Likely Geographic Distributional Shifts among Medically Important Tick Species and Tick-Associated Diseases under Climate Change in North America: A Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Tick-Associated Diseases of Humans in North America
2.1. Lyme Disease
2.2. Babesiosis
2.3. Anaplasmosis and Ehrlichiosis
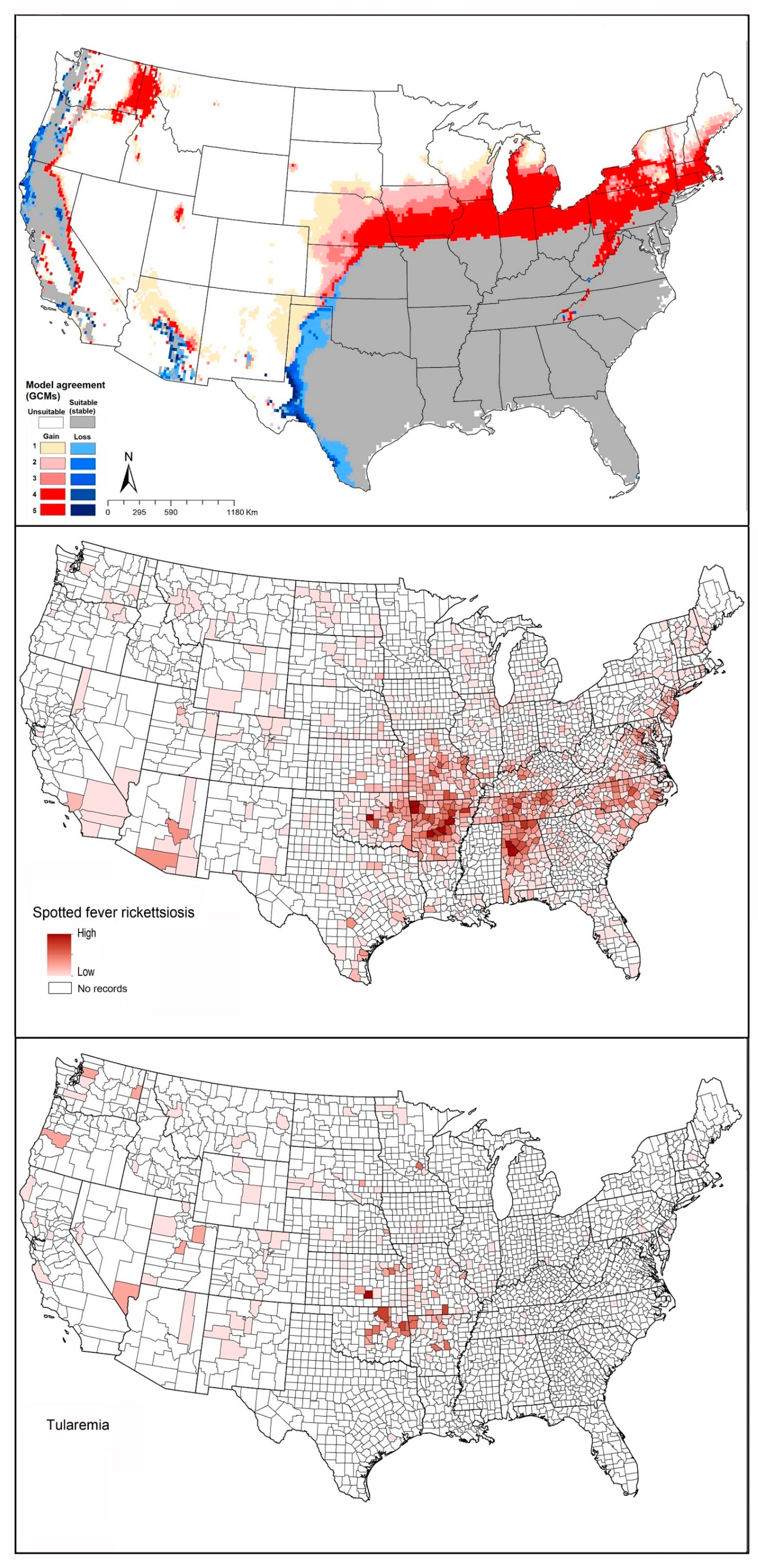
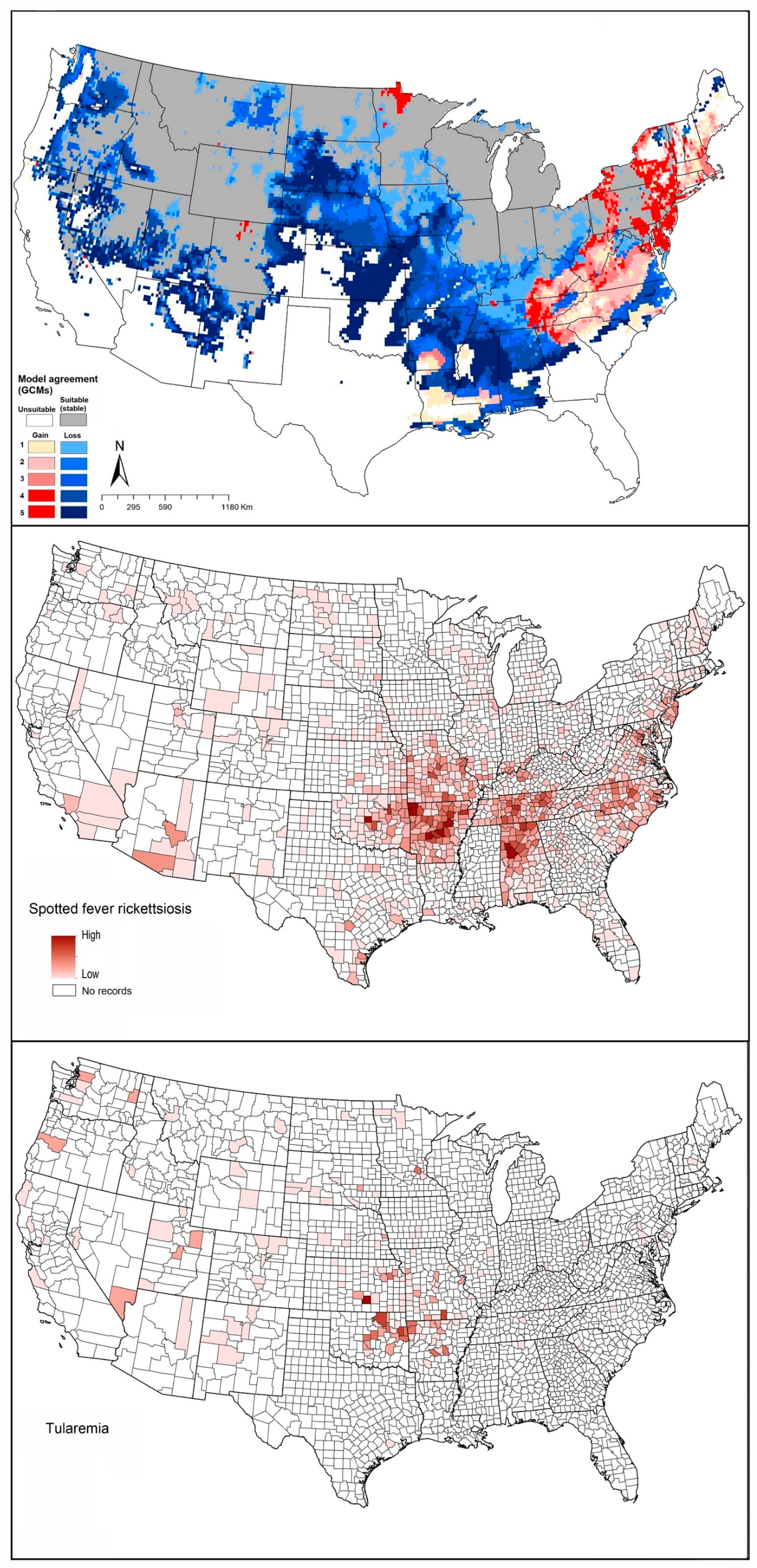
2.4. Spotted Fever Rickettsiosis (SFR)
2.5. Tularemia
2.6. Powassan Virus
3. Methods
4. Results
4.1. Geographic Distribution of Tick Vector Species
4.1.1. Ixodes scapularis (Black-Legged Tick)
4.1.2. Ixodes pacificus (Western Blacklegged Tick)
4.1.3. Ixodes cookei (Groundhog Tick or Woodchuck Tick)
4.1.4. Rhipicephalus sanguineus (Brown Dog Tick)
4.1.5. Amblyomma maculatum (Gulf Coast Tick)
4.1.6. Amblyomma americanum (Lone Star Tick)
4.1.7. Dermacentor andersoni (Rocky Mountain Wood Tick)
4.1.8. Dermacentor variabilis (American Dog Tick)
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogden, N.H.; Lindsay, L.R. Effects of climate and climate change on vectors and vector-borne diseases: Ticks are different. Trends Parasitol. 2016, 32, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Sonenshine, D.E.; Roe, R.M. Biology of Ticks; Oxford University Press: Oxford, UK, 2013; Volume 2. [Google Scholar]
- Bowman, A.S.; Nuttall, P.A. Ticks: Biology, Disease and Control; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Climate Central. 2019 Wrapped Up the Warmest Decade on Record. Climate Central. 2020. Available online: https://www.climatecentral.org/gallery/graphics/2019-wrapped-up-the-warmest-decade-on-record (accessed on 15 August 2020).
- Bush, E.; Lemmen, D.S. Canada’s Changing Climate Report; Government of Canada: Ottawa, ON, Canada, 2019.
- Cuervo-Robayo, A.P.; Ureta, C.; Gómez-Albores, M.A.; Meneses-Mosquera, A.K.; Téllez-Valdés, O.; Martínez-Meyer, E. One hundred years of climate change in Mexico. PLoS ONE 2020, 15, e0209808. [Google Scholar] [CrossRef]
- Merten, H.A.; Durden, L.A. A state-by-state survey of ticks recorded from humans in the United States. J. Vector Ecol. 2000, 25, 102–113. [Google Scholar]
- CDC. Regions Where Ticks Live. Centers for Disease Controls and Prevetion. 2020. Available online: https://www.cdc.gov/ticks/geographic_distribution.html (accessed on 15 August 2020).
