Effect of the Genotypic Variation of an Aphid Host on the Endosymbiont Associations in Natural Host Populations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Aphid Sampling and DNA Extraction
2.2. Microsatellite Genotyping of Aphid Individuals
2.3. Molecular Screening of Bacterial Endosymbionts and Parasitism in Aphid Individuals
2.4. Data Analysis
3. Results
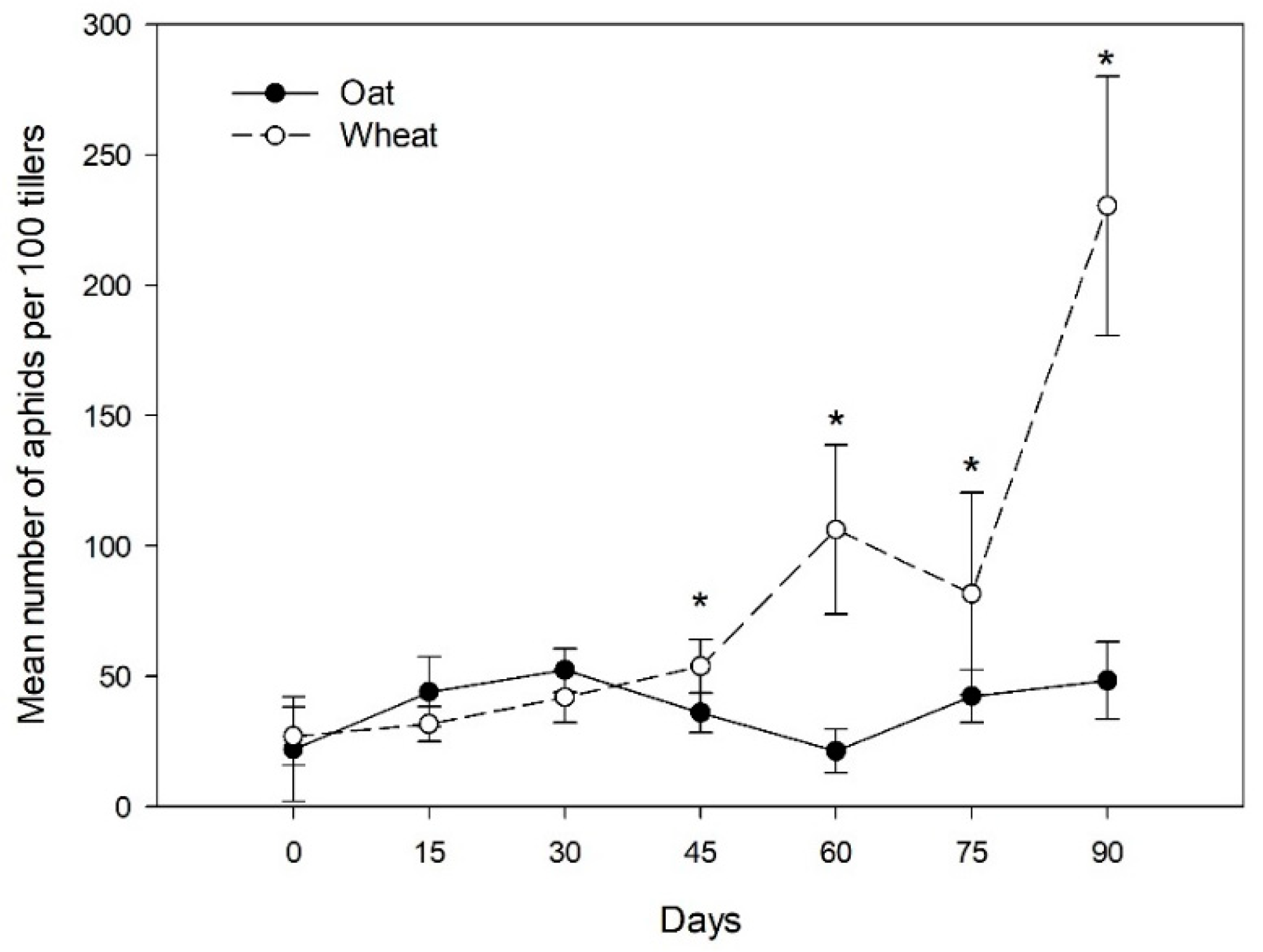
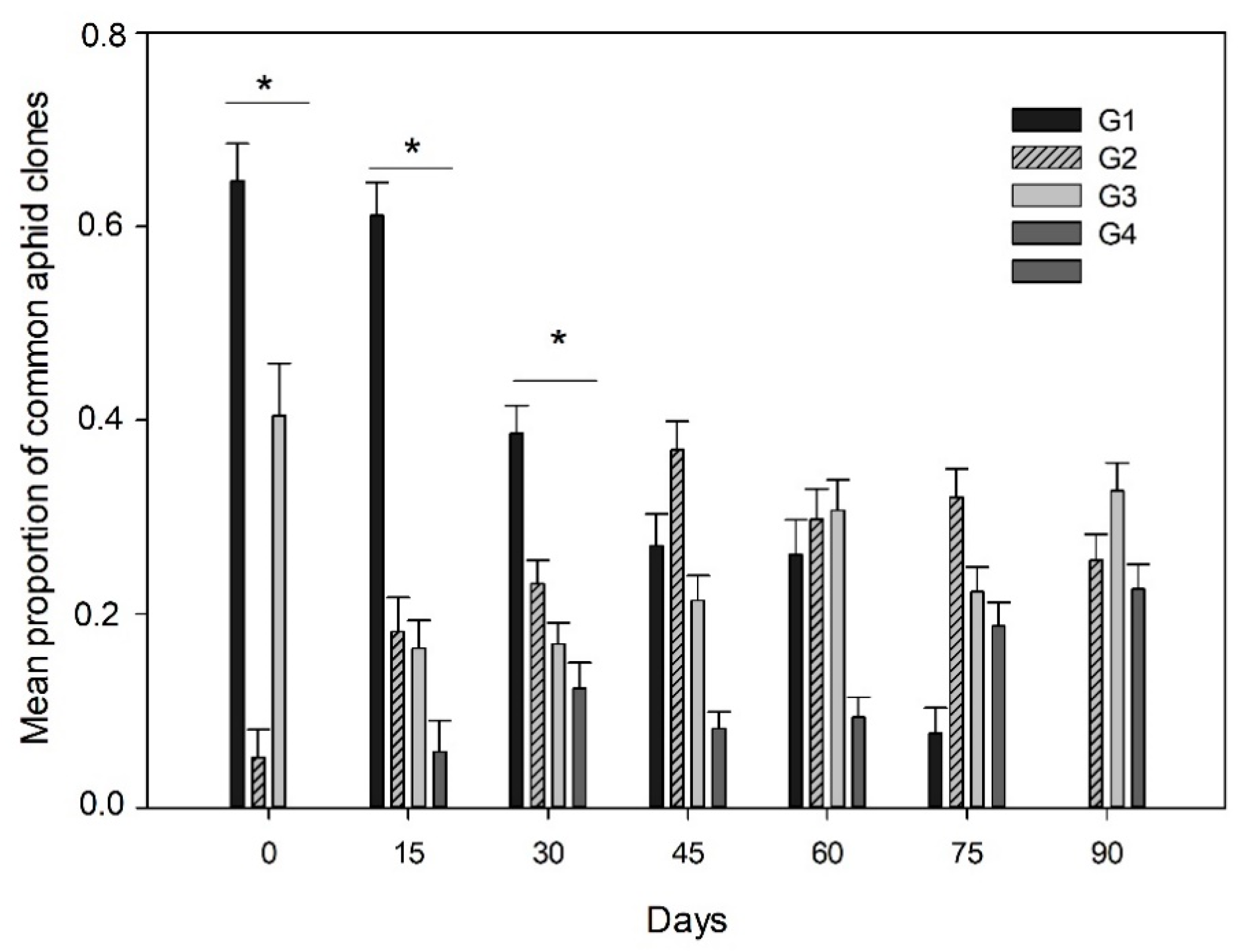
3.1. Aphid Density and Prevalence of Aphid Clones
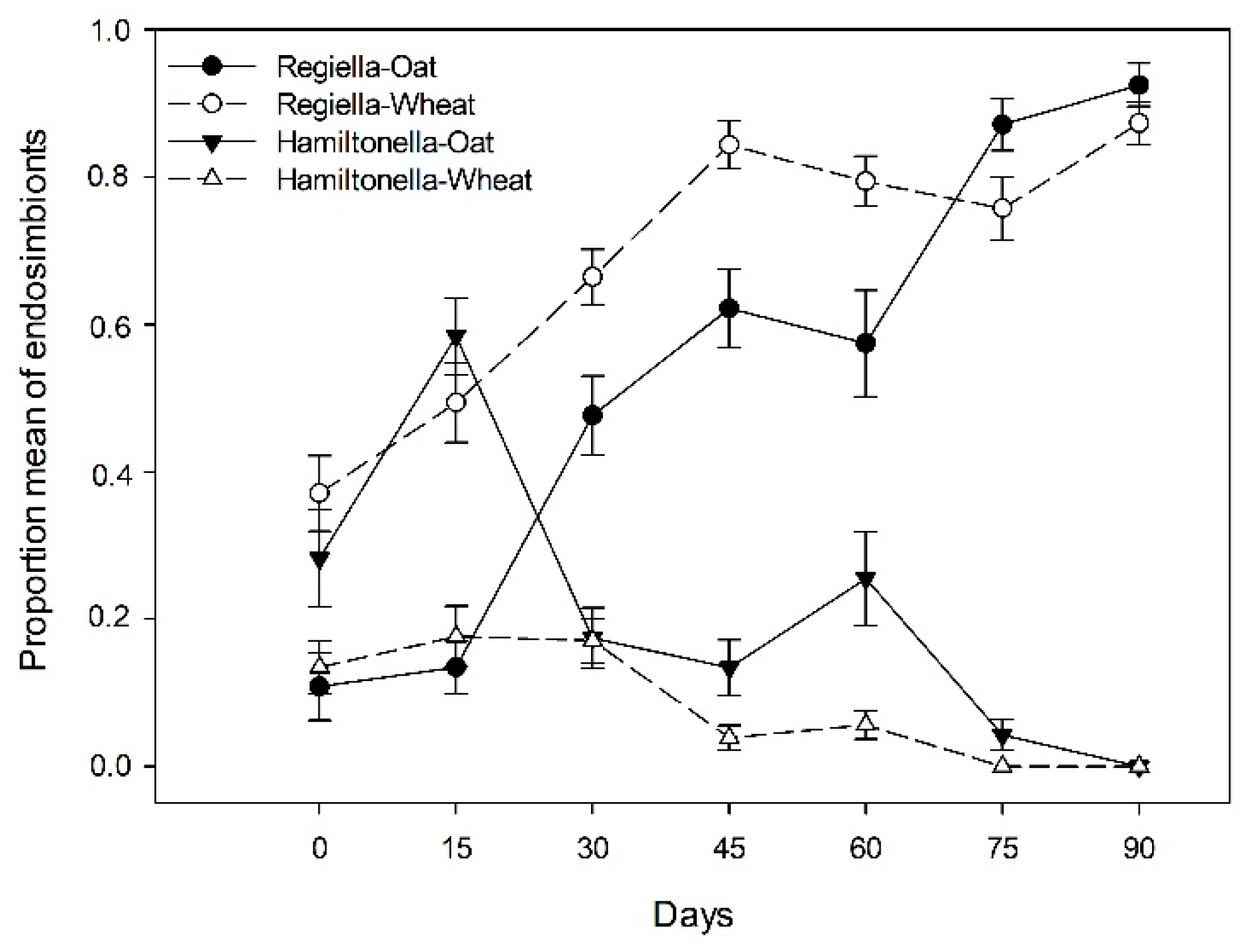
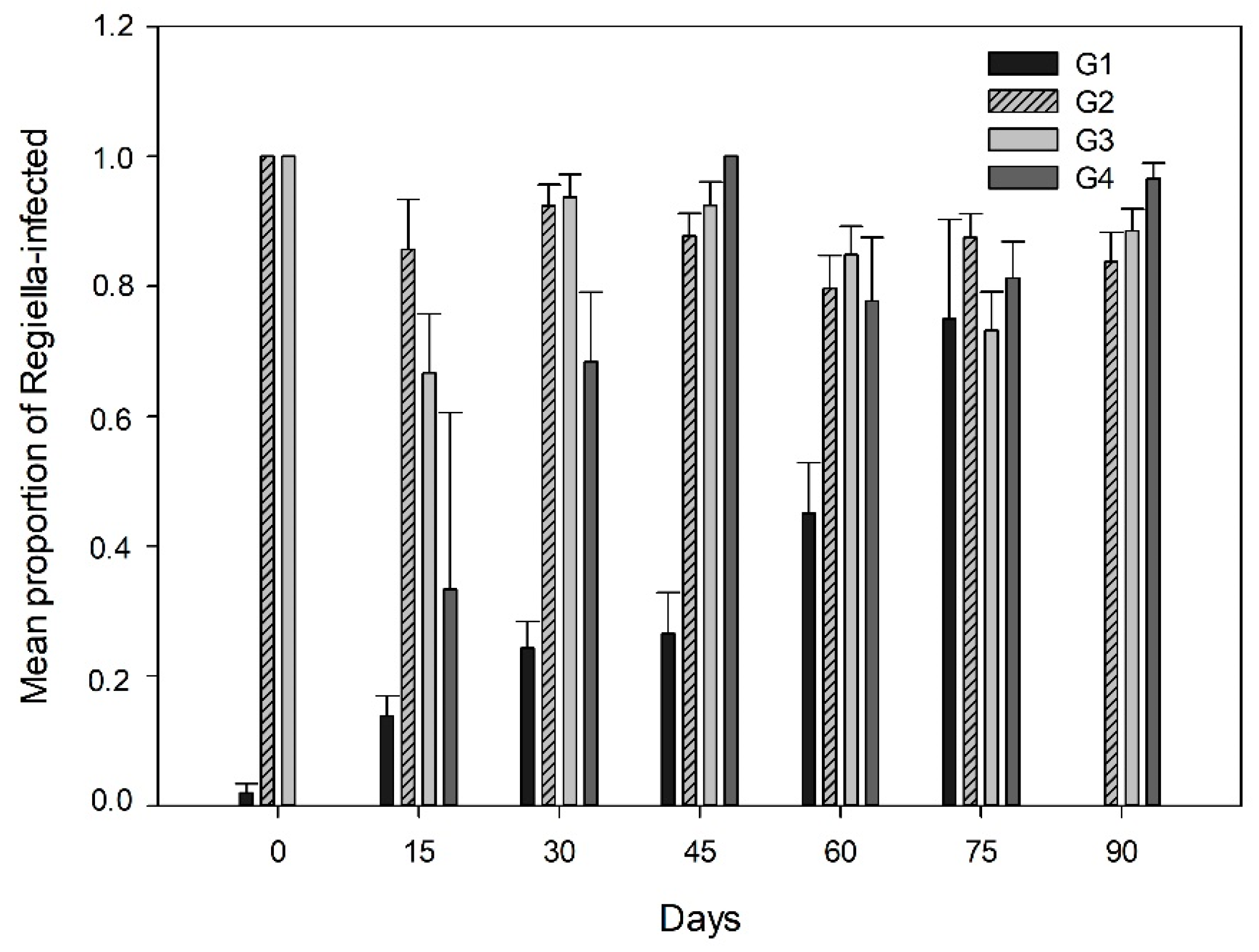
3.2. Endosymbiont Infections and Parasitism in the Aphid Clones
4. Discussion
Relationship among Endosymbiont Infections and Parasitism Rates in the Field
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Ethics Statements
References
- Oliver, K.M.; Degnan, P.H.; Burke, G.R.; Moran, N.A. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Èntomol. 2010, 55, 247–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, K.M.; Russell, J.A.; Moran, N.A.; Hunter, M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 2003, 100, 1803–1807. [Google Scholar] [CrossRef] [Green Version]
- Oliver, K.M.; Moran, N.A.; Hunter, M.S. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc. Natl. Acad. Sci. USA 2005, 102, 12795–12800. [Google Scholar] [CrossRef] [Green Version]
- Scarborough, C.L.; Ferrari, J.; Godfray, H.C.J. Aphid protected from pathogen by endosymbiont. Science 2005, 310, 1781. [Google Scholar] [CrossRef]
- Parker, B.J.; Spragg, C.J.; Altincicek, B.; Gerardo, N.M. Symbiont-mediated protection against fungal pathogens in pea aphids: A role for pathogen specificity? Appl. Environ. Microbiol. 2013, 79, 2455–2458. [Google Scholar] [CrossRef] [Green Version]
- Łukasik, P.; Guo, H.; Van Asch, M.; Ferrari, J.; Godfray, H.C.J. Protection against a fungal pathogen conferred by the aphid facultative endosymbionts Rickettsia and Spiroplasma is expressed in multiple host genotypes and species and is not influenced by co-infection with another symbiont. J. Evol. Biol. 2013, 26, 2654–2661. [Google Scholar] [CrossRef] [PubMed]
