Anti-Inflammatory Effect of Wasp Venom in BV-2 Microglial Cells in Comparison with Bee Venom
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of WV and BV
2.2. Cell Culture
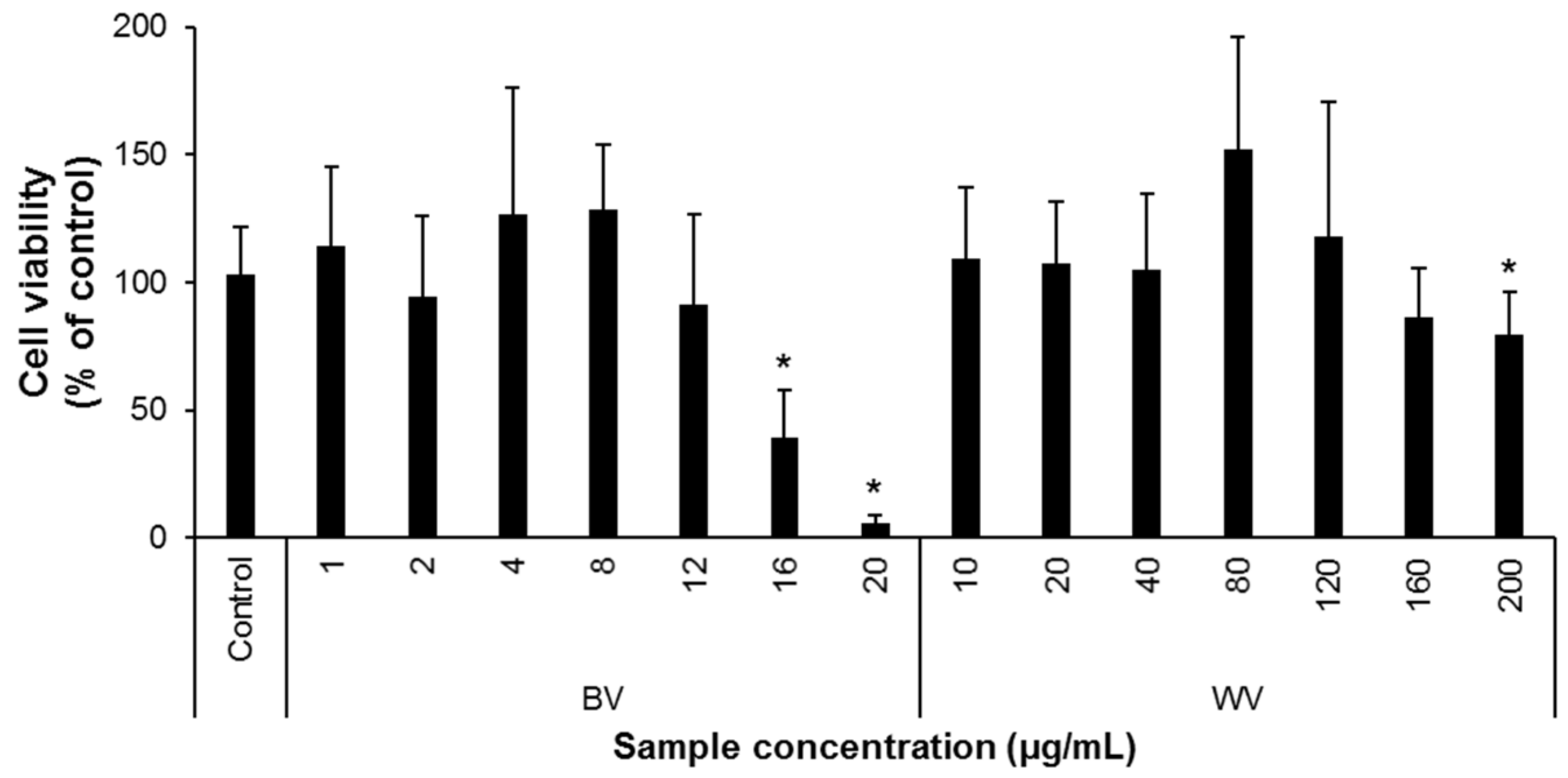
2.3. Cell Viability
2.4. Determination of Nitric Oxide (NO) and Proinflammatory Cytokine Levels in Culture Medium
2.5. Western Blot Analysis
2.6. Luciferase Reporter Assay
2.7. Statistical Analysis
3. Results and Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perrard, A.; Arca, M.; Rome, Q.; Muller, F.; Tan, J.; Bista, S.; Nugroho, H.; Baudoin, R.; Baylac, M.; Silvain, J.F.; et al. Geographic Variation of Melanisation Patterns in a Hornet Species: Genetic Differences, Climatic Pressures or Aposematic Constraints? PLoS ONE 2014, 9, e94162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinet, C.; Suppo, C.; Darrouzet, E. Rapid spread of the invasive yellow-legged hornet in France: The role of human-mediated dispersal and the effects of control measures. J. Appl. Ecol. 2017, 54, 205–215. [Google Scholar] [CrossRef]
- Budge, G.E.; Hodgetts, J.; Jones, E.P.; Ostoja-Starzewski, J.C.; Hall, J.; Tomkies, V.; Semmence, N.; Brown, M.; Wakefield, M.; Stainton, K. The invasion, provenance and diversity of Vespa velutina Lepeletier (Hymenoptera: Vespidae) in Great Britain. PLoS ONE 2017, 12, e0185172. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, J.; Okuyama, H.; Kiyoshi, T.; Takeuchi, T.; Martin, S.J. Origins of Vespa velutina hornets that recently invaded Iki Island, Japan and Jersey Island, UK. Mitochondrial. DNA Part A 2019, 30, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Laurino, D.; Lioy, S.; Carisio, L.; Manino, A.; Porporato, M. Vespa velutina: An Alien Driver of Honey Bee