Termite Assemblage and Damage on Tree Trunks in Fast-Growing Teak Plantations of Different Age: A Case Study in West Java, Indonesia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
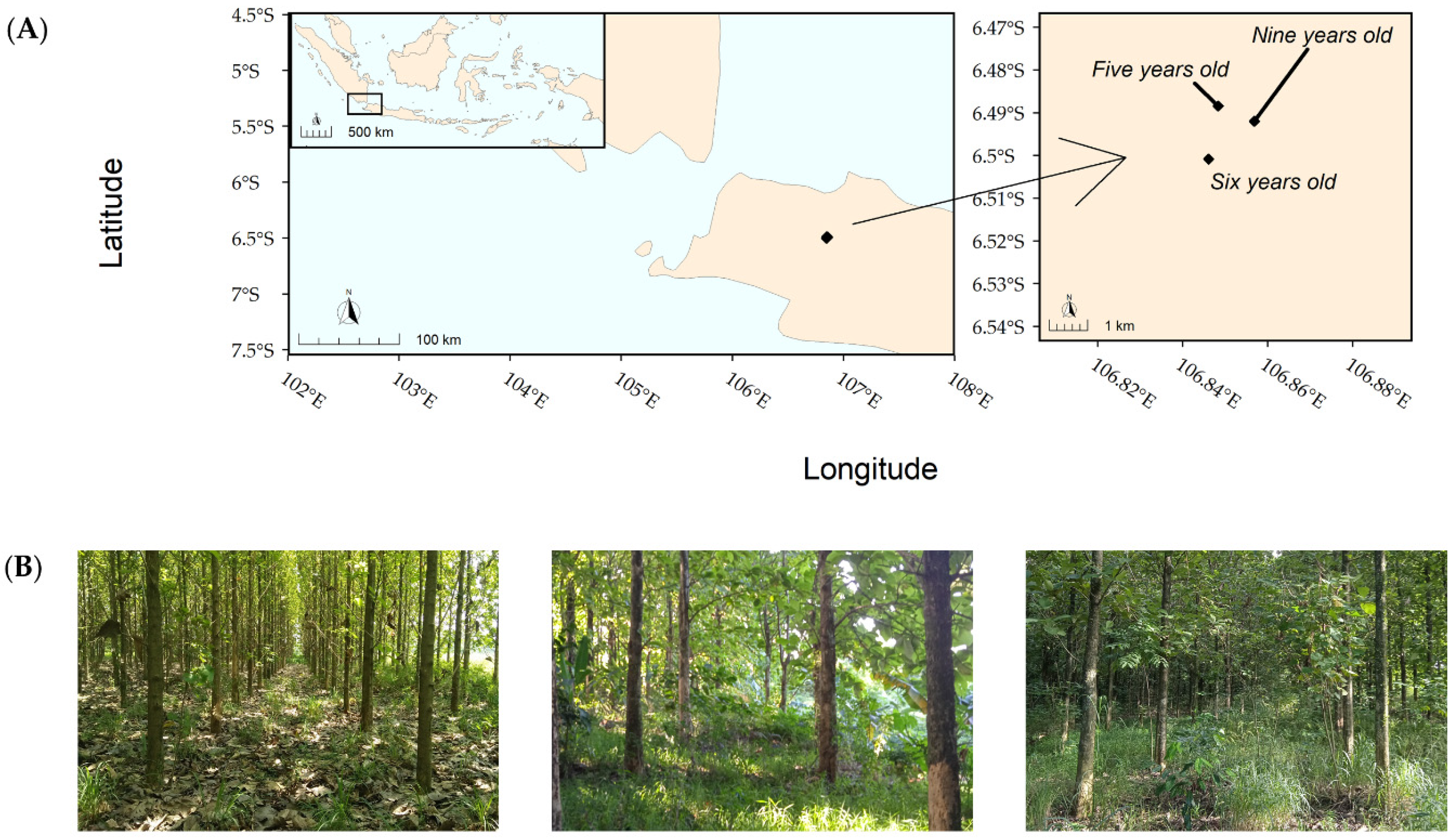
2.1. Sampling Locations
- Five years old (location A): The plantation location was 6°48′84″ S, 106°84′83″ E, at an altitude of approximately 140 m.a.s.l. This was the youngest plantation available. The spacing was 3 m with a moderate canopy cover and a tree height of 6–7 m. The leaf litter was substantial, with almost no herbaceous plants or shrubs present.