- Montllor, C.B.; Maxmen, A.; Purcell, A.H. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Èntomol. 2002, 27, 189–195. [Google Scholar] [CrossRef]
- Tsuchida, T.; Koga, R.; Fukatsu, T. Host plant specialization governed by facultative symbiont. Science 2004, 303, 1989. [Google Scholar] [CrossRef] [Green Version]
- Tsuchida, T.; Koga, R.; Matsumoto, S.; Fukatsu, T. Interspecific symbiont transfection confers a novel ecological trait to the recipient insect. Biol. Lett. 2010, 7, 245–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, J.; Scarborough, C.L.; Godfray, H.C.J. Genetic variation in the effect of a facultative symbiont on host-plant use by pea aphids. Oecologia 2007, 153, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Haselkorn, T.S.; Jaenike, J. Macroevolutionary persistence of heritable endosymbionts: Acquisition, retention and expression of adaptive phenotypes in Spiroplasma. Mol. Ecol. 2015, 24, 3752–3765. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Vilchez, I.; Mateos, M. Spiroplasma bacteria enhance survival of drosophila hydei attacked by the parasitic wasp leptopilina heterotoma. PLoS ONE 2010, 5, e12149. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Butler, S.; Sanchez, G.; Mateos, M. Male killing Spiroplasma protects Drosophila melanogaster against two parasitoid wasps. Heredity 2013, 112, 399–408. [Google Scholar] [CrossRef]
- Oliver, K.M.; Perlman, S.J. Toxin-mediated protection against natural enemies by insect defensive symbionts. Adv. Insect Physiol. 2020, 58, 277–316. [Google Scholar] [CrossRef]
- Oliver, K.M.; Smith, A.H.; Russell, J.A. Defensive symbiosis in the real world-advancing ecological studies of heritable, protective bacteria in aphids and beyond. Funct. Ecol. 2013, 28, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Zytynska, S.E.; Weisser, W.W. The natural occurrence of secondary bacterial symbionts in aphids. Ecol. Entomol. 2016, 41, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.H.; Łukasik, P.; O’Connor, M.P.; Lee, A.; Mayo, G.; Drott, M.T.; Doll, S.; Tuttle, R.; Disciullo, R.A.; Messina, A.; et al. Patterns, causes and consequences of defensive microbiome dynamics across multiple scales. Mol. Ecol. 2015, 24, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Campos, J.; Moran, N.A.; Hunter, M.S. Population dynamics of defensive symbionts in aphids. Proc. R. Soc. B 2007, 275, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Feldhaar, H. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol. Èntomol. 2011, 36, 533–543. [Google Scholar] [CrossRef]
- White, J.A. Evolution: A bacterially mediated swap meet for adaptive traits. Curr. Biol. 2013, 23, 723–725. [Google Scholar] [CrossRef] [Green Version]
- Henry, L.M.; Peccoud, J.; Simon, J.-C.; Hadfield, J.D.; Maiden, M.J.; Ferrari, J.; Godfray, H.C.J. Horizontally transmitted symbionts and host colonization of ecological niches. Curr. Biol. 2013, 23, 1713–1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zepeda-Paulo, F.; Villegas, C.; Lavandero, B. Host genotype-endosymbiont associations and their relationship with aphid parasitism at the field level. Ecol. Èntomol. 2016, 42, 86–95. [Google Scholar] [CrossRef]
- Łukasik, P.; Dawid, M.A.; Ferrari, J.; Godfray, H.C.J. The diversity and fitness effects of infection with facultative endosymbionts in the grain aphid, Sitobion avenae. Oecologia 2013, 173, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, D.A.; Zepeda-Paulo, F.; Ramírez, C.C.; Lavandero, B.; Figueroa, C.C. Diversity, frequency, and geographic distribution of facultative bacterial endosymbionts in introduced aphid pests. Insect Sci. 2017, 24, 511–521. [Google Scholar] [CrossRef]
- Ye, Z.; Vollhardt, I.M.G.; Parth, N.; Rubbmark, O.; Traugott, M. Facultative bacterial endosymbionts shape parasitoid food webs in natural host populations: A correlative analysis. J. Anim. Ecol. 2018, 87, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Fakhour, S.; Ambroise, J.; Renoz, F.; Foray, V.; Gala, J.-L.; Hance, T. A large-scale field study of bacterial communities in cereal aphid populations across Morocco. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Xiao, J.H.; Wu, Y.Q.; Huang, D.W. Diversity of bacterial symbionts in populations ofsitobion miscanthi (Hemiptera: Aphididae) in China. Environ. Èntomol. 2014, 43, 605–611. [Google Scholar] [CrossRef]
