Old Parasitoids for New Mealybugs: Host Location Behavior and Parasitization Efficacy of Anagyrus vladimiri on Pseudococcus comstocki
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
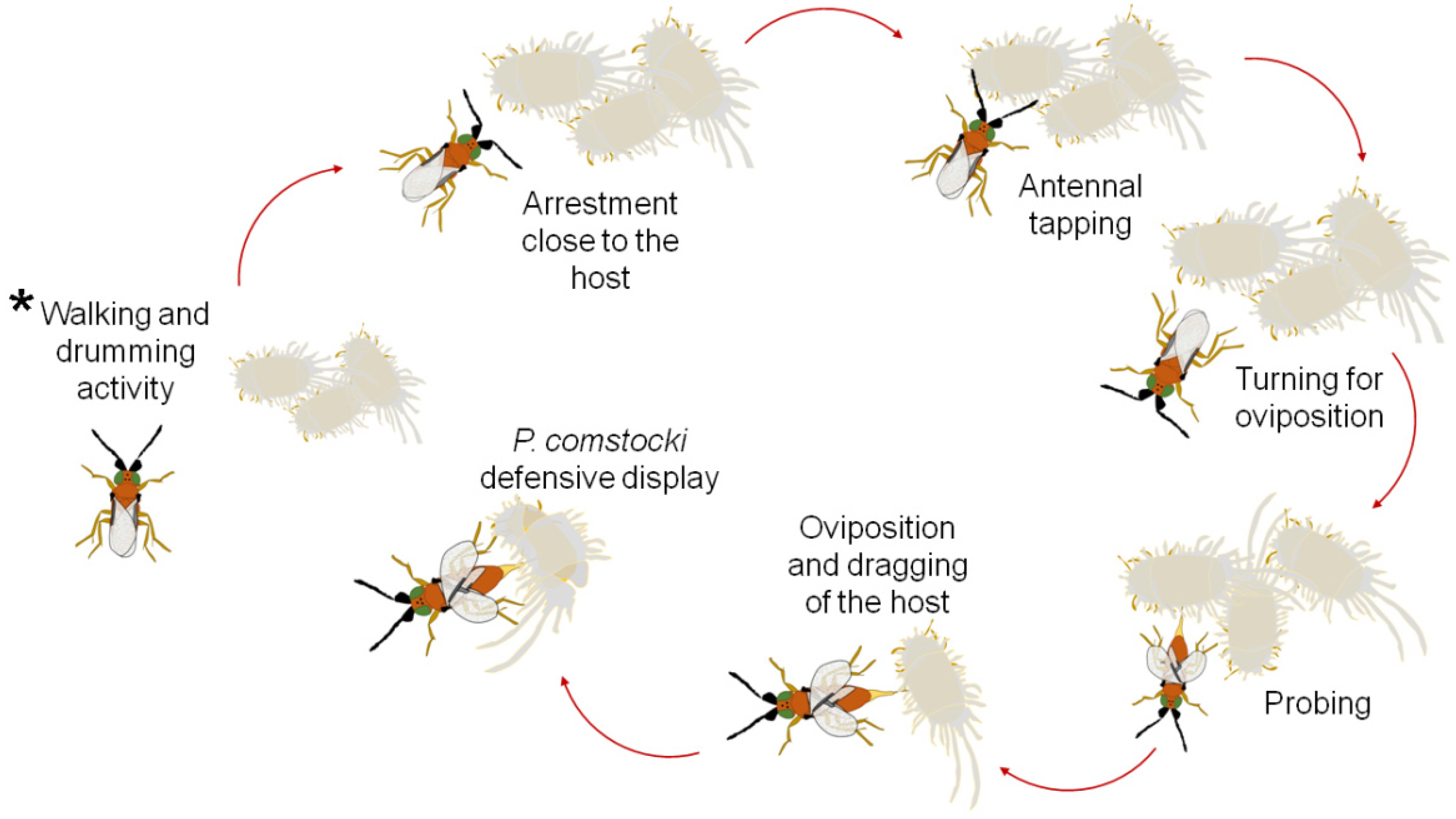
2.1. Insect Rearing and General Observations
2.2. Oviposition Behavior, Host Preferences, Host Suitability and Quality of the Parasitoid Progeny
2.2.1. No-Choice Tests
2.2.2. Two-Choice Tests
2.3. Statistical Analysis
3. Results
3.1. No-Choice Tests
3.2. Two-Choice Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wan, H.H.; Song, B.Z.; Tang, G.B.; Zhang, J.; Yao, Y.C. What are the effects of aromatic plants and meteorological factors on Pseudococcus comstocki and its predators in pear orchards? Agrofor. Syst. 2015, 89, 537–547. [Google Scholar] [CrossRef]
- Pellizzari, G.; Duso, C.; Rainato, A.; Pozzebon, A.; Zanini, G. Phenology, ethology and distribution of Pseudococcus comstocki, an invasive pest in northeastern Italy. Bull. Insect. 2012, 65, 209–215. [Google Scholar]
- Mazzeo, G.; Longo, S.; Pellizzari, G.; Porcelli, F.; Suma, P.; Russo, A. Exotic scale insects (Coccoidea) on ornamental plants in Italy: A never-ending story. Acta Zool. Bulg. 2014, 6, 55–61. [Google Scholar]
- Guerrieri, E.; Pellizzari, G. Parasitoids of Pseudococcus Comstocki in Italy Clausenia purpurea and Chrysoplatycerus splendens First Records from Europe. Bull. Insect. 2009, 62, 179–182. [Google Scholar]
- Negishi, T.; Ishiwatari, T.; Asano, S. Sex pheromone of the Comstock mealybug, Pseudococcus comstocki Kuwana; bioassay method, male response-habits to the sex pheromone. Jpn. J. Appl. Entomol. Zool. 1980, 24, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Sawamura, N.; Narai, Y. Effect of temperature on development and reproductive potential of two mealybug species Planococcus kraunhiae (Kuwana) and Pseudococcus comstocki (Kuwana) (Homoptera: Pseudococcidae). Jpn. J. Appl. Entomol. Zool. 2008. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Xu, Z.; Li, S.; Xu, W.; Li, H.; Sheng, X.; Jin, W.; Wang, Y.; Zhao, Y. Life table of the experimental population of Comstock mealybug, Pseudococcus comstocki (Hemiptera: Pseudococcidae), at different temperatures. Acta Entomol. Sin. 2012, 55, 1362–1367. [Google Scholar]
- Jeon, H.Y.; Kim, D.S.; Cho, M.R.; Chang, Y.D.; Yiem, M.S. Temperature-dependent Development of Pseudococcus comstocki. Korean J. Appl. Entomol. 2004, 42, 43–51. [Google Scholar]
- Kairo, M.T.K.; Paraiso, O.; Gautam, R.D.; Peterkin, D.D. Cryptolaemus montrouzieri (Mulsant) (Coccinellidae: Scymninae): A review of biology, ecology, and use in biological control with particular reference to potential impact on non-target organisms. CAB Rev. 2013, 8, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Malausa, T.; Delaunay, M.; Fleisch, A.; Groussier-Bout, G.; Warot, S.; Crochard, D.; Guerrieri, E.; Delvare, G.; Pellizzari, G.; Kaydan, M.B. Investigating biological control agents for controlling invasive populations of the mealybug Pseudococcus comstocki in France. PLoS ONE 2016, 11, e0157965. [Google Scholar] [CrossRef]
