between nectar availability and nectarivore density in urban and natural environments. Urban Ecosyst. 2015, 18, 503–515. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Turo, K.J.; Riley, C.B.; Inocente, E.A.; Tian, J.; Hoekstra, N.C.; Piermarini, P.M.; Gardiner, M.M. Can urban greening increase vector abundance in cities? The impact of mowing, local vegetation, and landscape composition on adult mosquito populations. Urban Ecosyst. 2019, 22, 827–839. [Google Scholar] [CrossRef]
- Little, E.; Biehler, D.; Leisnham, P.T.; Jordan, R.; Wilson, S.; LaDeau, S.L. Socio-ecological mechanisms supporting high densities of Aedes albopictus (Diptera: Culicidae) in Baltimore, MD. J. Med. Entomol. 2017, 54, 1183–1192. [Google Scholar] [CrossRef] [Green Version]
- Katz, G.; Leisnham, P.T.; LaDeau, S.L. Aedes albopictus body size differs across neighborhoods with varying infrastructural abandonment. J. Med. Entomol. 2020, 57, 615–619. [Google Scholar] [CrossRef]
- Murrell, E.G.; Damal, K.; Lounibos, L.P.; Juliano, S.A. Distributions of competing container mosquitoes depend on detritus types, nutrient ratios, and food availability. Ann. Entomol. Soc. Am. 2011, 104, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Godefroid, S.; Koedam, N. Distribution pattern of the flora in a peri-urban forest: An effect of the city-forest ecotone. Landsc. Urban Plan. 2003, 65, 169–185. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Carreiro, M.M. Controls on mass loss and nitrogen dynamics of oak leaf litter along an urban-rural land-use gradient. Oecologia 2003, 135, 288–298. [Google Scholar] [CrossRef]
- Carreiro, M.M.; Howe, K.; Parkhurst, D.F.; Pouyat, R.V. Variation in quality and decomposability of red oak leaf litter along an urban-rural gradient. Biol. Fertil. Soils 1999, 30, 258–268. [Google Scholar] [CrossRef]
- Dorendorf, J.; Wilken, A.; Eschenbach, A.; Jensen, K. Urban-induced changes in tree leaf litter accelerate decomposition. Ecol. Process. 2015, 4, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Yee, D.A.; Juliano, S.A. Consequences of detritus type in an aquatic microsystem: Effects on water quality, micro-organisms and performance of the dominant consumer. Freshw. Biol. 2006, 51, 448–459. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, M.G.; Walker, E.D. Indirect effects of soluble nitrogen on growth of Ochlerotatus triseriatus larvae in container habitats. J. Med. Entomol. 2006, 43, 677–688. [Google Scholar] [CrossRef]
- Sota, T. Performance of Aedes albopictus and A. riversi larvae (Diptera: Culicidae) in waters that contain tannic acid and decaying leaves: Is the treehole species better adapted to treehole water? Ann. Entomol. Soc. Am. 1993, 86, 450–457. [Google Scholar] [CrossRef]
- Carpenter, S.R. Stemflow chemistry: Effects on population dynamics of detritivorous mosquitoes in tree-hole ecosystems. Oecologia 1982, 53, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Westby, K.M.; Juliano, S.A. No detectable role for predators mediating effects of aquatic habitat size and permanence on populations and communities of container-dwelling mosquitoes. Ecol. Entomol. 2017, 42, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.M.; Knight, T.M. Drought-induced mosquito outbreaks in wetlands. Ecol. Lett. 2003, 6, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Juliano, S.A.; Stoffregen, T.L. Effects of habitat drying on size at and time to metamorphosis in the tree hole mosquito Aedes triseriatus. Oecologia 1994, 97, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Leberfinger, K.; Bohman, I.; Herrmann, J. Drought impact on stream detritivores: Experimental effects on leaf litter breakdown and life cycles. Hydrobiologia 2010, 652, 247–254. [Google Scholar] [CrossRef]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef]
