Plant Pathogen Invasion Modifies the Eco-Evolutionary Host Plant Interactions of an Endangered Checkerspot Butterfly
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
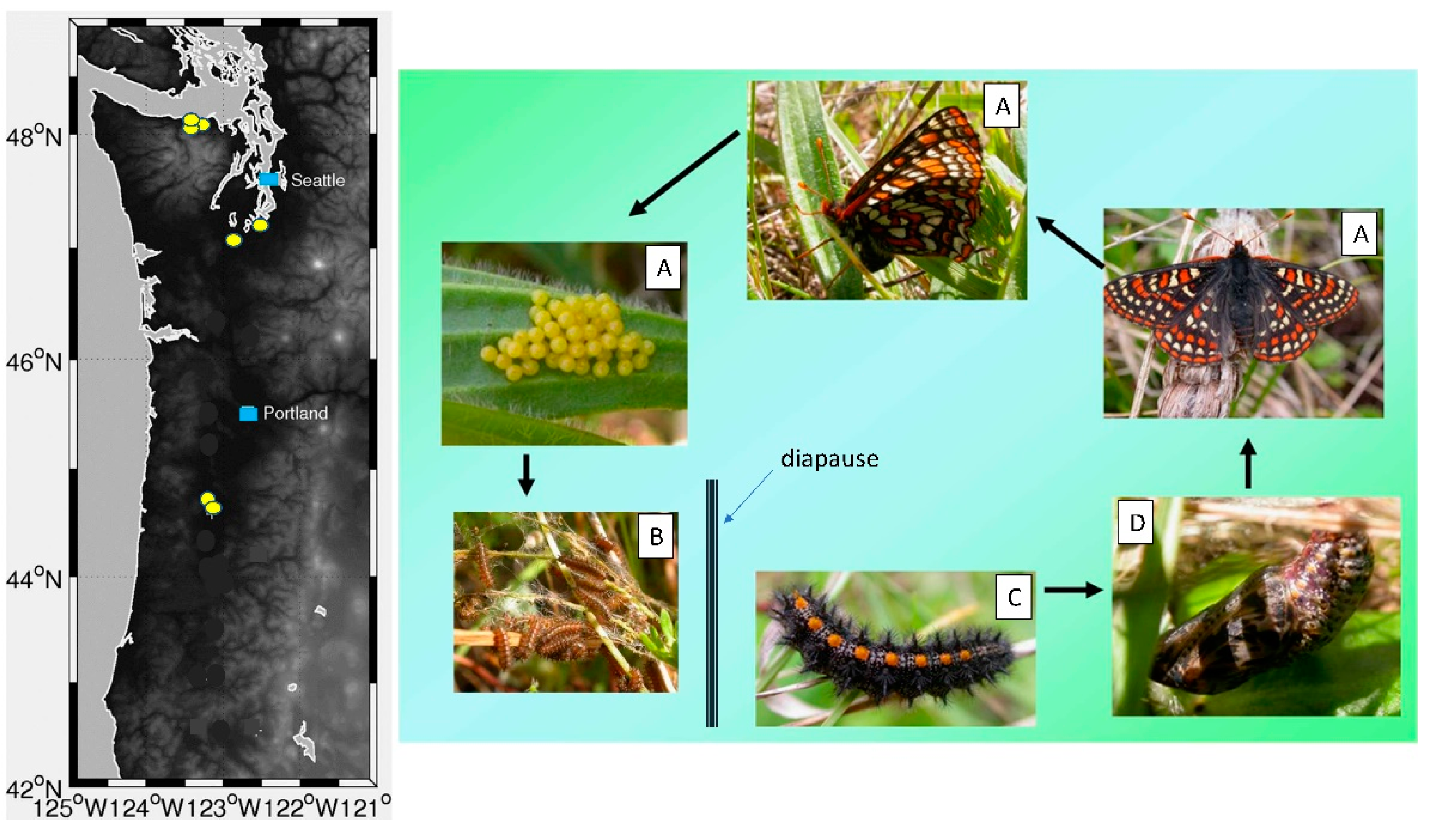
2.1. Taylor’s Checkerspot Georgraphic Distribution, Developmental Timing, and Life History
2.2. Butterfly Development, Larval Foodplant Availability, and P. plantaginis Disease
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hubbes, M. The American elm and Dutch elm disease. For. Chron. 1999, 75, 265–273. [Google Scholar] [CrossRef]
- Grünwald, N.J.; Garbelotto, M.; Goss, E.M.; Heungens, K.; Prospero, S. Emergence of the sudden oak death pathogen Phytophthora ramorum. Trends Microbiol. 2012, 20, 131–138. [Google Scholar] [CrossRef]
- Rigling, D.; Prospero, S. Cryphonectria parasitica, the causal agent of chestnut blight: Invasion history, population biology and disease control. Mol. Plant Pathol. 2018, 19, 7–20. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Lau, J.A.; Carroll, S.P. Evolutionary responses of natives to introduced species: What do introductions tell us about natural communities? Ecol. Lett. 2006, 9, 357–374. [Google Scholar] [CrossRef]
- Alexander, H.M. Disease in natural plant populations, communities, and ecosystems: Insights into ecological and evolutionary processes. Plant Dis. 2010, 94, 492–503. [Google Scholar] [CrossRef]
- Cavers, P.B.; Bassett, I.J.; Crompton, C.W. The biology of Canadian weeds: 47. Plantago lanceolata L. Can. J. Plant Sci. 1980, 60, 1269–1282. [Google Scholar] [CrossRef]
- Wolff, K.; Schaal, B. Chloroplast DNA variation within and among five Plantago species. J. Evol. Biol. 2002, 5, 325–344. [Google Scholar] [CrossRef]
- Stone, J.K.; Severns, P.M.; Miller, N. Pyrenopeziza plantaginis new to North America. N. Am. Fungi 2011, 6, 1–4. [Google Scholar] [CrossRef]
- Bruzzese, E.; Hasan, S. Host specificity of the rust Phragmidium violaceurn, a potential biological control agent of European blackberry. Ann. Appl. Biol. 1986, 108, 585–596. [Google Scholar] [CrossRef]
- Gomez, D.R.; Evans, K.J.; Baker, J.; Harvey, P.R.; Scott, E.S. Dynamics of introduced populations of Phragmidium violaceum and implications for biological control of European blackberry in Australia. Appl. Environ. Microbiol. 2008, 74, 5504–5510. [Google Scholar] [CrossRef]
- Severns, P.M.; Stone, J.K. Pathogen invasion generates an evolutionary trap for an endangered checkerspot butterfly dependent on an exotic host plant. Biol. Invasion 2016, 18, 3623–3633. [Google Scholar] [CrossRef]
- Severns, P.M.; Warren, A.D. Saving an imperiled butterfly, Euphydryas editha taylori (Taylor’s checkerspot), by selectively conserving and eliminating exotic plants. Anim. Conserv. 2008, 11, 476–483. [Google Scholar] [CrossRef]
- Dornfeld, E.J. The Butterflies of Oregon; Timber Press: Forest Grove, OR, USA, 1980; 276p. [Google Scholar]
- Danby, W.H. The foodplant of Melitaea taylori Edw. Can. Entomol. 1890, 22, 121–122. [Google Scholar] [CrossRef]