- Andreason, S.A.; Triapitsyn, S.V.; Perring, T.M. Untangling the Anagyrus pseudococci species complex (Hymenoptera: Encyrtidae), parasitoids of worldwide importance for biological control of mealybugs (Hemiptera: Pseudococcidae): Genetic data corroborates separation of two new, previously misidentified species. Biol. Control 2019, 129, 65–82. [Google Scholar]
- Lucchi, A.; Benelli, G. Towards pesticide-free farming? Sharing needs and knowledge promotes Integrated Pest Management. Environ. Sci. Pollut. Res. 2018, 25, 13439–13445. [Google Scholar] [CrossRef] [Green Version]
- Van Driesche, R.G.; Bellotti, A.; Herrera, C.J.; Castillo, J.A. Host preferences of two encyrtid parasitoids for the Columbian Phenacoccus spp. of cassava mealybugs. Entomol. Exp. Appl. 1987, 43, 261–266. [Google Scholar] [CrossRef]
- Romano, D.; Stefanini, C.; Canale, A.; Benelli, G. Artificial blood feeders for mosquito and ticks—Where from, where to? Acta Trop. 2018, 183, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.S.; Copland, M.J.W. Host preference and progeny sex ratio in a solitary koinobiont mealybug endoparasitoid, Anagyrus pseudococci (Girault), in response to its host stage. Biocontrol Sci. Technol. 1997, 7, 449–456. [Google Scholar] [CrossRef]
- Chong, J.-H.; Oetting, R.D. Specificity of Anagyrus sp. nov. nr. sinope and Leptomastix dactylopii for six mealybug species. BioControl 2007, 52, 289–308. [Google Scholar] [CrossRef]
- Romano, D.; Kavallieratos, N.G.; Athanassiou, C.G.; Stefanini, C.; Canale, A.; Benelli, G. Impact of geographical origin and rearing medium on mating success and lateralization in the rice weevil, Sitophilus oryzae (L.) (Coleoptera: Curculionidae). J. Stored Prod. Res. 2016, 69, 106–112. [Google Scholar] [CrossRef]
- Bokononganta, A.H.; Neuenschwander, P.; Vanalphen, J.J.M.; Vos, M. Host stage selection and sex allocation by Anagyrus mangicola (Hymenoptera: Encyrtidae), a parasitoid of the mango mealybug, Rastrococcus invadens (Homoptera: Pseudococcidae). Biol. Control 1995, 5, 479–486. [Google Scholar] [CrossRef]
- Sagarra, L.A.; Vincent, C.; Stewart, R.K. Suitability of nine mealybug species (Homoptera: Pseudococcidae) as hosts for the parasitoid Anagyrus kamali (Hymenoptera: Encyrtidae). Fla. Entomol. 2001, 84, 112–116. [Google Scholar] [CrossRef]
- Benelli, G.; Desneux, N.; Romano, D.; Conte, G.; Messing, R.H.; Canale, A. Contest experience enhances aggressive behaviour in a fly: When losers learn to win. Sci. Rep. 2015, 5, 9347. [Google Scholar] [CrossRef] [Green Version]
- Benelli, G.; Romano, D.; Desneux, N.; Messing, R.H.; Canale, A. Sex differences in fighting-induced hyperaggression in a fly. Anim. Behav. 2015, 104, 165–174. [Google Scholar] [CrossRef]
- Sokal, R.R. Single classification analysis of variance. In Biometry: The Principles and Practice of Statistics in Biological Research, 1st ed.; Rolfes, M., Ed.; W.H. Freeman and Company: New York, NY, USA, 1981; pp. 207–246. [Google Scholar]
- Cocco, A.; da Silva, V.C.P.; Benelli, G.; Botton, M.; Lucchi, A.; Lentini, A. Sustainable management of the vine mealybug in organic vineyards. J. Pest Sci. 2021, 94, 153–185. [Google Scholar] [CrossRef]
- Gullan, P.J.; Kosztarab, M. Adaptations in scale insects. Annu. Rev. Entomol. 1997, 42, 23–50. [Google Scholar] [CrossRef]