- Clow, K.M.; Leighton, P.A.; Ogden, N.H.; Lindsay, L.R.; Michel, P.; Pearl, D.L.; Jardine, C.M. Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. PLoS ONE 2017, 12, e0189393. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Eisen, L.; Ogden, N.H.; Beard, C.B. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and Lyme disease in North America. Med. Entomol. 2015, 53, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Monzón, J.D.; Atkinson, E.G.; Henn, B.M.; Benach, J.L. Population and evolutionary genomics of Amblyomma americanum, an expanding arthropod disease vector. Genome Biol. Evol. 2016, 8, 1351–1360. [Google Scholar] [CrossRef]
- Raghavan, R.K.; Peterson, A.T.; Cobos, M.E.; Ganta, R.; Foley, D. Current and future distribution of the lone star tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS ONE 2019, 14, e0209082. [Google Scholar] [CrossRef]
- Boorgula, G.; Peterson, A.; Foley, D.; Ganta, R.; Raghavan, R. Assessing the current and future potential geographic distribution of the American dog tick, Dermacentor variabilis (Say) (Acari: Ixodidae) in North America. PLoS ONE 2020, 15, e0237191. [Google Scholar] [CrossRef]
- Alkishe, A.; Cobos, M.E.; Peterson, A.T.; Samy, A.M. Recognizing sources of uncertainty in disease vector ecological niche models: An example with the tick Rhipicephalus sanguineus sensu lato. Perspect. Ecol. Conserv. 2020, 18, 91–102. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering and Medicine. Global Health Impacts of Vector-Borne Diseases: Workshop Summary; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- CDC. Lyme and Other Tickborne Disease Increasing. Centers for Disease Control and Prevention. 2019. Available online: https://www.cdc.gov/media/dpk/diseases-and-conditions/lyme-disease/index.html (accessed on 15 August 2020).
- Bouchard, C.; Dibernardo, A.; Koffi, J.; Wood, H.; Leighton, P.A.; Lindsay, L.R. Increased risk of tick-borne diseases with climate and environmental changes. Can. Commun. Dis. Rep. 2019, 45, 81–89. [Google Scholar] [CrossRef]
- Sosa-Gutierrez, C.G.; Vargas-Sandoval, M.; Torres, J.; Gordillo-Pérez, G. Tick-borne rickettsial pathogens in questing ticks, removed from humans and animals in Mexico. J. Vet. Sci. 2016, 17, 353–360. [Google Scholar] [CrossRef] [PubMed]
- CDC. Geographic Distribution of Ticks that Bite Humans. 2019. Available online: https://wwwnc.cdc.gov/eid/article/26/4/19-1629_article (accessed on 15 August 2020).
- Bacon, R.; Kugeler, K.J.; Mead, P.S. Surveillance for Lyme Disease—United States, 1992–2006; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2008.
- Steere, A.C.; Coburn, J.; Glickstein, L. The emergence of Lyme disease. J. Clin. Investig. 2004, 113, 1093–1101. [Google Scholar] [CrossRef]
- CDC. Tickborne Disease Surveillance Data Summary. Centers for Disease Control and Prevention. 2019. Available online: https://www.cdc.gov/ticks/data-summary/index.html (accessed on 16 August 2020).
- Kugeler, K.J.; Farley, G.M.; Forrester, J.D.; Mead, P.S. Geographic distribution and expansion of human Lyme disease, United States. Emerg. Infect. Dis. 2015, 21, 1455–1457. [Google Scholar] [CrossRef] [PubMed]
- Koffi, J.; Gasmi, S. Surveillance for Lyme disease in Canada: 2009–2015. Online J. Public Health Inform. 2019, 11, e409. [Google Scholar] [CrossRef]
- Illoldi-Rangel, P.; Rivaldi, C.-L.; Sissel, B.; Trout Fryxell, R.; Gordillo-Pérez, G.; Rodríguez-Moreno, A.; Williamson, P.; Montiel-Parra, G.; Sánchez-Cordero, V.; Sarkar, S. Species distribution models and ecological suitability analysis for potential tick vectors of Lyme disease in Mexico. J. Trop. Med. 2012, 2012, 959101. [Google Scholar] [CrossRef]
- Gordillo-Pérez, G.; Torres, J.; Solórzano-Santos, F.; Garduño-Bautista, V.; Tapia-Conyer, R.; Muñoz, O. Estudio seroepidemiológico de borreliosis de Lyme en la Ciudad de México y el noreste de la República Mexicana. Salud Pública México 2003, 45, 351–355. [Google Scholar]
- Ord, R.L.; Lobo, C.A. Human babesiosis: Pathogens, prevalence, diagnosis, and treatment. Curr. Clin. Microbiol. Rep. 2015, 2, 173–181. [Google Scholar] [CrossRef]
- Vannier, E.; Krause, P.J. Human babesiosis. N. Engl. J. Med. 2012, 366, 2397–2407. [Google Scholar] [CrossRef]
- Sanchez, E.; Vannier, E.; Wormser, G.P.; Hu, L.T. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: A review. JAMA 2016, 315, 1767–1777. [Google Scholar] [CrossRef]
- Akel, T.; Mobarakai, N. Hematologic manifestations of babesiosis. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.B.; Herwaldt, B.L. Babesiosis surveillance—United States, 2011–2015. MMWR Surveill. Summ. 2019, 68, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Scott, C.M. Human babesiosis caused by Babesia duncani has widespread distribution across Canada. Healthcare 2018, 6, 49. [Google Scholar] [CrossRef]
- Peniche-Lara, G.; Balmaceda, L.; Perez-Osorio, C.; Munoz-Zanzi, C. Human Babesiosis, Yucatán State, Mexico, 2015. Emerg. Infect. Dis. 2018, 24, 2061–2062. [Google Scholar] [CrossRef]
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2010, 30, 261–292. [Google Scholar] [CrossRef]
- Horowitz, R.; Richards, A.L.; DuLaney, M.; Ericson, M.E.; Green, C.; Lubelczyk, C.; Munderloh, U.; Nicolson, G.L.; Paddock, C.; Perdue, S.S. Report of the Other Tick-Borne Diseases and Co-Infections Subcommittee to the Tick-Borne Disease Working Group; U.S. Department of Health and Human Services, Tick-Borne Disease Working Group: Washington, DC, USA, 2020.