- Six years old (location B): The plantation location was 6°50′0″ S, 1065°84′50″ E, at an altitude of approximately 150–145 m.a.s.l. The spacing was 3–4 m with a moderate canopy cover and a tree height of 8–12 m. The herbaceous layer and leaf litter were substantial. Some shrubs were also present.
- Nine years old (location C): The plantation location was 6°49′20″ S, 106°85′60″ E, at an altitude of approximately 135–128 m.a.s.l. The spacing was 4 m with a dense canopy cover and a tree height of 10–15 m. The herbaceous layer and leaf litter were substantial. Some shrubs were also present.
2.2. Survey of Termites and Damaged Teakwood Trunk
2.3. Termite Identification Process
2.4. Data Analysis
3. Results
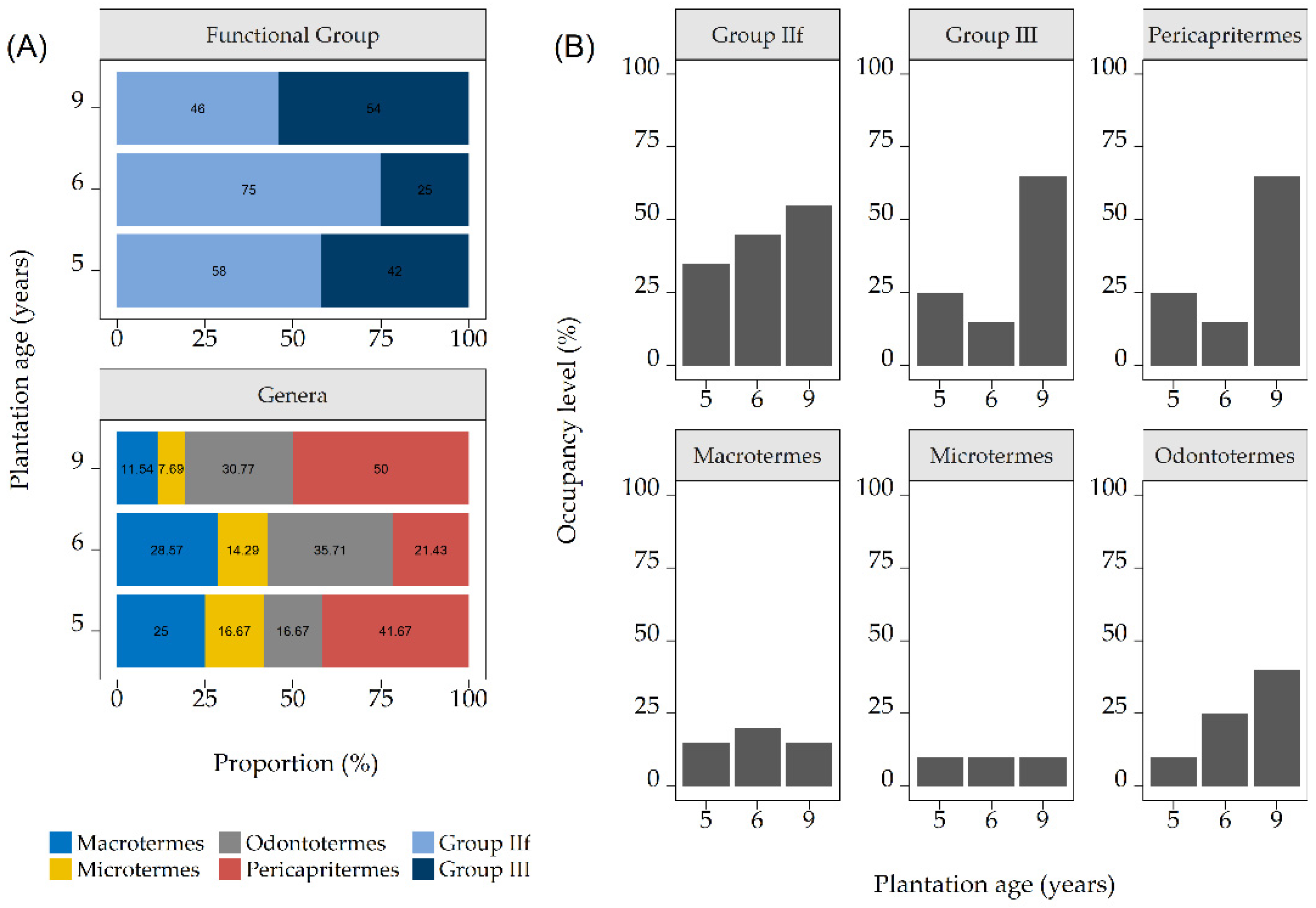
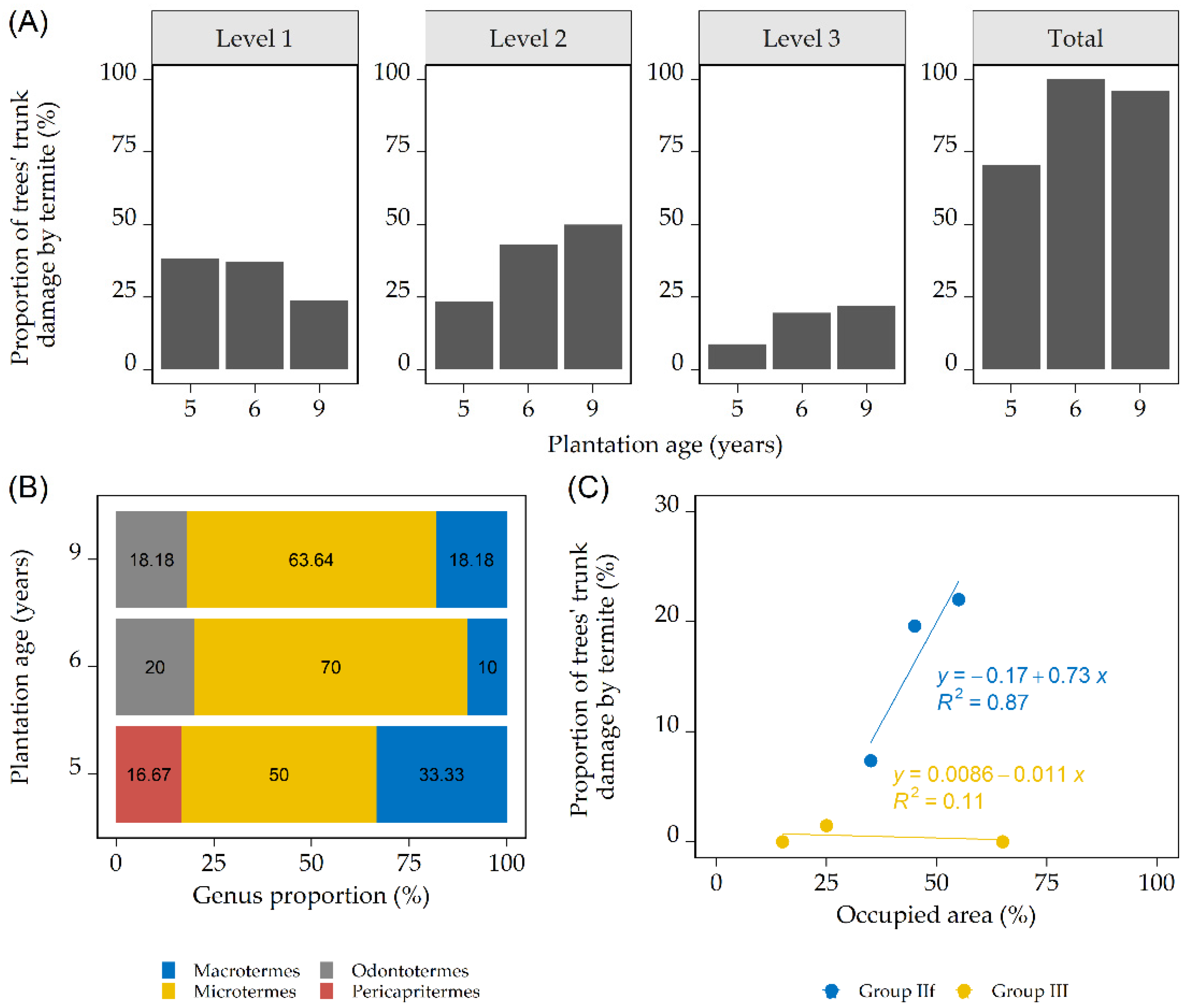
3.1. Termite Identity, Functional Group, and Termite Damage on the Trunks
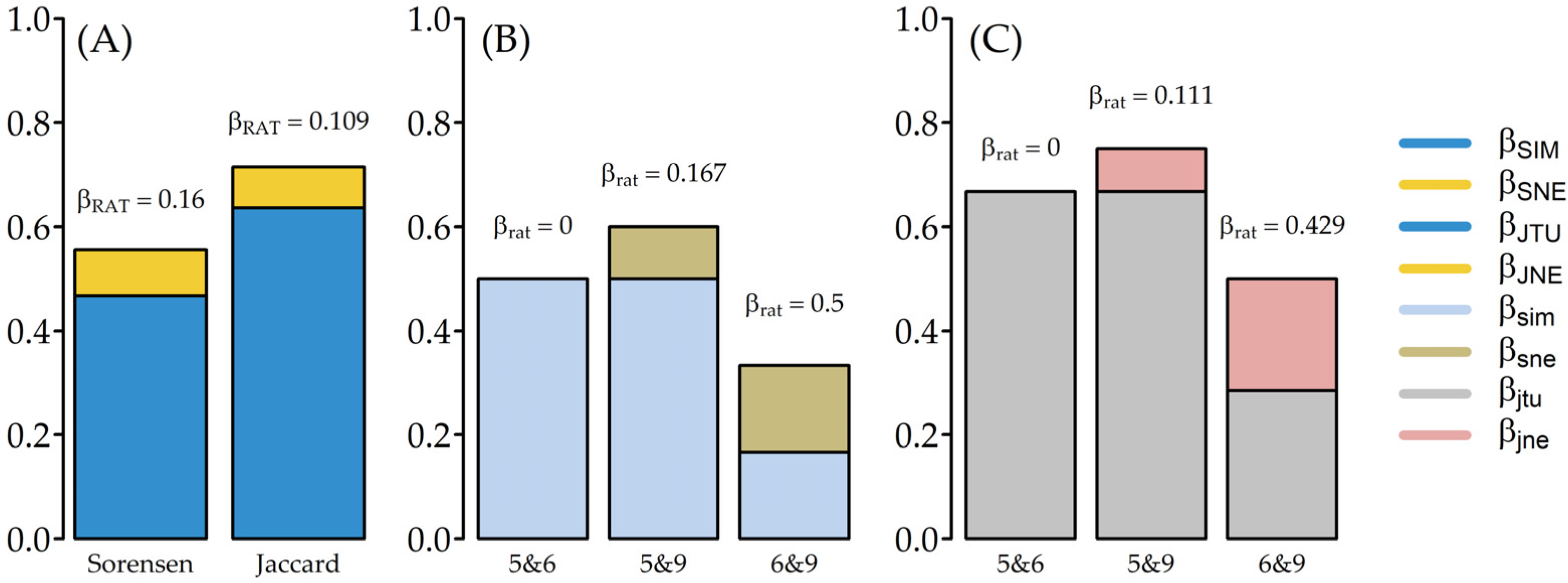
3.2. Termite Distribution and Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jouquet, P.; Bottinelli, N.; Shanbhag, R.R.; Bourguignon, T.; Traoré, S.; Abbasi, S.A. Termites: The neglected soil engineers of tropical soils. Soil Sci. 2016, 181, 157–165. [Google Scholar] [CrossRef]
- Bignell, D.E. Termites as Soil Engineers and Soil Processors. In Intestinal Microorganisms of Termites and Other Invertebrates; König, H., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 6, pp. 183–220. [Google Scholar]
- Lee, C.Y. Subterranean termite pests and their control in the urban environment in Malaysia. Sociobiology 2002, 40, 3–9. [Google Scholar]
- Evans, T.A.; Iqbal, N. Termite (order Blattodea, infraorder Isoptera) baiting 20 years after commercial release. Pest Manag. Sci. 2015, 71, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Cowie, R.H.; Logan, J.W.M.; Wood, T.G. Termite (Isoptera) damage and control in tropical forestry with special reference to Africa and Indo-Malaysia: A review. Bull. Entomol. Res. 1989, 79, 173–184. [Google Scholar] [CrossRef]
- Mill, A.E. Termites as Agricultural Pests in Amazônia, Brazil. Outlook Agric. 1992, 21, 41–46. [Google Scholar] [CrossRef]
- Junqueira, L.K.; Florencio, D.F. Termite Damage in Agriculture Areas and Implanted Forests: An Ecological Approach. In Termites and Sustainable Management; Khan, M.A., Ahmad, W., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 38–50. ISBN 978-3-319-68725-4. [Google Scholar]
