Rearing Tenebrio molitor and Alphitobius diaperinus Larvae on Seed Cleaning Process Byproducts
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Byproducts
2.3. Experimental Design
2.4. Statistical Analysis
3. Results
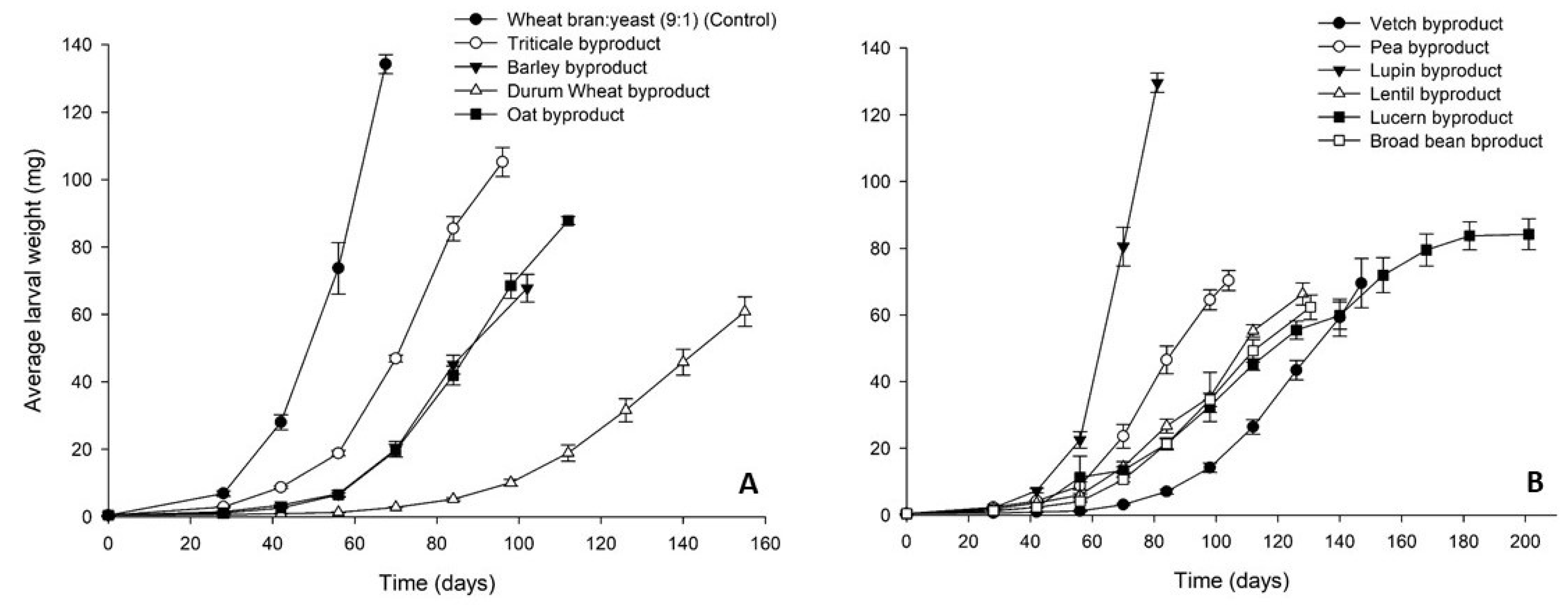
3.1. Tenebrio molitor
3.2. Alphitobius diaperinus
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bedoić, R.; Ćosić, B.; Duić, N. Technical potential and geographic distribution of agricultural residues, co-products and by-products in the European Union. Sci. Total Environ. 2019, 686, 568–579. [Google Scholar] [CrossRef]
- Varelas, V. Food wastes as a potential new source for edible insect mass production for food and feed: A review. Fermentation 2019, 5, 81. [Google Scholar] [CrossRef] [Green Version]
- Gasco, L.; Biancarosa, I.; Liland, N.S. From waste to feed: A review of recent knowledge on insects as producers of protein and fat for animal feeds. Curr. Opin. Green Sustain. Chem. 2020, 23, 67–79. [Google Scholar] [CrossRef]
- IPIFF (International Platform of Insects for Food and Feed). Building Bridges between the Insect Production Chain, Research and Policymakers; IPIFF: Brussels, Belgium, 2019; p. 20. [Google Scholar]
- Roffeis, M.; Wakefield, M.E.; Almeida, J.; Valada, T.R.A.; Devic, E.; Koné, N.G.; Kenis, M.; Nacambo, S.; Fitches, E.C.; Koko, G.K.D.; et al. Life cycle cost assessment of insect based feed production in West Africa. J. Clean. Prod. 2018, 199, 792–806. [Google Scholar] [CrossRef] [Green Version]
- Gahukar, R.T. Edible insects farming: Efficiency and impact on family livelihood, food security, and environment compared with livestock and crops. In Insects as Sustainable Food Ingredients: Production, Processing and Food Applications; Dossey, A.T., Morales-Ramos, J.A., Guadalupe Rojas, M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 85–111. [Google Scholar]
- Arru, B.; Furesi, R.; Gasco, L.; Madau, F.A.; Pulina, P. The introduction of insect meal into fish diet: The first economic analysis on European sea bass farming. Sustainability 2019, 11, 1697. [Google Scholar] [CrossRef] [Green Version]
- Cadinu, L.A.; Barra, P.; Torre, F.; Delogu, F.; Madau, F.A. Insect rearing: Potential, challenges, and circularity. Sustainability 2020, 12, 4567. [Google Scholar] [CrossRef]
- Van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef] [Green Version]
- EU Commission. Towards a Circular Economy: A Zero Waste Programme for Europe. 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A52014DC0398 (accessed on 11 February 2021).
- EU Commission. A New Circular Economy Action Plan: For a Cleaner and More Competitive Europe. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1583933814386&uri=COM:2020:98:FIN (accessed on 11 February 2021).