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging tick-borne diseases. Clin. Microbiol. Rev. 2020, 33, e00083-18. [Google Scholar] [CrossRef] [PubMed]
- CDC. Anaplasmosis—Epidemiology and Statistics. Centers for Disease Control and Prevention. 2019. Available online: https://www.cdc.gov/anaplasmosis/stats/index.html (accessed on 16 August 2020).
- Uminski, K.; Kadkhoda, K.; Houston, B.L.; Lopez, A.; MacKenzie, L.J.; Lindsay, R.; Walkty, A.; Embil, J.; Zarychanski, R. Anaplasmosis: An emerging tick-borne disease of importance in Canada. IDCases 2018, 14, e00472. [Google Scholar] [CrossRef]
- CDC. Rocky Mountain Spotted Fever (RMSF)-Transmission. Centers for Disease Control and Prevention. 2018. Available online: https://www.cdc.gov/rmsf/transmission/index.html (accessed on 16 August 2020).
- CDC. Spotted Fever Rickettsiosis (Rickettsia spp.). Centers for Disease Control and Prevention. 2010. Available online: https://wwwn.cdc.gov/nndss/conditions/spotted-fever-rickettsiosis/case-definition/2010/ (accessed on 16 August 2020).
- CDC. Rocky Mountain Spotted Fever (RMSF), Centers for Disease Control and Prevention. 2019. Available online: https://www.cdc.gov/ticks/tickbornediseases/rmsf.html (accessed on 16 August 2020).
- Openshaw, J.J.; Swerdlow, D.L.; Krebs, J.W.; Holman, R.C.; Mandel, E.; Harvey, A.; Haberling, D.; Massung, R.F.; McQuiston, J.H. Rocky Mountain spotted fever in the United States, 2000–2007: Interpreting contemporary increases in incidence. Am. J. Trop. Med. Hyg. 2010, 83, 174–182. [Google Scholar] [CrossRef]
- Humphreys, F.; Campbell, A. Plague, Rocky Mountain spotted fever, and tularaemia surveys in Canada. Can. J. Public Health 1947, 38, 124–130. [Google Scholar]
- Wood, H.; Artsob, H. Spotted fever group rickettsiae: A brief review and a Canadian perspective. Zoonoses Public Health 2012, 59, 65–79. [Google Scholar] [CrossRef]
- Stromdahl, E.Y.; Jiang, J.; Vince, M.; Richards, A.L. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group rickettsiae in the United States. Vector-Borne Zoonotic Dis. 2011, 11, 969–977. [Google Scholar] [CrossRef]
- Teng, J.; Lindsay, L.; Bartlett, K.; Klinkenberg, B.; Dibernardo, A.; Wood, H.; Morshed, M. Prevalence of tick-borne pathogens in the South Okanagan, British Columbia: Active surveillance in ticks (Dermacentor andersoni) and deer mice (Peromyscus maniculatus). B. C. Med. J. 2011, 53, 122–127. [Google Scholar]
- Álvarez-Hernández, G.; Roldán, J.F.G.; Milan, N.S.H.; Lash, R.R.; Behravesh, C.B.; Paddock, C.D. Rocky Mountain spotted fever in Mexico: Past, present, and future. Lancet Infect. Dis. 2017, 17, e189–e196. [Google Scholar] [CrossRef]
- Francis, E. Symptoms, diagnosis and pathology of tularemia. J. Am. Med. Assoc. 1928, 91, 1155–1161. [Google Scholar] [CrossRef]
- McKee, P.H.; Calonje, E.; Lazar, A.; Brenn, T. McKee’s Pathology of the Skin with Clinical Correlations, 4th ed.; Elsevier Mosby: Philadelphia, PA, USA, 2012; pp. 288–295. [Google Scholar]
- CDC. Tularemia. Centers fro Disease Control and Prevention. 2018. Available online: https://www.cdc.gov/tularemia/ (accessed on 18 August 2020).
- Mörner, T. The ecology of tularaemia. Rev. Sci. Tech. Off. Int. Épizooties 1992, 11, 1123. [Google Scholar]
- USDA. Tularemia. United States Department of Agriculture. 2020. Available online: https://www.aphis.usda.gov/aphis/ourfocus/wildlifedamage/programs/nwrc/nwdp/ct_tularemia (accessed on 18 August 2020).
- Penn, R.L. Francisella tularensis (Tularemia). Princ. Pract. Infect. Dis. 2015, 2, 2590–2602. [Google Scholar]
- Alberta Health. Public Health Disease Management Guidelines; Goverment of Alberta: Edmonton, AB, Canada, 2018.
- Clarke, R. Tularemia, a Potentially Serious and Life Threatening Disease. Canadian Cattleman. 2018. Available online: https://www.canadiancattlemen.ca/vet-advice/tularemia-a-potentially-serious-and-life-threatening-disease/ (accessed on 18 August 2020).