- Moore, C.G. Aedes albopictus in the United States: Current status and prospects for further spread. J. Am. Mosq. Control Assoc. 1999, 15, 221–227. [Google Scholar]
- Metzger, M.E.; Yoshimizu, M.H.; Padgett, K.A.; Hu, R.; Kramer, V.L.; Ritchie, S. Detection and establishment of Aedes Aegypti and Aedes albopictus (Diptera: Culicidae) mosquitoes in California, 2011-2015. J. Med. Entomol. 2017, 54, 533–543. [Google Scholar] [CrossRef]
- Englbrecht, C.; Gordon, S.; Venturelli, C.; Rose, A.; Geier, M. Evaluation of BG-sentinel trap as a management tool to reduce Aedes albopictus nuisance in an urban environment in Italy. J. Am. Mosq. Control Assoc. 2015, 31, 16–25. [Google Scholar] [CrossRef]
- Li, Y.; Kamara, F.; Zhou, G.; Puthiyakunnon, S.; Li, C.; Liu, Y.; Zhou, Y.; Yao, L.; Yan, G.; Chen, X.-G. Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl. Trop. Dis. 2014, 8, e3301. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, Y.; Suwonkerd, W.; Chawprom, S.; Prajakwong, S.; Takagi, M. Different spatial distribution of Aedes aegypti and Aedes albopictus along an urban-rural gradient and the relating environmental factors examined in three villages in northern Thailand. J. Am. Mosq. Control Assoc. 2006, 22, 222–228. [Google Scholar] [CrossRef]
- Higa, Y. Dengue vectors and their spatial distribution. Trop. Med. Health 2011, 39, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Honório, N.A.; Silva, W.C.; Leite, P.J.; Gonçalves, J.M.; Lounibos, L.P.; Lourenço-de-Oliveira, R. Dispersal of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in an urban endemic dengue area in the State of Rio de Janeiro, Brazil. Memórias do Inst. Oswaldo Cruz 2003, 98, 191–198. [Google Scholar] [CrossRef]
- Wimberly, M.C.; Davis, J.K.; Evans, M.V.; Hess, A.; Newberry, P.M.; Solano-Asamoah, N.; Murdock, C.C. Land cover affects microclimate and temperature suitability for arbovirus transmission in an urban landscape. PLoS Negl. Trop. Dis. 2020, 14, e0008614. [Google Scholar] [CrossRef] [PubMed]
- Murrell, E.G.; Juliano, S.A. Predation resistance does not trade off with competitive ability in early-colonizing mosquitoes. Oecologia 2013, 173, 1033–1042. [Google Scholar] [CrossRef] [Green Version]
- Murrell, E.G.; Ives, A.R.; Juliano, S.A. Intrinsic and extrinsic drivers of succession: Effects of habitat age and season on an aquatic insect community. Ecol. Entomol. 2014, 39, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Reiskind, M.H.; Wilson, M.L. Culex restuans (Diptera: Culicidae) oviposition behavior determined by larval habitat quality and quantity in southeastern Michigan. J. Med. Entomol. 2004, 41, 179–186. [Google Scholar] [CrossRef]
- Alto, B.W.; Juliano, S.A. Temperature effects on the dynamics of Aedes albopictus (Diptera: Culicidae) populations in the laboratory. J. Med. Entomol. 2001, 38, 548–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiner, R.C.; Perkins, T.A.; Barker, C.M.; Niu, T.; Chaves, L.F.; Ellis, A.M.; George, D.B.; Le Menach, A.; Pulliam, J.R.C.; Bisanzio, D.; et al. A systematic review of mathematical models of mosquito-borne pathogen transmission: 1970–2010. J. R. Soc. Interface 2013, 10, 20120921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westby, K.M.; Juliano, S.A.; Medley, K.A. Aedes albopictus (Diptera: Culicidae) has not become the dominant species in artificial container habitats in a temperate forest more than a decade after establishment. J. Med. Entomol. 2020, 1–6. [Google Scholar] [CrossRef]
- Ferreira-de-Lima, V.H.; Câmara, D.C.P.; Honório, N.A.; Lima-Camara, T.N. The Asian tiger mosquito in Brazil: Observations on biology and ecological interactions since its first detection in 1986. Acta Trop. 2020, 205, 105386. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, C.E.; Hobbs, R.J. Time for a change: Dynamic urban ecology. Trends Ecol. Evol. 2012, 27, 179–188. [Google Scholar] [CrossRef]