- Vorburger, C.; Sandrock, C.; Gouskov, A.; Castañeda, L.E.; Ferrari, J. Genotypic variation and the role of defensive endosymbionts in an all-parthenogenetic host-parasitoid interaction. Evolution 2009, 63, 1439–1450. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Sieber, R.; Zimmermann, Y.S.; Vorburger, C. Development specificity and sublethal effects of symbiont-conferred resistance to parasitoids in aphids Functional. Ecology 2012, 26, 207–215. [Google Scholar]
- Asplen, M.K.; Bano, N.; Brady, C.M.; Desneux, N.; Hopper, K.R.; Malouines, C.; Oliver, K.M.; White, J.A.; Heimpel, G.E. Specialization of bacterial endosymbionts that protect aphids from parasitoids. Ecol. Entomol. 2014, 39, 736–739. [Google Scholar] [CrossRef]
- Wang, D.; Shi, X.; Dai, P.; Liu, D.; Dai, X.; Shang, Z.; Ge, Z.; Meng, X. Comparison of fitness traits and their plasticity on multiple plants for Sitobion avenae infected and cured of a secondary endosymbiont. Sci. Rep. 2016, 6, 23177. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Liu, D.; Zhang, R.; Zhai, Y.; Huang, X.; Wang, D.; Shi, X. Effects of a presumably protective endosymbiont on life-history characters and their plasticity for its host aphid on three plants. Ecol. Evol. 2018, 8, 13004–13013. [Google Scholar] [CrossRef]
- Ramírez-Cáceres, G.E.; Moya-Hernández, M.G.; Quilodrán, M.; Nespolo, R.F.; Ceballos, R.; Villagra, C.A.; Ramírez, C.C. Harbouring the secondary endosymbiont Regiella insecticola increases predation risk and reproduction in the cereal aphid Sitobion avenae. J. Pest Sci. 2019, 92, 1039–1047. [Google Scholar] [CrossRef]
- Starý, P. Alternative host and parasitoid in first method in aphid pest management in glasshouses. J. Appl. Èntomol. 1993, 116, 187–191. [Google Scholar] [CrossRef]
- Sunnucks, P.; Hales, D.F. Numerous transposed sequences of mitochondrial cytochrome oxidase I-II in aphids of the genus Sitobion (Hemiptera: Aphididae). Mol. Biol. Evol. 1996, 13, 510–524. [Google Scholar] [CrossRef]