- Boggs, W. Rising Number of Human Tularemia Cases in Four U.S. States. American College of Emergency Physicians (ACEP). 2016. Available online: https://www.acepnow.com/rising-number-of-human-tularemia-cases-in-four-u-s-states/ (accessed on 18 August 2020).
- Nakazawa, Y.; Williams, R.; Peterson, A.T.; Mead, P.; Staples, E.; Gage, K.L. Climate change effects on plague and tularemia in the United States. Vector-Borne Zoonotic Dis. 2007, 7, 529–540. [Google Scholar] [CrossRef]
- Dhama, K.; Pawaiya, R.V.S.; Chakraborty, S.; Tiwari, R.; Verma, A.K. Powassan virus (POWV) infection in animals and humans: A review. Asian J. Anim. Vet. Adv. 2014, 9, 177–189. [Google Scholar] [CrossRef]
- Main, A.; Carey, A.; Downs, W. Powassan virus in Ixodes cookei and Mustelidae in New England. J. Wildl. Dis. 1979, 15, 585–591. [Google Scholar] [CrossRef] [PubMed]
- EI Khoury, M.Y.; Camargo, J.F.; Wormser, G.P. Changing epidemiology of Powassan encephalitis in North America suggests the emergence of the deer tick virus subtype. Expert Rev. Anti-Infect. Ther. 2013, 11, 983–985. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ebel, G.D.; Campbell, E.N.; Goethert, H.K.; Spielman, A.; Telford, S., 3rd. Enzootic transmission of deer tick virus in New England and Wisconsin sites. Am. J. Trop. Med. Hyg. 2000, 63, 36–42. [Google Scholar] [CrossRef]
- CDC. Powassan Virus. Centers for Disease Control and Prevention. 2019. Available online: https://www.cdc.gov/powassan/statistics.html (accessed on 18 August 2020).
- Corrin, T.; Greig, J.; Harding, S.; Young, I.; Mascarenhas, M.; Waddell, L.A. Powassan virus, a scoping review of the global evidence. Zoonoses Public Health 2018, 65, 595–624. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef]
- Phillips, V.C.; Zieman, E.A.; Kim, C.-H.; Stone, C.M.; Tuten, H.C.; Jiménez, F.A. Documentation of the expansion of the gulf coast tick (Amblyomma maculatum) and Rickettsia parkeri: First report in Illinois. J. Parasitol. 2020, 106, 9–13. [Google Scholar] [CrossRef]
- Maestas, L.P.; Reeser, S.R.; McGay, P.J.; Buoni, M.H. Surveillance for Amblyomma maculatum (Acari: Ixodidae) and Rickettsia parkeri (Rickettsiales: Rickettsiaceae) in the state of Delaware, and their public health implications. J. Med. Entomol. 2020, 57, 979–983. [Google Scholar] [CrossRef]
- Florin, D.A.; Brinkerhoff, R.J.; Gaff, H.; Jiang, J.; Robbins, R.G.; Eickmeyer, W.; Butler, J.; Nielsen, D.; Wright, C.; White, A. Additional US collections of the Gulf Coast tick, Amblyomma maculatum (Acari: Ixodidae), from the State of Delaware, the first reported field collections of adult specimens from the State of Maryland, and data regarding this tick from surveillance of migratory songbirds in Maryland. Syst. Appl. Acarol. 2014, 19, 257–262. [Google Scholar] [CrossRef]
- Mays, S.; Houston, A.; Trout Fryxell, R. Specifying pathogen associations of Amblyomma maculatum (Acari: Ixodidae) in western Tennessee. J. Med. Entomol. 2016, 53, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Pagac, B.B.; Miller, M.K.; Mazzei, M.C.; Nielsen, D.H.; Jiang, J.; Richards, A.L. Rickettsia parkeri and Rickettsia montanensis, Kentucky and Tennessee, USA. Emerg. Infect. Dis. 2014, 20, 1750–1752. [Google Scholar] [CrossRef]
- James, A.M.; Freier, J.E.; Keirans, J.E.; Durden, L.A.; Mertins, J.W.; Schlater, J.L. Distribution, seasonality, and hosts of the Rocky Mountain wood tick in the United States. J. Med. Entomol. 2006, 43, 17–24. [Google Scholar] [CrossRef]
- Dergousoff, S.J.; Galloway, T.D.; Lindsay, L.R.; Curry, P.S.; Chilton, N.B. Range expansion of Dermacentor variabilis and Dermacentor andersoni (Acari: Ixodidae) near their northern distributional limits. J. Med. Entomol. 2013, 50, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Eisen, L.; Beard, C.B. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol. 2016, 53, 349–386. [Google Scholar] [CrossRef]
- CDC. Tick Surveillance. Centers for Disease Control and Prevention. 2020. Available online: https://www.cdc.gov/ticks/surveillance/index.html (accessed on 18 August 2020).