- Severns, P.M.; Grosboll, D. Patterns of Reproduction in Four Washington State Populations of Taylor’s Checkerspot (Euphydryas editha taylori) during the Spring of 2010. The Nature Conservancy, Olympia, WA. 2011. Available online: https://cascadiaprairieoak.org/documents/Patterns-of-Reproduction-Taylors-Checkerspot.pdf (accessed on 3 March 2021).
- Bennett, V.J.; Pack, S.M.; Smith, W.P.; Betts, M.G. Sex-biased dispersal in a rare butterfly and the implications for its conservation. J. Insect Conserv. 2013, 17. [Google Scholar] [CrossRef]
- Severns, P.M.; Breed, G.A. Behavioral consequences of exotic host plant adoption and the differing roles of male harassment on female movement in two checkerspot butterflies. Behav. Ecol. Sociobiol. 2014, 68, 805–814. [Google Scholar] [CrossRef]
- Severns, P.M.; Breed, G.A. Male harassment influences female movements and genetic architecture in a fragmented metapopulation. Ecography 2018, 41, 2045–2054. [Google Scholar] [CrossRef]
- Wahlberg, N. The phylogenetics and biochemistry of host-plant specialization in melitaeine butterflies (lepidoptera: Nymphalidae). Evolution 2001, 55, 522–537. [Google Scholar] [CrossRef]
- Stamp, N.E. New oviposition plant for Euphydryas phaeton (Nymphalidae). J. Lepid. Soc. 1979, 33, 203–204. [Google Scholar]
- Singer, M.C.; Thomas, C.D.; Parmesan, C. Rapid human-induced evolution of insect host associations. Nature 1993, 366, 681–683. [Google Scholar] [CrossRef]
- Camara, M.D. A recent host range expansion in Junonia coenia Hübner (Nymphalidae): Oviposition preference, survival, growth, and chemical defense. Evolution 1997, 51, 873–884. [Google Scholar] [CrossRef]
- Fuchs, A.; Bowers, M.D. Patterns of iridoid glycoside production and induction in Plantago lanceolata and the importance of plant age. J. Chem. Ecol. 2004, 30, 1723–1741. [Google Scholar] [CrossRef]
- Severns, P.M. Interactions between two endangered butterflies and invasive, exotic grasses in western Oregon, USA. Endanger. Species Update 2008, 25, 35–40. [Google Scholar]
- Bennett, N.L.; Singer, M.C.; Severns, P.M.; Parmesan, C. Geographic mosaics of phenology, host preference, adult size and microhabitat choice predict butterfly resilience to climate warming. Oikos 2015, 124, 41–53. [Google Scholar] [CrossRef]
- Dunwiddie, P.W.; Hann, N.L.; Linders, M.; Bakker, J.D.; Fimberl, C.; Thomas, T.B. Intertwined fates: Opportunities and challenges in the linked recovery of two rare species. Nat. Areas J. 2016, 36, 207–215. [Google Scholar] [CrossRef]
- Haan, N.L.; Bakker, J.D.; Dunwiddie, P.W.; Linders, M.J. Instar-specific effects of host plants on survival of endangered butterfly larvae. Ecol. Entomol. 2018, 43, 742–753. [Google Scholar] [CrossRef]
- Singer, M.; Wee, B. Spatial pattern in checkerspot butterfly—host plant association at local, metapopulation and regional scales. Annales Zool. Fennici 2005, 42, 347–361. [Google Scholar]