- Simon, J.C.; Baumann, S.; Sunnucks, P.; Hebert, P.; Pierre, J.S.; Le Gallic, J.F.; Dedryver, C.A. Reproductive mode and population genetic structure of the cereal aphid Sitobion avenae studied using phenotypic and microsatellite markers. Mol. Ecol. 1999, 8, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.C.C.; Massonnet, B.; Simon, J.-C.; Prunier-Leterme, N.; Dolatti, L.; Llewellyn, K.S.; Figueroa, C.C.; Ramirez, C.C.; Blackman, R.L.; Estoup, A.; et al. Cross-species amplification of microsatellite loci in aphids: Assessment and application. Mol. Ecol. Notes 2004, 4, 104–109. [Google Scholar] [CrossRef]
- Zepeda-Paulo, F.; Ortiz-Martínez, S.; Silva, A.X.; Lavandero, B. Low bacterial community diversity in two introduced aphid pests revealed with 16S rRNA amplicon sequencing. PeerJ 2018, 6, e4725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peccoud, J.; Bonhomme, J.; Mahéo, F.; De La Huerta, M.; Cosson, O.; Simon, J.-C. Inheritance patterns of secondary symbionts during sexual reproduction of pea aphid biotypes. Insect Sci. 2013, 21, 291–300. [Google Scholar] [CrossRef]
- Ye, Z.; Vollhardt, I.M.G.; Girtler, S.; Wallinger, C.; Tomanovic, Z.; Traugott, M. An effective molecular approach for assessing cereal aphid-parasitoid-endosymbiont networks. Sci. Rep. 2017, 7, 3138. [Google Scholar] [CrossRef]
- Ortiz-Martínez, S.; Pierre, J.-S.; Van Baaren, J.; Le Lann, C.; Zepeda-Paulo, F.; Lavandero, B. Interspecific competition among aphid parasitoids: Molecular approaches reveal preferential exploitation of parasitized hosts. Sci. Rep. 2019, 9, 19641. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Green, P. Package ‘lme4’, Version 1 17, CRAN; R Foundation for STATISTICAL Computing: Vienna, Austria, 2018. [Google Scholar]
- Crawley, M.J. The R Book; John Wiley & Sons: Chichester, UK, 2012. [Google Scholar]
- Fox, J.; Friendly, M.; Weisberg, S. Hypothesis tests for multivariate linear models using the car package. R. J. 2013, 5, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Hothorn, T.; Bretz, F.; Westfall, P.; Heiberger, R.M.; Schuetzenmeister, A.; Scheibe, S.; Hothorn, M.T. Package ‘Multcomp’ Simultaneous Inference in General Parametric Models; Project for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Sandstrom, J.P.; Russell, J.A.; White, J.P.; Moran, N.A. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 2001, 10, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Darby, A.C.; Douglas, A.E. Elucidation of the transmission patterns of an insect-borne bacterium. Appl. Environ. Microbiol. 2003, 69, 4403–4407. [Google Scholar] [CrossRef] [Green Version]