- Scott, J.D.; Clark, K.L.; Foley, J.E.; Anderson, J.F.; Bierman, B.C.; Durden, L.A. Extensive distribution of the Lyme disease bacterium, Borrelia burgdorferi sensu lato, in multiple tick species parasitizing avian and mammalian hosts across Canada. Healthcare 2018, 6, 131. [Google Scholar] [CrossRef]
- Soberón, J.; Peterson, A.T. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundations for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011; Volume 49. [Google Scholar]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Guerra, M.; Walker, E.; Jones, C.; Paskewitz, S.; Cortinas, M.R.; Stancil, A.; Beck, L.; Bobo, M.; Kitron, U. Predicting the risk of Lyme disease: Habitat suitability for Ixodes scapularis in the north central United States. Emerg. Infect. Dis. 2002, 8, 289–297. [Google Scholar] [CrossRef]
- Davis, R.S.; Ramirez, R.A.; Anderson, J.L.; Bernhardt, S.A. Distribution and habitat of Ixodes pacificus (Acari: Ixodidae) and prevalence of Borrelia burgdorferi in Utah. J. Med. Entomol. 2015, 52, 1361–1367. [Google Scholar] [CrossRef]
- Eisen, L.; Eisen, R.; Lane, R. Seasonal activity patterns of Ixodes pacificus nymphs in relation to climatic conditions. Med. Vet. Entomol. 2002, 16, 235–244. [Google Scholar] [CrossRef]
- Gasmi, S.; Bouchard, C.; Ogden, N.H.; Adam-Poupart, A.; Pelcat, Y.; Rees, E.E.; Milord, F.; Leighton, P.A.; Lindsay, R.L.; Koffi, J.K. Evidence for increasing densities and geographic ranges of tick species of public health significance other than Ixodes scapularis in Québec, Canada. PLoS ONE 2018, 13, e0201924. [Google Scholar] [CrossRef] [PubMed]
- INSPQ. Ixodes cookei, or Groundhog Tick. Institut National de Santé Publique du Québec, Public Health Expertise and Reference Centre. 2021. Available online: https://www.inspq.qc.ca/en/ixodes-cookei-or-groundhog-tick#:~:text=The%20seasonal%20distribution%20of%20Ixodescookei%20ticks%20is%20the%20summer (accessed on 20 August 2020).
- Louly, C.C.B.; Fonseca, I.N.; Oliveira, V.F.d.; Linhares, G.F.C.; Menezes, L.B.d.; Borges, L.M.F. Seasonal dynamics of Rhipicephalus sanguineus (Acari: Ixodidae) in dogs from a police unit in Goiania, Goias, Brazil. Ciência Rural 2007, 37, 464–469. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasites Vectors 2010, 3, 26. [Google Scholar] [CrossRef]
- Cortinas, R.; Spomer, S. Lone star tick (Acari: Ixodidae) occurrence in Nebraska: Historical and current perspectives. J. Med. Entomol. 2013, 50, 244–251. [Google Scholar] [CrossRef]
- Scifres, C.J.; Oldham, T.W.; Teel, P.D.; Drawe, D.L. Gulf coast tick (Amblyomma maculatum) populations and responses to burning of coastal prairie habitats. Southwest. Nat. 1988, 33, 55–64. [Google Scholar] [CrossRef]
- Koch, H.G. Survival of the lone star tick, Amblyomma americanum (Acari: Ixodidae), in contrasting habitats and different years in southeastern Oklahoma, USA. J. Med. Entomol. 1984, 21, 69–79. [Google Scholar] [CrossRef]
- Schulze, T.L.; Jordan, R.A.; Hung, R.W. Effects of microscale habitat physiognomy on the focal distribution of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. Environ. Entomol. 2002, 31, 1085–1090. [Google Scholar] [CrossRef]
- Eisen, L. Seasonal pattern of host-seeking activity by the human-biting adult life stage of Dermacentor andersoni (Acari: Ixodidae). J. Med. Entomol. 2007, 44, 359–366. [Google Scholar] [CrossRef] [PubMed]
- ESU. Rocky Mountain Wood Tick. Tick Research Lab of Pennsylvania, East Stroudsburg University. 2021. Available online: https://www.ticklab.org/rocky-mountain-wood-tick (accessed on 20 August 2020).
- Sonenshine, D.E.; Levy, G.F. Ecology of the American Dog Tick, Dermacentor variabilis, in a Study Area in Virginia. 2. Distribution in Relation to Vegetative Types. Ann. Entomol. Soc. Am. 1972, 65, 1175–1182. [Google Scholar] [CrossRef]
- Dennis, D.T.; Nekomoto, T.S.; Victor, J.C.; Paul, W.S.; Piesman, J. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J. Med. Entomol. 1998, 35, 629–638. [Google Scholar] [CrossRef]
- Lane, R.; Piesman, J.; Burgdorfer, W. Lyme borreliosis: Relation of its causative agent to its vectors and hosts in North America and Europe. Annu. Rev. Entomol. 1991, 36, 587–609. [Google Scholar] [CrossRef]
- CDC. Tickborne Diseases of the United States. Centers for Disease Control and Prevention. 2019. Available online: https://www.cdc.gov/ticks/tickbornediseases/tickID.html (accessed on 20 August 2020).