- EU Commission Regulation 2017/893 of 24 May 2017 Amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as Regards the Provisions on Processed Animal Protein. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0893&from=EN (accessed on 11 February 2021).
- European Food Safety Authority (EFSA) NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens); Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Scientific opinion on the safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, 6343. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J.; González, E.A.; Hernández, A.R.; Pino, J.M. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to recycle organic wastes and as feed for broiler chickens. J. Econ. Entomol. 2002, 95, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.A.B.; Van Broekhoven, S.; Van Huis, A.; Van Loon, J.J.A. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar]
- Kim, S.Y.; Kim, H.G.; Yoon, H.J.; Lee, K.Y.; Kim, N.J. Nutritional analysis of alternative feed ingredients and their effects on the larval growth of Tenebrio molitor (Coleoptera: Tenebrionidae). Entomol. Res. 2017, 47, 194–202. [Google Scholar] [CrossRef]
- Shu, W.T.; Kok, S.L.; Jiun, Y.L. Effects of food wastes on yellow mealworm Tenebrio molitor larval nutritional profiles and growth performances. Exam. Mar. Biol. Oceanogr. 2018, 2, 173–178. [Google Scholar]
- Mancini, S.; Fratini, F.; Turchi, B.; Mattioli, S.; Bosco, A.D.; Tuccinardi, T.; Nozic, S.; Paci, G. Former foodstuff products in Tenebrio molitor rearing: Effects on growth, chemical composition, microbiological load, and antioxidant status. Animals 2019, 9, 484. [Google Scholar] [CrossRef] [Green Version]
- Stull, V.J.; Kersten, M.; Bergmans, R.S.; Patz, J.A.; Paskewitz, S. Crude protein, amino acid, and iron content of Tenebrio molitor (Coleoptera, Tenebrionidae) reared on an agricultural byproduct from maize production: An exploratory study. Ann. Entomol. Soc. Am. 2019, 112, 533–543. [Google Scholar] [CrossRef]
- Harsányi, E.; Juhász, C.; Kovács, E.; Huzsvai, L.; Pintér, R.; Fekete, G.; Varga, Z.I.; Aleksza, L.; Gyuricza, C. Evaluation of organic wastes as substrates for rearing Zophobas morio, Tenebrio molitor, and Acheta domesticus larvae as alternative feed supplements. Insects 2020, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Riudavets, J.; Castañé, C.; Agustí, N.; del Arco, L.; Diaz, I.; Castellari, M. Development and biomass composition of Ephestia kuehniella (Lepidoptera: Pyralidae), Tenebrio molitor (Coleoptera: Tenebrionidae), and Hermetia illucens (Diptera: Stratiomyidae) reared on different byproducts of the agri-food industry. J. Insect Sci. 2020, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Ruschioni, S.; Loreto, N.; Foligni, R.; Mannozzi, C.; Raffaelli, N.; Zamporlini, F.; Pasquini, M.; Roncolini, A.; Cardinali, F.; Osimani, A.; et al. Addition of olive pomace to feeding substrate affects growth performance and nutritional value of mealworm (Tenebrio molitor L.) larvae. Foods 2020, 9, 317. [Google Scholar] [CrossRef] [Green Version]