- Eggleton, P.; Bignell, D.E.; Hauser, S.; Dibog, L.; Norgrove, L.; Madong, B. Termite diversity across an anthropogenic disturbance gradient in the humid forest zone of West Africa. Agric. Ecosyst. Environ. 2002, 90, 189–202. [Google Scholar] [CrossRef]
- Gathorne-Hardy, F.J.; Syaukani, S.; Inward, D.J.G. Recovery of termite (Isoptera) assemblage structure from shifting cultivation in Barito Ulu, Kalimantan, Indonesia. J. Trop. Ecol. 2006, 22, 605–608. [Google Scholar] [CrossRef]
- Bonar, S.A.; Fehmi, J.S.; Mercado-Silva, N. An Overview of Sampling Issues in Species Diversity and Abundance Surveys. In Biological Dversity: Frontiers in Measurement and Assesment; Magurran, A.E., Mcgill, B.J., Eds.; Oxford University Press: New York, NY, USA, 2011; pp. 11–24. [Google Scholar]
- Lepage, M.; Darlington, J.P.E.C. Population Dynamics of Termites. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 333–361. [Google Scholar]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Guimara, P.; Guimara, P.R.; Ulrich, W. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos 2008, 117, 1227–1239. [Google Scholar] [CrossRef]
- Hui, C.; McGeoch, M.A. Zeta Diversity as a Concept and Metric That Unifies Incidence-Based Biodiversity Patterns. Am. Nat. 2014, 184, 684–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef] [Green Version]
- Gould, M.S.; Lowe, A.J.; Clarke, G.P. The frequency of termite (Isoptera) damage to tree species in Namakutwa Forest, Tanzania. Sociobiology 1993, 23, 189–198. [Google Scholar]
- Mandal, B.K.; Bashar, K.; Howlader, A.J.; Rahman, K.M.Z. Incidence of termite infestation to tree species in Jahangirnager university campus, Bangladesh. Bangladesh J. Life Sci. 2010, 22, 7–15. [Google Scholar]
- Harris, W.V. Termites. Their Recognition and Control, 2nd ed.; Longman: London, UK, 1971; ISBN 0582466563. [Google Scholar]
- Harris, W.V. Termites and forestry. Emp. For. Rev. 1955, 34, 160–166. [Google Scholar]
- Adi, D.S.; Sudarmanto, S.; Ismadi, I.; Gopar, M.; Darmawan, T.; Amin, Y.; Dwianto, W.; Witjaksono, W. Evaluation of the wood quality of platinum teak wood. Teknol. Indones. 2016, 39, 36–44. [Google Scholar] [CrossRef]
- Hastuti, I. Frequency of attack of insect Inger-Inger (Neotermes tectonae Damm) in teak stand in RPH curah jati BKPH Grajagan KPH South Banyuwangi. In Proceedings of the Seminar Nasional Agroteknologi 2019; Pusat Penelitian dan Penerbitan UIN Sunan Gunung Djati Bandung: Bandung, Indonesia, 2019; pp. 299–305. (In Indonesian) [Google Scholar]
- Intari, S.E. Effects of Neotermes tectonae Damm attack on the quality and quantity of teak timber in the Kebonharjo Forest Division, Central Java. Bul. Penelit. Hutan 1990, 530, 25–35. [Google Scholar]