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Peña, A.; Horak, I.G. The Hard Ticks of the World; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-007-7496-4. [Google Scholar]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef]
- Dantas-Torres, F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): From taxonomy to control. Vet. Parasitol. 2008, 152, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Paddock, C.D.; Sumner, J.W.; Comer, J.A.; Zaki, S.R.; Goldsmith, C.S.; Goddard, J.; McLellan, S.L.; Tamminga, C.L.; Ohl, C.A. Rickettsia parkeri: A newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 2004, 38, 805–811. [Google Scholar] [CrossRef]
- Sumner, J.W.; Durden, L.A.; Goddard, J.; Stromdahl, E.Y.; Clark, K.L.; Reeves, W.K.; Paddock, C.D. Gulf coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg. Infect. Dis. 2007, 13, 751. [Google Scholar] [CrossRef]
- Paddock, C.D.; Goddard, J. The evolving medical and veterinary importance of the Gulf Coast tick (Acari: Ixodidae). J. Med. Entomol. 2015, 52, 230–252. [Google Scholar] [CrossRef]
- Teel, P.; Ketchum, H.; Mock, D.; Wright, R.; Strey, O. The Gulf Coast tick: A review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J. Med. Entomol. 2010, 47, 707–722. [Google Scholar] [CrossRef]
- Goddard, J.; Varela-Stokes, A.S. Role of the lone star tick, Amblyomma americanum (L.), in human and animal diseases. Vet. Parasitol. 2009, 160, 1–12. [Google Scholar] [CrossRef]
- Diaz, J.H. Red Meat Allergies after Lone Star Tick (Amblyomma americanum) Bites. South. Med. J. 2020, 113, 267–274. [Google Scholar] [CrossRef]
- Means, R.; White, D. New distribution records of Amblyomma americanum (L.) (Acari: Ixodidae) in New York state. J. Vector Ecol. J. Soc. Vector Ecol. 1997, 22, 133–145. [Google Scholar]
- Keirans, J.E.; Lacombe, E.H. First records of Amblyomma americanum, Ixodes (Ixodes) dentatus, and Ixodes (Ceratixodes) uriae (Acari: Ixodidae) from Maine. J. Parasitol. 1998, 84, 629–631. [Google Scholar] [CrossRef]
- Barrett, A.W.; Noden, B.H.; Gruntmeir, J.M.; Holland, T.; Mitcham, J.R.; Martin, J.E.; Johnson, E.M.; Little, S.E. County scale distribution of Amblyomma americanum (Ixodida: Ixodidae) in Oklahoma: Addressing local deficits in tick maps based on passive reporting. J. Med. Entomol. 2015, 52, 269–273. [Google Scholar] [CrossRef]
- Brown, H.E.; Yates, K.F.; Dietrich, G.; MacMillan, K.; Graham, C.B.; Reese, S.M.; Helterbrand, W.S.; Nicholson, W.L.; Blount, K.; Mead, P.; et al. An acarologic survey and Amblyomma americanum distribution map with implications for tularemia risk in Missouri. Am. J. Trop. Med. Hyg. 2011, 84, 411–419. [Google Scholar] [CrossRef]
- Springer, Y.P.; Eisen, L.; Beati, L.; James, A.M.; Eisen, R.J. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J. Med Entomol. 2014, 51, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. Rocky Mountain spotted fever. Lancet Infect. Dis. 2007, 7, 724–732. [Google Scholar] [CrossRef]
- Atif, F.A. Anaplasma marginale and Anaplasma phagocytophilum: Rickettsiales pathogens of veterinary and public health significance. Parasitol. Res. 2015, 114, 3941–3957. [Google Scholar] [CrossRef] [PubMed]
- Brackney, M.M.; Marfin, A.A.; Staples, J.E.; Stallones, L.; Keefe, T.; Black, W.C.; Campbell, G.L. Epidemiology of Colorado tick fever in Montana, Utah, and Wyoming, 1995–2003. Vector-Borne Zoonotic Dis. 2010, 10, 381–385. [Google Scholar] [CrossRef]
- Dennis, D.T.; Inglesby, T.V.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Fine, A.D.; Friedlander, A.M.; Hauer, J.; Layton, M. Tularemia as a biological weapon: Medical and public health management. JAMA 2001, 285, 2763–2773. [Google Scholar] [CrossRef]
- CDC. Colorado Tick Fever. Centers for Disease Control and Prevention. 2018. Available online: https://www.cdc.gov/coloradotickfever/transmission.html (accessed on 20 August 2020).
- CDC. Colorado Tick Fever (CTF). Centers for Disease Control and Prevention. 2018. Available online: https://www.cdc.gov/coloradotickfever/statistics.html (accessed on 20 August 2020).
- Wood, H.; Dillon, L.; Patel, S.N.; Ralevski, F. Prevalence of Rickettsia species in Dermacentor variabilis ticks from Ontario, Canada. Ticks Tick-Borne Dis. 2016, 7, 1044–1046. [Google Scholar] [CrossRef]
- Yunik, M.E.; Galloway, T.D.; Lindsay, L.R. Ability of unfed Dermacentor variabilis (Acari: Ixodidae) to survive a second winter as adults in Manitoba, Canada, near the northern limit of their range. J. Med. Entomol. 2015, 52, 138–142. [Google Scholar] [CrossRef]
- James, A.; Burdett, C.; McCool, M.; Fox, A.; Riggs, P. The geographic distribution and ecological preferences of the A merican dog tick, Dermacentor variabilis (Say), in the USA. Med. Vet. Entomol. 2015, 29, 178–188. [Google Scholar] [CrossRef]
- Peterson, A.T.; Navarro-Sigüenza, A.G.; Martínez-Meyer, E. Digital Accessible Knowledge and well-inventoried sites for birds in Mexico: Baseline sites for measuring faunistic change. PeerJ 2016, 4, e2362. [Google Scholar] [CrossRef]
- Sousa-Baena, M.S.; Garcia, L.C.; Peterson, A.T. Completeness of digital accessible knowledge of the plants of Brazil and priorities for survey and inventory. Divers. Distrib. 2014, 20, 369–381. [Google Scholar] [CrossRef]