- Mandrioli, M.; Manicardi, G. Evolving aphids: One genome-one organism insects or holobionts. Invertebr. Surviv. J. 2013, 10, 1–6. [Google Scholar]
- Vorburger, C.; Lancaster, M.; Sunnucks, P. Environmentally related patterns of reproductive modes in the aphid Myzus persicae and the predominance of two ‘superclones’ in Victoria, Australia. Mol. Ecol. 2003, 12, 3493–3504. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.C.; Simon, J.-C.; Le Gallic, J.-F.; Prunier-Leterme, N.; Briones, L.M.; Dedryver, C.-A.; Niemeyer, H.M. Genetic structure and clonal diversity of an introduced pest in Chile, the cereal aphid Sitobion avenae. Heredity 2005, 95, 24–33. [Google Scholar] [CrossRef]
- Zepeda-Paulo, F.A.; Simon, J.C.; Ramirez, C.C.; Fuentes-Contreras, E.; Margaritopoulos, J.T.; Wilson, A.C.C.; Sorenson, C.E.; Briones, L.M.; Azevedo, R.; Ohashi, D.V.; et al. The invasion route for an insect pest species: The tobacco aphid in the New World. Mol. Ecol. 2010, 19, 4738–4752. [Google Scholar] [CrossRef]
- Gilabert, A.; Dedryver, C.-A.; Stoeckel, S.; Plantegenest, M.; Simon, J.-C. Longitudinal clines in the frequency distribution of ‘super-clones’ in an aphid crop pest. Bull. Èntomol. Res. 2015, 105, 694–703. [Google Scholar] [CrossRef]
- Henry, L.M.; Maiden, M.C.J.; Ferrari, J.; Godfray, H.C.J. Insect life history and the evolution of bacterial mutualism. Ecol. Lett. 2015, 18, 516–525. [Google Scholar] [CrossRef]
- Ferrari, J.; West, J.A.; Via, S.; Godfray, H.C.J. Population genetic structure and secondary symbionts in host-associated populations of the pea aphid complex. Evolution 2012, 66, 375–390. [Google Scholar] [CrossRef]
- Łukasik, P.; Guo, H.; Van Asch, M.; Henry, L.M.; Godfray, H.C.J.; Ferrari, J. Horizontal transfer of facultative endosymbionts is limited by host relatedness. Evolution 2015, 69, 2757–2766. [Google Scholar] [CrossRef]