- Ridwan, R.; Handayani, T.; Riastiwi, I.; Witjaksono, W. Tetraploid teak seedling was more tolerant to drought stress than its diploid seedling. J. Penelit. Kehutan. Wallacea 2018, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pramasari, D.A.; Wahyuni, I.; Adi, D.S.; Amin, Y.; Darmawan, T.; Dwianto, W. Effect of Age on Chemical Component of Platinum Teak Wood—A Fast Growing Teak Wood From Lipi. In Proceedings of the The 6th International Symposium of IWoRS; Hartono, R., Ed.; Indonesian Wood Research Society (IWoRS): Medan, Indonesia, 2014; pp. 211–216. [Google Scholar]
- BMKG. Forecast of 2018/2019 Rainy Season in Indonesia; BMKG: Jakarta, Indonesia, 2018. (In Indonesian) [Google Scholar]
- Jones, D.T.; Eggleton, P. Sampling termite assemblages in tropical forests: Testing a rapid biodiversity assessment protocol. J. Appl. Ecol. 2000, 37, 191–203. [Google Scholar] [CrossRef]
- Himmi, S.K.; Wikantyoso, B.; Ismayati, M.; Fajar, A.; Meisyara, D.; Krishanti, N.P.R.A.; Zulfiana, D.; Lestari, A.S.; Tarmadi, D.; Kartika, T.; et al. Termite assemblage structure in Batam Island, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 361, 012026. [Google Scholar] [CrossRef]
- Kartika, T.; Himmi, S.K.; Wikantyoso, B.; Lestari, A.S.; Ismayati, M.; Zulfiana, D.; Krishanti, N.P.R.A.; Zulfitri, A.; Prianto, A.H.; Yusuf, S. Biodiversity of Termites and Fungi in Two Botanical Gardens in Batam, Riau Island Province and Kuningan, West Java Province. IOP Conf. Ser. Earth Environ. Sci. 2018, 166, 012008. [Google Scholar] [CrossRef]
- Jones, D.T.; Susilo, F.X.; Bignell, D.E.; Hardiwinoto, S.; Gillison, A.N.; Eggleton, P. Termite assemblage collapse along a land-use intensification gradient in lowland central Sumatra, Indonesia. J. Appl. Ecol. 2003, 40, 380–391. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.T.; Prasetyo, A.H. A Survey of The Termites (Insecta: Isoptera) of Tabalong District, South Kalimantan, Indonesia. Raffles Bull. Zool. 2002, 50, 117–128. [Google Scholar]
- Ahmad, M. Key to the Indomalayan Termites. Biologia 1958, 4, 33–198. [Google Scholar]
- Ahmad, M. Termites (Isoptera) of Thailand. Bull. Am. Mus. Nat. Hist. 1965, 131, 1–120. [Google Scholar]
- Thapa, R.S. Termites of Sabah (East Malaysia); Sabah Forest Department: Sandakan, Malaysia, 1982. [Google Scholar]
- Tho, Y.P. Termites of Peninsular Malaysia; Malayan forest records; Kirton, L.G., Ed.; Forest Research Institute Malaysia: Kepong, Malaysia, 1992; ISBN 9789839592146. [Google Scholar]