- Duso, C. Bioecological study on Planococcus ficus (Sign.) in Veneto. Boll. Lab. Entomol. Agrar. Silvestri’ 1989, 46, 3–20. [Google Scholar]
- Sagarra, L.A.; Vincent, C. Influence of Host Stage on Oviposition, Development, Sex Ratio, and Survival of Anagyrus kamali Moursi (Hymenoptera: Encyrtidae), a Parasitoid of the Hibiscus Mealybug, Maconellicoccus hirsutus Green (Homoptera: Pseudococcidae). Biol. Control 1999, 15, 51–56. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, J.; Lu, Y.; Xia, T. Effects of temperature and host stage on the parasitization rate and offspring sex ratio of Aenasius bambawalei Hayat in Phenacoccus solenopsis Tinsley. PeerJ 2016, 4, e1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messing, R.H.; Wright, M.G. Biological control of invasive species: Solution or pollution? Front. Ecol. Environ. 2006, 4, 132–140. [Google Scholar] [CrossRef]
- Parry, D. Beyond Pandora’s box: Quantitatively evaluating non-target effects of parasitoids in classical biological control. In Ecological Impacts of Non-Native Invertebrates and Fungi on Terrestrial Ecosystems; Springer: Berlin/Heidelberg, Germany, 2008; pp. 47–58. [Google Scholar]
- Bugila, A.A.A.; Franco, J.C.; da Silva, E.B.; Branco, M. Suitability of five mealybug species (Hemiptera, Pseudococcidae) as hosts for the solitary parasitoid Anagyrus sp. nr. pseudococci (Girault) (Hymenoptera: Encyrtidae). Biocontrol Sci. Technol. 2015, 25, 108–120. [Google Scholar] [CrossRef]
- Bugila, A.A.A.; Branco, M.; da Silva, E.B.; Franco, J.C. Host selection behaviour and specificity of the solitary parasitoid of mealybugs Anagyrus sp. nr. pseudococci (Girault) (Hymenoptera, Encyrtidae). Biocontrol Sci. Technol. 2014, 24, 22–38. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricciardi, R.; Zeni, V.; Michelotti, D.; Di Giovanni, F.; Cosci, F.; Canale, A.; Zang, L.-S.; Lucchi, A.; Benelli, G. Old Parasitoids for New Mealybugs: Host Location Behavior and Parasitization Efficacy of Anagyrus vladimiri on Pseudococcus comstocki. Insects 2021, 12, 257. https://doi.org/10.3390/insects12030257
Ricciardi R, Zeni V, Michelotti D, Di Giovanni F, Cosci F, Canale A, Zang L-S, Lucchi A, Benelli G. Old Parasitoids for New Mealybugs: Host Location Behavior and Parasitization Efficacy of Anagyrus vladimiri on Pseudococcus comstocki. Insects. 2021; 12(3):257. https://doi.org/10.3390/insects12030257
Chicago/Turabian StyleRicciardi, Renato, Valeria Zeni, Davide Michelotti, Filippo Di Giovanni, Francesca Cosci, Angelo Canale, Lian-Sheng Zang, Andrea Lucchi, and Giovanni Benelli. 2021. "Old Parasitoids for New Mealybugs: Host Location Behavior and Parasitization Efficacy of Anagyrus vladimiri on Pseudococcus comstocki" Insects 12, no. 3: 257. https://doi.org/10.3390/insects12030257
APA StyleRicciardi, R., Zeni, V., Michelotti, D., Di Giovanni, F., Cosci, F., Canale, A., Zang, L.-S., Lucchi, A., & Benelli, G. (2021). Old Parasitoids for New Mealybugs: Host Location Behavior and Parasitization Efficacy of Anagyrus vladimiri on Pseudococcus comstocki. Insects, 12(3), 257. https://doi.org/10.3390/insects12030257