- Luo, C.; Luo, K.; Meng, L.; Wan, B.; Zhao, H.; Hu, Z. Ecological impact of a secondary bacterial symbiont on the clones of Sitobion avenae (Fabricius) (Hemiptera: Aphididae). Sci. Rep. 2017, 7, 40754. [Google Scholar] [CrossRef] [Green Version]
- Castañeda, L.E.; Sandrock, C.; Vorburger, C. Variation and covariation of life history traits in aphids are related to infection with the facultative bacterial endosymbiont Hamiltonella defensa. Biol. J. Linn. Soc. 2010, 100, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Russell, A.J.; Moran, N.A. Costs and benefits of symbiont infection in aphids: Variation among symbionts and across temperatures. Proc. R. Soc. B 2005, 273, 603–610. [Google Scholar] [CrossRef] [Green Version]
- Hughes, G.L.; Rasgon, J.L. Transinfection: A method to investigateWolbachia-host interactions and control arthropod-borne disease. Insect Mol. Biol. 2014, 23, 141–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sochard, C.; LeClair, M.; Simon, J.-C.; Outreman, Y. Host plant effects on the outcomes of defensive symbioses in the pea aphid complex. Evol. Ecol. 2019, 33, 651–669. [Google Scholar] [CrossRef]
- Sochard, C.; Morlière, S.; Toussaint, G.; Outreman, Y.; Sugio, A.; Simon, J. Examination of the success rate of secondary symbiont manipulation by microinjection methods in the pea aphid system. Èntomol. Exp. Appl. 2020, 168, 174–183. [Google Scholar] [CrossRef]
- Ferrari, J.; Vavre, F. Bacterial symbionts in insects or the story of communities affecting communities. Philos. Trans. R. Soc. B 2011, 366, 1389–1400. [Google Scholar] [CrossRef] [Green Version]
- Simon, J.-C.; Peccoud, J. Rapid evolution of aphid pests in agricultural environments. Curr. Opin. Insect Sci. 2018, 26, 17–24. [Google Scholar] [CrossRef]
- Thierry, M.; Becker, N.; Hajri, A.; Reynaud, B.; Lett, J.-M.; Delatte, H. Symbiont diversity and non-random hybridization among indigenous (Ms) and invasive (B) biotypes of Bemisia tabaci. Mol. Ecol. 2011, 20, 2172–2187. [Google Scholar] [CrossRef]