- Singer, M.C.; Parmesan, C. Lethal trap created by adaptive evolutionary response to an exotic resource. Nature 2018, 557, 238–241. [Google Scholar] [CrossRef]
- James, D.G.; Nunnallee, D. Life Histories of Cascadia Butterflies; Oregon State University Press: Corvallis, OR, USA, 2011; 447p. [Google Scholar]
- Breed, G.A.; Severns, P.M. Low relative error in consumer-grade GPS units make them ideal for measuring small-scale animal movement patterns. PeerJ 2015, 3, e1205. [Google Scholar] [CrossRef]
- Godt, M.J.W.; Caplow, F.; Hamrick, J.L. Allozyme diversity in the federally threatened golden paintbrush, Castilleja levisecta (Scrophulariaceae). Conserv. Gen. 2005, 6, 87–99. [Google Scholar] [CrossRef]
- Sandlin, I.J. Hybridization between Castilleja levisecta and C. hispida: Implications for Pacific Northwest Prairie Management. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2018. Available online: https://ir.library.oregonstate.edu/concern/graduate_thesis_or_dissertations/bg257m03z (accessed on 3 March 2021).
- Stone, J.K.; Coop, L.B.; Manter, D.K. Predicting effects of climate change on Swiss needle cast disease severity in Pacific Northwest forests. Can. J. Plant Pathol. 2008, 30, 169–176. [Google Scholar] [CrossRef]
- Garrett, K.A.; Forbes, G.A.; Savary, S.; Skelsey, P.; Sparks, A.H.; Valdivia, C.; Van Bruggen, A.H.C.; Willocquet, L.; Djurle, A.; Duveiller, E.; et al. Complexity in climate-change impacts: An analytical framework for effects mediated by plant disease. Plant Pathol. 2011, 60, 15–30. [Google Scholar] [CrossRef]
- Maron, J.L.; Marler, M.; Klironomos, J.N.; Cleveland, C.C. Soil fungal pathogens and the relationship between plant diversity and productivity. Ecol. Lett. 2011, 14, 36–41. [Google Scholar] [CrossRef]
- Koziol, L.; Schultz, P.A.; House, G.L.; Bauer, J.T.; Middleton, E.L.; Bever, J.D. The plant microbiome and native plant restoration: The example of native mycorrhizal fungi. BioScience 2018, 68, 996–1006. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Severns, P.M.; Guzman-Martinez, M. Plant Pathogen Invasion Modifies the Eco-Evolutionary Host Plant Interactions of an Endangered Checkerspot Butterfly. Insects 2021, 12, 246. https://doi.org/10.3390/insects12030246
Severns PM, Guzman-Martinez M. Plant Pathogen Invasion Modifies the Eco-Evolutionary Host Plant Interactions of an Endangered Checkerspot Butterfly. Insects. 2021; 12(3):246. https://doi.org/10.3390/insects12030246
Chicago/Turabian StyleSeverns, Paul M., and Melinda Guzman-Martinez. 2021. "Plant Pathogen Invasion Modifies the Eco-Evolutionary Host Plant Interactions of an Endangered Checkerspot Butterfly" Insects 12, no. 3: 246. https://doi.org/10.3390/insects12030246
APA StyleSeverns, P. M., & Guzman-Martinez, M. (2021). Plant Pathogen Invasion Modifies the Eco-Evolutionary Host Plant Interactions of an Endangered Checkerspot Butterfly. Insects, 12(3), 246. https://doi.org/10.3390/insects12030246