- Morales-Ramos, J.A.; Rojas, M.G.; Kelstrup, H.C.; Emery, V. Self-selection of agricultural by-products and food ingredients by Tenebrio molitor (Coleoptera: Tenebrionidae) and impact on food utilization and nutrient intake. Insects 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Van Broekhoven, S.; Oonincx, D.G.A.B.; van Huis, A.; van Loon, J.J.A. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef]
- Gianotten, N.; Soetemans, L.; Bastiaens, L. Agri-food side-stream inclusions in the diet of Alphitobius diaperinus Part 1: Impact on larvae growth performance parameters. Insects 2020, 11, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soetemans, L.; Gianotten, N.; Bastiaens, L. Agri-food side-stream inclusion in the diet of Alphitobius diaperinus. Part 2: Impact on larvae composition. Insects 2020, 11, 190. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists International, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Waldbauer, G.P. The consumption and utilization of food by insects. Adv. Insect Phys. 1968, 5, 229–288. [Google Scholar]
- Psofakis, P.; Karapanagiotidis, I.T.; Malandrakis, E.E.; Golomazou, E.; Exadactylos, A.; Mente, E. Effect of fishmeal replacement by hydrolyzed feather meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and growth-related gene expression of gilthead seabream (Sparus aurata). Aquaculture 2020, 521, 735006. [Google Scholar] [CrossRef]
- Han, C.S.; Dingemanse, N.J. You are what you eat: Diet shapes body composition, personality and behavioural stability. BMC Evol. Biol. 2017, 17, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Psofakis, P.; Athanassiou, C.G. Evaluation of various commodities for the development of the yellow mealworm, Tenebrio molitor. Sci. Rep. 2020, 10, 11224. [Google Scholar] [CrossRef] [PubMed]
- Vohra, P.; Shariff, G.; Robinson, D.D.; Qualset, C.O.; Gale, G.A.E. Nutritional evaluation of triticale, wheat and rice grain using red flour beetle (Triboltum castaneum) larvae and chickens. Nutr. Rep. Int. 1978, 18, 289–300. [Google Scholar]
- Shariff, G.; Vohra, P.; Qualset, C.O. Further studies on the nutritional evaluation of wheat, triticale, and rice grains using the red flour beetle. Cereal Chem. 1981, 58, 86–89. [Google Scholar]
- Lucas, M.M.; Stoddard, F.L.; Annicchiarico, P.; Frías, J.; Martínez-Villaluenga, C.; Sussmann, D.; Duranti, M.; Seger, A.; Zander, P.M.; Pueyo, J.J. The future of lupin as a protein crop in Europe. Front. Plant Sci. 2015, 6, 705. [Google Scholar] [CrossRef] [PubMed]