- Donovan, S.E.; Eggleton, P.; Bignell, D.E. Gut content analysis and a new feeding group classification of termites. Ecol. Entomol. 2001, 26, 356–366. [Google Scholar] [CrossRef]
- Jones, D.T.; Eggleton, P. Global Biogeography of Termites: A Compilation of Sources. In Biology of Termites: A Modern Synthesis; Bignell, D.E., Roisin, Y., Lo, N., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 477–498. ISBN 978-90-481-3976-7. [Google Scholar]
- Himmi, S.K.; Wikantyoso, B.; Fajar, A.; Tarmadi, D.; Yusuf, S. Functional structures of termite assemblage in changing habitats and ecosystems. IOP Conf. Ser. Earth Environ. Sci. 2020, 591, 012049. [Google Scholar] [CrossRef]
- Latombe, G.; McGeoch, M.A.; Nipperess, D.; Hui, C. zetadiv: An R package for computing compositional change across multiple sites, assemblages or cases. bioRxiv 2018, 324897. [Google Scholar] [CrossRef] [Green Version]
- McGeoch, M.A.; Latombe, G.; Andrew, N.R.; Nakagawa, S.; Nipperess, D.A.; Roigé, M.; Marzinelli, E.M.; Campbell, A.H.; Vergés, A.; Thomas, T.; et al. Measuring continuous compositional change using decline and decay in zeta diversity. Ecology 2019, 100, e02832. [Google Scholar] [CrossRef]
- Scheiner, S.M.; Chiarucci, A.; Fox, G.A.; Helmus, M.R.; McGlinn, D.J.; Willig, M.R. The underpinnings of the relationship of species richness with space and time. Ecol. Monogr. 2011, 81, 195–213. [Google Scholar] [CrossRef] [Green Version]
- Si, X.; Baselga, A.; Ding, P. Revealing Beta-Diversity Patterns of Breeding Bird and Lizard Communities on Inundated Land-Bridge Islands by Separating the Turnover and Nestedness Components. PLoS ONE 2015, 10, e0127692. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; ISBN 978-0-387-98140-6. [Google Scholar]
- Hidayat, M.R.; Endris, W.M. Effect of a rubber plantation on termite diversity in Melawi, West Kalimantan, Indonesia. Agric. Nat. Resour. 2018, 52, 439–444. [Google Scholar] [CrossRef]
- Neoh, K.B.; Bong, L.J.; Muhammad, A.; Itoh, M.; Kozan, O.; Takematsu, Y.; Yoshimura, T. The Impact of Tropical Peat Fire on Termite Assemblage in Sumatra, Indonesia: Reduced Complexity of Community Structure and Survival Strategies. Environ. Entomol. 2016, 45, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Eggleton, P.; Tayasu, I. Feeding groups, lifetypes and the global ecology of termites. Ecol. Res. 2001, 16, 941–960. [Google Scholar] [CrossRef]