- LaDeau, S.L.; Leisnham, P.T.; Biehler, D.; Bodner, D. Higher mosquito production in low-income neighborhoods of Baltimore and Washington, DC: Understanding ecological drivers and mosquito-borne disease risk in temperate cities. Int. J. Environ. Res. Public Health 2013, 10, 1505–1526. [Google Scholar] [CrossRef] [Green Version]
- Shragai, T.; Harrington, L.C. Aedes albopictus (Diptera: Culicidae) on an invasive edge: Abundance, spatial distribution, and habitat usage of larvae and pupae across urban and socioeconomic environmental gradients. J. Med. Entomol. 2019, 56, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Juliano, S.A. Species interactions among larval mosquitoes: Context dependence across habitat gradients. Annu. Rev. Entomol. 2009, 54, 37–56. [Google Scholar] [CrossRef] [Green Version]
- Rey, J.R.; Nishimura, N.; Wagner, B.; Braks, M.A.H.; O’Connell, S.M.; Lounibos, L.P. Habitat segregation of mosquito arbovirus vectors in south Florida. J. Med. Entomol. 2006, 43, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.G.; Francy, D.B.; Eliason, D.A.; Monath, T. Aedes albopictus in the United States: Rapid spread of a potential disease vector. J. Am. Mosq. Control Assoc. 1988, 4, 356–361. [Google Scholar] [CrossRef]
- Moore, C.G.; Francy, D.B.; Eliason, D.A.; Bailey, R.E.; Campos, E.G. Aedes albopictus and other container-inhabiting mosquitoes in the United States: Results of an eight-city survey. J. Am. Mosq. Control Assoc. 1990, 6, 173–178. [Google Scholar] [PubMed]
- Andreadis, T.G. A survey of mosquitoes breeding in used tire stockpiles in Connecticut. J. Am. Mosq. Control Assoc. 1988, 4, 256–260. [Google Scholar]
- Beier, J.C.; Travis, M.; Patricoski, C.; Kranzfelder, J. Habitat segregation among larval mosquitoes (Diptera: Culicidae) in tire yards in Indiana, USA. J. Med. Entomol. 1983, 20, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Delatte, H.; Gimonneau, G.; Triboire, A.; Fontenille, D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J. Med. Entomol. 2009, 46, 33–41. [Google Scholar] [CrossRef]
- Alto, B.W.; Juliano, S.A. Precipitation and temperature effects on populations of Aedes albopictus (Diptera: Culicidae): Implications for range expansion. J. Med. Entomol. 2001, 38, 646–656. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.A.; Comeau, G.; Monaghan, A.J.; Williamson, D.J.; Ernst, K.C. Effects of desiccation stress on adult female longevity in Aedes aegypti and Ae. albopictus (Diptera: Culicidae): Results of a systematic review and pooled survival analysis. Parasit. Vectors 2018, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Juliano, S.A.; O’Meara, G.F.; Morrill, J.R.; Cutwa, M.M. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia 2002, 130, 458–469. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, K.S.; Kesavaraju, B.; Juliano, S.A. Condition-specific competition in container mosquitoes: The role of noncompeting life-history stages. Ecology 2005, 86, 3289–3295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellborn, G.A.; Skelly, D.K.; Werner, E.E. Mechanisms creating community structure across a freshwater habitat gradient. Annu. Rev. Ecol. Syst. 1996, 27, 337–363. [Google Scholar] [CrossRef] [Green Version]
- Aspbury, A.S.; Juliano, S.A. Negative effects of habitat drying and prior exploitation on the detritus resource in an ephemeral aquatic habitat. Oecologia 1998, 115, 137–148. [Google Scholar] [CrossRef]
- Fish, D.; Carpenter, S.R. Leaf litter and larval mosquito dynamics in tree-hole ecosystems. Ecology 1982, 63, 283–288. [Google Scholar] [CrossRef]