- Himler, A.G.; Adachi-Hagimori, T.; Hunter, M.S.; Bergen, J.E.; Kozuch, A.; Kelly, E.S.; Tabashnik, E.B.; Chiel, E.; Duckworth, E.V.; Dennehy, T.J.; et al. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 2011, 332, 254–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raymond, L.; Ortiz-Martínez, S.A.; Lavandero, B. Temporal variability of aphid biological control in contrasting landscape contexts. Biol. Control 2015, 90, 148–156. [Google Scholar] [CrossRef]
- Ortiz-Martínez, S.; Staudacher, K.; Baumgartner, V.; Traugott, M.; Lavandero, B. Intraguild predation is independent of landscape context and does not affect the temporal dynamics of aphids in cereal fields. J. Pest Sci. 2019, 93, 235–249. [Google Scholar] [CrossRef]
- Hansen, A.K.; Vorburger, C.; Moran, N.A. Genomic basis of endosymbiont-conferred protection against an insect parasitoid. Genome Res. 2011, 22, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Vorburger, C.; Gehrer, L.; Rodriguez, P. A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biol. Lett. 2009, 6, 109–111. [Google Scholar] [CrossRef] [Green Version]
- Heyworth, E.R.; Smee, M.R.; Ferrari, J. Aphid facultative symbionts aid recovery of their obligate symbiont and their host after heat stress. Front. Ecol. Evol. 2020, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Doremus, M.R.; Oliver, K.M. Aphid heritable symbiont exploits defensive mutualism. Appl. Environ. Microbiol. 2017, 83, e03276-16. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zepeda-Paulo, F.; Lavandero, B. Effect of the Genotypic Variation of an Aphid Host on the Endosymbiont Associations in Natural Host Populations. Insects 2021, 12, 217. https://doi.org/10.3390/insects12030217
Zepeda-Paulo F, Lavandero B. Effect of the Genotypic Variation of an Aphid Host on the Endosymbiont Associations in Natural Host Populations. Insects. 2021; 12(3):217. https://doi.org/10.3390/insects12030217
Chicago/Turabian StyleZepeda-Paulo, Francisca, and Blas Lavandero. 2021. "Effect of the Genotypic Variation of an Aphid Host on the Endosymbiont Associations in Natural Host Populations" Insects 12, no. 3: 217. https://doi.org/10.3390/insects12030217
APA StyleZepeda-Paulo, F., & Lavandero, B. (2021). Effect of the Genotypic Variation of an Aphid Host on the Endosymbiont Associations in Natural Host Populations. Insects, 12(3), 217. https://doi.org/10.3390/insects12030217