- Tayasu, I. Use of carbon and nitrogen isotope ratios in termite research. Ecol. Res. 1998, 13, 377–387. [Google Scholar] [CrossRef]
- Beekman, H.A.J.M. Forest Damage and Protection in Indonesia; H. Veenman: Wageningen, The Netherlands, 1947. (In Dutch) [Google Scholar]
- Subyanto. Control of Neotermes Tectonae Damm. (Isoptera: Kalotermitidae) on Teak using Insecticide FASTAC 15 EC, 1st ed.; Forestry Department UGM. Pen. No. UGM/58/M/09/01; Universitas Gadjah Mada: Yogyakarta, Indonesia, 1989. (In Indonesian) [Google Scholar]
- Luke, S.H.; Fayle, T.M.; Eggleton, P.; Turner, E.C.; Davies, R.G. Functional structure of ant and termite assemblages in old growth forest, logged forest and oil palm plantation in Malaysian Borneo. Biodivers. Conserv. 2014, 23, 2817–2832. [Google Scholar] [CrossRef] [Green Version]
- Eggleton, P. Global Patterns of Termite Diversity. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 25–51. [Google Scholar]
- Brauman, A.; Bignell, D.E.; Tayasu, I. Soil-Feeding Termites: Biology, Microbial Associations and Digestive Mechanisms. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 233–259. [Google Scholar]
- Tayasu, I.; Abe, T.; Eggleton, P.; Bignell, D.E. Nitrogen and carbon isotope ratios in termites: An indicator of trophic habit along the gradient from wood-feeding to soil-feeding. Ecol. Entomol. 1997, 22, 343–351. [Google Scholar] [CrossRef]
- Aanen, D.K. As you reap, so shall you sow: Coupling of harvesting and inoculating stabilizes the mutualism between termites and fungi. Biol. Lett. 2006, 2, 209–212. [Google Scholar] [CrossRef]
- Chiu, C.; Ou, J.; Chen, C.; Li, H. Fungal nutrition allocation enhances mutualism with fungus-growing termite. Fungal Ecol. 2019, 41, 92–100. [Google Scholar] [CrossRef]
- Chiu, C.I.; Yang, M.M.; Li, H.F. Structure and function of subterranean gallery systems of soil-feeding termites Pericapritermes nitobei and Sinocapritermes mushae. Insectes Sociaux 2015, 62, 393–400. [Google Scholar] [CrossRef]
- Mackay, W.P.; Blizzard, J.H.; Miller, J.J.; Whitford, W.G. Analysis of above-ground gallery construction by the subterranean termite Gnathamitermes tubiformans (Isoptera: Termitidae). Environ. Entomol. 1985, 14, 470–474. [Google Scholar] [CrossRef]
- Prastyaningsih, S.R.; Hardiwinoto, S.; Musyafa, M.; Koranto, C.A.D. Diversity of termites (Isoptera) on industrial forest plantation of eucalyptus pellita stands of tropical ecosystem in Riau, Indonesia. Biodiversitas 2020, 21, 5498–5505. [Google Scholar] [CrossRef]