- Kaufman, M.G.; Goodfriend, W.; Kohler-Garrigan, A.; Walker, E.D.; Klug, M.J. Soluble nutrient effects on microbial communities and mosquito production in Ochlerotatus triseriatus habitats. Aquat. Microb. Ecol. 2002, 29, 73–88. [Google Scholar] [CrossRef]
- Moore, J.C.; Berlow, E.L.; Coleman, D.C.; De Suiter, P.C.; Dong, Q.; Hastings, A.; Johnson, N.C.; McCann, K.S.; Melville, K.; Morin, P.J.; et al. Detritus, trophic dynamics and biodiversity. Ecol. Lett. 2004, 7, 584–600. [Google Scholar] [CrossRef]
- Leisnham, P.T.; Slaney, D.P.; Weinstein, P.; Slaney, D.P.; Lester, P.J.; Weinstein, P.; Heath, A.C.G. Mosquito density, macroinvertebrate diversity, and water chemistry in water-filled containers: Relationships to land use. N. Z. J. Zool. 2007, 34, 203–218. [Google Scholar] [CrossRef]
- Kling, L.J.; Juliano, S.A.; Yee, D.A. Larval mosquito communities in discarded vehicle tires in a forested and unforested site: Detritus type, amount, and water nutrient differences. J. Vector Ecol. 2007, 32, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Ostrofsky, M.L. Effect of tannins on leaf processing and conditioning rates in aquatic ecosystems: An empirical approach. Can. J. Fish. Aquat. Sci. 1993, 50, 1176–1180. [Google Scholar] [CrossRef]
- David, J.P.; Rey, D.; Pautou, M.P.; Meyran, J.C. Differential toxicity of leaf litter to dipteran larvae of mosquito developmental sites. J. Invertebr. Pathol. 2000, 75, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.E.; Yee, D.A. Response of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) survival, life history, and population growth to oak leaf and acorn detritus. J. Med. Entomol. 2019, 1–8. [Google Scholar] [CrossRef]
- Costanzo, K.S.; Mormann, K.; Juliano, S.A. Asymmetrical competition and patterns of abundance of Aedes albopictus and Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2005, 42, 559–570. [Google Scholar] [CrossRef] [Green Version]








| Year | Type | A. albopictus | A. triseriatus | A. japonicus | A. hendersoni | C. restuans | C. pipiens | An. Barberi | T. rutilus |
|---|---|---|---|---|---|---|---|---|---|
| 2017 | Rural | 716 | 1019 | 36 | 79 | 0 | 0 | 14 | 6 |
| Suburban | 5235 | 25 | 0 | 0 | 0 | 0 | 0 | 5 | |
| Urban | 3845 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | |
| 2018 | Rural | 519 | 2199 | 246 | 254 | 0 | 1 | 0 | 6 |
| Suburban | 4873 | 2 | 9 | 48 | 616 | 268 | 0 | 2 | |
| Urban | 4867 | 7 | 0 | 13 | 159 | 651 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westby, K.M.; Adalsteinsson, S.A.; Biro, E.G.; Beckermann, A.J.; Medley, K.A. Aedes albopictus Populations and Larval Habitat Characteristics across the Landscape: Significant Differences Exist between Urban and Rural Land Use Types. Insects 2021, 12, 196. https://doi.org/10.3390/insects12030196
Westby KM, Adalsteinsson SA, Biro EG, Beckermann AJ, Medley KA. Aedes albopictus Populations and Larval Habitat Characteristics across the Landscape: Significant Differences Exist between Urban and Rural Land Use Types. Insects. 2021; 12(3):196. https://doi.org/10.3390/insects12030196
Chicago/Turabian StyleWestby, Katie M., Solny A. Adalsteinsson, Elizabeth G. Biro, Alexis J. Beckermann, and Kim A. Medley. 2021. "Aedes albopictus Populations and Larval Habitat Characteristics across the Landscape: Significant Differences Exist between Urban and Rural Land Use Types" Insects 12, no. 3: 196. https://doi.org/10.3390/insects12030196
APA StyleWestby, K. M., Adalsteinsson, S. A., Biro, E. G., Beckermann, A. J., & Medley, K. A. (2021). Aedes albopictus Populations and Larval Habitat Characteristics across the Landscape: Significant Differences Exist between Urban and Rural Land Use Types. Insects, 12(3), 196. https://doi.org/10.3390/insects12030196