- Musco, N.; Cutrignelli, M.I.; Calabrò, S.; Tudisco, R.; Infascelli, F.; Grazioli, R.; Lo Presti, V.; Gresta, F.; Chiofalo, B. Comparison of nutritional and antinutritional traits among different species (L. albus L., L. luteus L., L. angustifolius L.) and varieties of lupin seeds. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1227–1241. [Google Scholar] [CrossRef] [Green Version]
- Abraham, E.M.; Ganopoulos, I.; Madesis, P.; Mavromatis, A.; Mylona, P.; Nianiou-Obeidat, I.; Parissi, Z.; Polidoros, A.; Tani, E.; Vlachostergios, D. The use of lupin as a source of protein in animal feeding: Genomic tools and breeding approaches. Int. J. Mol. Sci. 2019, 20, 851. [Google Scholar] [CrossRef] [Green Version]
- Bähr, M.; Fechner, A.; Hasenkopf, K.; Mittermaier, S.; Jahreis, G. Chemical composition of dehulled seeds of selected lupin cultivars in comparison to pea and soya bean. LWT Food Sci. Technol. 2014, 59, 587–590. [Google Scholar] [CrossRef]
- Hosen, M.; Khan, A.R.; Hossain, M. Growth and development of the lesser mealworm, Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae) on cereal flours. Pak. J. Biol. Sci. 2004, 7, 1505–1508. [Google Scholar]
- Sen, S.; Makkar, H.; Becker, K. Alfalfa saponins and their implication in animal nutrition. J. Agric. Food Chem. 1998, 46, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Wina, E.; Muetzel, S.; Becker, K. The impact of saponins or saponin-containing plant materials on ruminant production. J. Agric. Food Chem. 2005, 53, 8093–8105. [Google Scholar]
- Tava, A.; Odoardi, M. Saponins from Medicago sp.: Chemical characterization and biological activity against insects. In Saponins Used in Food and Agriculture; Waller, G.R., Yamasaki, K., Eds.; Plenum Press: New York, NY, USA, 1996; pp. 97–109. [Google Scholar]
- Huang, Y.F.; Gao, X.L.; Nan, Z.B.; Zhang, Z.X. Potential value of the common vetch (Vicia sativa L.) as an animal feedstuff: A review. J. Anim. Physiol. Anim. Nutr. 2017, 101, 807–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Byproduct | Dry Matter (%) | Protein (% DM) | Price (€/ton) |
|---|---|---|---|
| Wheat bran (control diet ingredient) | 85.2 | 16.7 | 170 |
| Vetch (Vicia sativa) byproduct | 91.8 | 24.9 | 270 |
| Pea (Pisum sativum) byproduct | 91.9 | 28.2 | 220 |
| Lupin (Lupinus albus) byproduct | 93.3 | 33.5 | 300 |
| Triticale (Triticum sp. x Secale cereale) byproduct | 92.1 | 8.5 | 140 |
| Lentil (Lens culinaris) byproduct | 91.0 | 20.8 | 350 |
| Lucerne (Medicago sativa) byproduct | 95.8 | 13.3 | 100 |
| Broad bean (Vicia faba) byproduct | 91.0 | 27.3 | 220 |
| Barley (Hordeum vulgare) byproduct | 91.6 | 9.1 | 140 |
| Durum wheat (Triticum durum) byproduct | 91.0 | 11.0 | 170 |
| Oat (Avena sativa) byproduct | 90.4 | 12.3 | 140 |
| Yeast (control diet ingredient) | 97.9 | 50.0 | 8000 |