- Qian, H.; Ricklefs, R.E. A latitudinal gradient in large-scale beta diversity for vascular plants in North America. Ecol. Lett. 2007, 10, 737–744. [Google Scholar] [CrossRef]
- Liggins, L.; Booth, D.J.; Figueira, W.F.; Treml, E.A.; Tonk, L.; Ridgway, T.; Harris, D.A.; Riginos, C. Latitude-wide genetic patterns reveal historical effects and contrasting patterns of turnover and nestedness at the range peripheries of a tropical marine fish. Ecography 2015, 38, 1212–1224. [Google Scholar] [CrossRef]
- Hylander, K.; Nilsson, C.; Gunnar Jonsson, B.; Göthner, T. Differences in habitat quality explain nestedness in a land snail meta-community. Oikos 2005, 108, 351–361. [Google Scholar] [CrossRef]
- Logan, J.W.M.; Cowie, R.H.; Wood, T.G. Termite (Isoptera) control in agriculture and forestry by non-chemical methods: A review. Bull. Entomol. Res. 1990, 80, 309–330. [Google Scholar] [CrossRef]
- Wood, T.G.; Bednarzik, M.; Aden, H. Damage to crops by Microtermes najdensis (Isoptera, Macrotermitinae) in irrigated semi-desert areas of the Red Sea coast 1. The Tihama region of the Yemen Arab Republic. Trop. Pest Manag. 1987, 33, 142–150. [Google Scholar] [CrossRef]
- Nair, K.S.S.; Varma, R.V. Some ecological aspects of the termite problem in young eucalypt plantations in Kerala, India. For. Ecol. Manag. 1985, 12, 287–303. [Google Scholar] [CrossRef]
- Kirton, L.; Cheng, S. Ring-barking and root debarking of dipterocarp saplings by termites in an enrichment planting site in Malaysia. J. Trop. For. Sci. 2007, 19, 67–72. [Google Scholar]
- Pramono, A.A.; Fauzi, M.A.; Widyani, N.; Heriansyah, I.; Roshetko, J.M. Managing Smallholder Teak Plantations: Field Guide for Farmers; Center for International Forestry Research: Bogor, Indonesia, 2011; ISBN 9786028693547. [Google Scholar]
- Song, L.; Boithias, L.; Sengtaheuanghoung, O.; Oeurng, C.; Valentin, C.; Souksavath, B.; Sounyafong, P.; de Rouw, A.; Soulileuth, B.; Silvera, N.; et al. Understory limits surface runoff and soil loss in teak tree plantations of Northern Lao PDR. Water 2020, 12, 2327. [Google Scholar] [CrossRef]
- Wardell, D.A. Control of termites in nurseries and young plantations in africa: Established practices and alternative courses of action. Commonw. For. Rev. 1987, 66, 77–89. [Google Scholar]

| Damage Level | Teakwood Bark Condition |
|---|---|
| Level 1 | The trace of termite damage on the bark is observed, but neither gallery nor termites are present. |
| Level 2 | The bark has been disturbed and peeled, with a gallery observed but no termites present. |