- Owens, H.L.; Campbell, L.P.; Dornak, L.L.; Saupe, E.E.; Barve, N.; Soberón, J.; Ingenloff, K.; Lira-Noriega, A.; Hensz, C.M.; Myers, C.E. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol. Model. 2013, 263, 10–18. [Google Scholar] [CrossRef]
- Vail, S.G.; Smith, G. Air temperature and relative humidity effects on behavioral activity of blacklegged tick (Acari: Ixodidae) nymphs in New Jersey. J. Med. Entomol. 1998, 35, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- Despins, J.L. Effects of temperature and humidity on ovipositional biology and egg development of the tropical horse tick, Dermacentor (Anocentor) nitens. J. Med. Entomol. 1992, 29, 332–337. [Google Scholar] [CrossRef]
- Diyes, C.P.; Dergousoff, S.J.; Yunik, M.E.; Chilton, N.B. Reproductive output and larval survival of American dog ticks (Dermacentor variabilis) from a population at the northern distributional limit. Exp. Appl. Acarol. 2021, 83, 257–270. [Google Scholar] [CrossRef]
- Ogden, N.; Lindsay, L.; Beauchamp, G.; Charron, D.; Maarouf, A.; O’callaghan, C.; Waltner-Toews, D.; Barker, I. Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (Acari: Ixodidae) in the laboratory and field. J. Med. Entomol. 2004, 41, 622–633. [Google Scholar] [CrossRef]
- Randolph, S.E. The shifting landscape of tick-borne zoonoses: Tick-borne encephalitis and Lyme borreliosis in Europe. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001, 356, 1045–1056. [Google Scholar] [CrossRef]
- Bacon, R.; Kugeler, K.J.; Mead, P.S. Rare Disease Database. National Organization for Rare Disorders. 2009. Available online: https://rarediseases.org/rare-diseases/babesiosis/ (accessed on 23 August 2020).
- González, C.; Rebollar-Téllez, E.A.; Ibáñez-Bernal, S.; Becker-Fauser, I.; Martínez-Meyer, E.; Peterson, A.T.; Sánchez-Cordero, V. Current knowledge of Leishmania vectors in Mexico: How geographic distributions of species relate to transmission areas. Am. J. Trop. Med. Hyg. 2011, 85, 839–846. [Google Scholar] [CrossRef]
- Smith, R.P., Jr.; Elias, S.P.; Borelli, T.J.; Missaghi, B.; York, B.J.; Kessler, R.A.; Lubelczyk, C.B.; Lacombe, E.H.; Hayes, C.M.; Coulter, M.S. Human babesiosis, Maine, USA, 1995–2011. Emerg. Infect. Dis. 2014, 20, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- CDC. Babesiosis. Centers for Disease Control and Prevention. 2019. Available online: https://www.cdc.gov/ticks/tickbornediseases/babesiosis.html (accessed on 23 August 2020).
- Peterson, A.T. Mapping Disease Transmission Risk: Enriching Models Using Biogeography and Ecology; Johns Hopkins University Press: Baltimore, MD, USA, 2014. [Google Scholar]









| Tick Species | Primary Active Season | Geographic Distribution | Primary Habitat | Source |
|---|---|---|---|---|
| Ixodes scapularis | spring, summer, and fall | widely distributed across eastern United States | wooded vegetation, and sandy soils | [8,80] |
| I. pacificus | spring, and summer | Pacific coast of North America. | gambel oak, juniper (Juniperus spp.), sagebrush, and mixed grass habitat | [81,82] |
| I. cookei | summer | northeastern United States, southeastern Canada. | host’s nest or burrow | [83,84] |
| Rhipicephalus sanguineus | all seasons | broad, worldwide in the tropics and subtropics | diverse habitats, can live indoor | [14,85,86] |
| Amblyomma maculatum | spring and summer | Caribbean, south and central United States, Mexico, West Indies, Colombia, Venezuela, Peru | grasslands | [87,88] |
| A. americanum | summer and fall | Coastal areas along the Atlantic Ocean and Gulf of Mexico. | oak and pine forests | [8,87,89,90] |
| Dermacentor andersoni | spring | Southwestern Canada; Rocky Mountain states in the United States, at high elevations (1300–3000 m) | wooded habitat, grassland, low-growing vegetation | [8,91,92] |
| D. variabilis | spring and summer | Eastern United States, Pacific coast | mixed upland and mixed-oak, hickory-dominant forest | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkishe, A.; Raghavan, R.K.; Peterson, A.T. Likely Geographic Distributional Shifts among Medically Important Tick Species and Tick-Associated Diseases under Climate Change in North America: A Review. Insects 2021, 12, 225. https://doi.org/10.3390/insects12030225
Alkishe A, Raghavan RK, Peterson AT. Likely Geographic Distributional Shifts among Medically Important Tick Species and Tick-Associated Diseases under Climate Change in North America: A Review. Insects. 2021; 12(3):225. https://doi.org/10.3390/insects12030225
Chicago/Turabian StyleAlkishe, Abdelghafar, Ram K. Raghavan, and Andrew T. Peterson. 2021. "Likely Geographic Distributional Shifts among Medically Important Tick Species and Tick-Associated Diseases under Climate Change in North America: A Review" Insects 12, no. 3: 225. https://doi.org/10.3390/insects12030225
APA StyleAlkishe, A., Raghavan, R. K., & Peterson, A. T. (2021). Likely Geographic Distributional Shifts among Medically Important Tick Species and Tick-Associated Diseases under Climate Change in North America: A Review. Insects, 12(3), 225. https://doi.org/10.3390/insects12030225