| Substrate | FCR | SGR (% day−1) | ECR (€ ton−1 Larvae) |
|---|---|---|---|
| Wheat bran:yeast (9:1) (control) | 2.3 ± 0.1 cd | 8.6 ± 0.1 a | 2211.2 ± 83.5 a |
| Vetch byproduct | 7.9 ± 0.6 a | 3.5 ± 0.1 d | 2138.4 ± 148.8 a |
| Pea byproduct | 1.7 ± 0.1 d | 5.0 ± 0.2 bc | 380.6 ± 30.4 d |
| Lupin byproduct | 4.7 ± 1.0 abc | 7.2 ± 0.1 ab | 1418.4 ± 299.1 ab |
| Triticale byproduct | 2.6 ± 0.3 cd | 5.8 ± 0.2 ab | 361.1 ± 40.4 d |
| Lentil byproduct | 5.8 ± 0.4 ab | 4.0 ± 0.1 cd | 2020.7 ± 157.0 a |
| Lucerne byproduct | 12.6 ± 3.0 a | 2.7 ± 0.1 d | 1260.5 ± 300.5 abc |
| Broad bean byproduct | 2.9 ± 0.6 cd | 3.9 ± 0.1 cd | 629.4 ± 125.4 bcd |
| Barley byproduct | 3.5 ± 0.2 bc | 5.1 ± 0.1 abc | 493.5 ± 34.0 cd |
| Durum wheat byproduct | 7.6 ± 2.4 ab | 3.3 ± 0.1 d | 1284.8 ± 402.5 abc |
| Oat byproduct | 3.1 ± 0.2 bc | 4.8 ± 0.1 bc | 428.0 ± 21.6 d |
| Substrate | FCR | SGR (% day−1) | ECR (€ ton−1 Larvae) |
|---|---|---|---|
| Wheat bran:yeast (9:1) (control) | 2.3 ± 0.2 e | 7.8 ± 0.4 a | 2185.0 ± 171.5 b |
| Vetch byproduct | 15.2 ± 3.4 a | 5.1 ± 0.2 cde | 4092.3 ± 906.4 a |
| Pea byproduct | 5.4 ± 0.7 bcd | 6.2 ± 0.1 abc | 1192.6 ± 152.0 bc |
| Lupin byproduct | 2.7 ± 0.3 de | 7.3 ± 0.2 ab | 810.9 ± 84.5 cd |
| Triticale byproduct | 5.1 ± 0.2 cde | 5.4 ± 0.2 cde | 719.2 ± 26.0 cd |
| Lentil byproduct | 12.6 ± 1.2 a | 4.5 ± 0.4 e | 4410.2 ± 433.8 a |
| Lucerne byproduct | 16.6 ± 5.5 a | 4.6 ± 0.2 de | 1663.7 ± 550.2 bc |
| Broad bean byproduct | 10.7 ± 1.8 ab | 4.3 ± 0.4 e | 2361.9 ± 389.8 ab |
| Barley byproduct | 4.8 ± 0.8 cde | 5.7 ± 0.2 bcd | 667.3 ± 117.5 d |
| Durum wheat byproduct | 7.1 ± 0.5 abc | 4.6 ± 0.1 e | 1202.9 ± 81.2 bc |
| Oat byproduct | 5.4 ± 0.4 bcde | 5.4 ± 0.3 cde | 755.3 ± 55.0 cd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumbos, C.I.; Bliamplias, D.; Gourgouta, M.; Michail, V.; Athanassiou, C.G. Rearing Tenebrio molitor and Alphitobius diaperinus Larvae on Seed Cleaning Process Byproducts. Insects 2021, 12, 293. https://doi.org/10.3390/insects12040293
Rumbos CI, Bliamplias D, Gourgouta M, Michail V, Athanassiou CG. Rearing Tenebrio molitor and Alphitobius diaperinus Larvae on Seed Cleaning Process Byproducts. Insects. 2021; 12(4):293. https://doi.org/10.3390/insects12040293
Chicago/Turabian StyleRumbos, Christos I., Dimitrios Bliamplias, Marina Gourgouta, Vasilios Michail, and Christos G. Athanassiou. 2021. "Rearing Tenebrio molitor and Alphitobius diaperinus Larvae on Seed Cleaning Process Byproducts" Insects 12, no. 4: 293. https://doi.org/10.3390/insects12040293
APA StyleRumbos, C. I., Bliamplias, D., Gourgouta, M., Michail, V., & Athanassiou, C. G. (2021). Rearing Tenebrio molitor and Alphitobius diaperinus Larvae on Seed Cleaning Process Byproducts. Insects, 12(4), 293. https://doi.org/10.3390/insects12040293