| Level 3 | The bark has been disturbed and peeled, with both a gallery and termites present. |
| Feeding Group | Feeding Habit |
|---|---|
| I | Deadwood and grass feeders. The only group with flagellate protists in their guts |
| II | Feed on miscellaneous types of grass, dead wood, leaf litter, and micro-epiphytes |
| IIf | Feed on grass, dead wood, and leaf litter, with the help of fungal symbionts grown inside the nest (fungus-growing termites) |
| III | Feed on organically rich upper soil layers (humus feeders) |
| IV | Feed on organically impoverished soil or mineral soil (true soil feeders) |
| Family/Sub-Family | Genus | Species | Functional Group | Incidence Presence | ||
|---|---|---|---|---|---|---|
| A | B | C | ||||
| Macro-termitinae | Macrotermes | Macrotermes gilvus | IIf | ✓ | ✓ | ✓ |
| Microtermes | Microtermes insperatus | IIf | ✓ | ✓ | ✓ | |
| Odontotermes | Odontotermes grandiceps | IIf | ✓ | ✓ | ✓ | |
| Odontotermes javanicus | ✓ | ✓ | ||||
| Odontotermes karnyi | ✓ | |||||
| Odontotermes bilitoni | ✓ | |||||
| Odontotermes longignathus | ✓ | |||||
| Odontotermes sp1a | ✓ | |||||
| Miro-capritermitinae | Pericapritermes | Pericapritermes semarangi | III | ✓ | ||
| Pericapritermes mohri | ✓ | ✓ | ||||
| Pericapritermes latignathus | ✓ | |||||
| Pericapritermes Ab | ✓ | |||||
| Pericapritermes Bb | ✓ | |||||
| Species Richness | 6 | 6 | 9 | |||
| α diversity = 23/3; γ diversity = 13; β diversity = 39/23 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fajar, A.; Himmi, S.K.; Latif, A.; Tarmadi, D.; Kartika, T.; Guswenrivo, I.; Yusuf, S.; Yoshimura, T. Termite Assemblage and Damage on Tree Trunks in Fast-Growing Teak Plantations of Different Age: A Case Study in West Java, Indonesia. Insects 2021, 12, 295. https://doi.org/10.3390/insects12040295
Fajar A, Himmi SK, Latif A, Tarmadi D, Kartika T, Guswenrivo I, Yusuf S, Yoshimura T. Termite Assemblage and Damage on Tree Trunks in Fast-Growing Teak Plantations of Different Age: A Case Study in West Java, Indonesia. Insects. 2021; 12(4):295. https://doi.org/10.3390/insects12040295
Chicago/Turabian StyleFajar, Anugerah, Setiawan Khoirul Himmi, Astuti Latif, Didi Tarmadi, Titik Kartika, Ikhsan Guswenrivo, Sulaeman Yusuf, and Tsuyoshi Yoshimura. 2021. "Termite Assemblage and Damage on Tree Trunks in Fast-Growing Teak Plantations of Different Age: A Case Study in West Java, Indonesia" Insects 12, no. 4: 295. https://doi.org/10.3390/insects12040295