Colony Losses. Diversity 2020, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.P.; Conyers, C.; Tomkies, V.; Semmence, N.; Fouracre, D.; Wakefield, M.; Stainton, K. Managing incursions of Vespa velutina nigrithorax in the UK: An emerging threat to apiculture. Sci. Rep. 2020, 10, 19553. [Google Scholar] [CrossRef] [PubMed]
- Keeling, M.J.; Franklin, D.N.; Datta, S.; Brown, M.A.; Budge, G.E. Predicting the spread of the Asian hornet (Vespa velutina) following its incursion into Great Britain. Sci. Rep. 2017, 7, 6240. [Google Scholar] [CrossRef] [PubMed]
- Monceau, K.; Bonnard, O.; Moreau, J.; Thiery, D. Spatial distribution of Vespa velutina individuals hunting at domestic honeybee hives: Heterogeneity at a local scale. Insect Sci. 2014, 21, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Lioy, S.; Laurino, D.; Capello, M.; Romano, A.; Manino, A.; Porporato, M. Effectiveness and Selectiveness of Traps and Baits for Catching the Invasive Hornet Vespa velutina. Insects 2020, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Goka, K. Review of the invasive yellow-legged hornet, Vespa velutina nigrithorax (Hymenoptera: Vespidae), in Japan and its possible chemical control. Appl. Entomol. Zool. 2017, 52, 361–368. [Google Scholar] [CrossRef]
- Darrouzet, E.; Gevar, J.; Guignard, Q.; Aron, S. Production of Early Diploid Males by European Colonies of the Invasive Hornet Vespa velutina nigrithorax. PLoS ONE 2015, 10, e0136680. [Google Scholar]
- Ruiz-Cristi, I.; Berville, L.; Darrouzet, E. Characterizing thermal tolerance in the invasive yellow-legged hornet (Vespa velutina nigrithorax): The first step toward a green control method. PLoS ONE 2020, 15, e0239742. [Google Scholar]
- Habermann, E. Bee and wasp venoms. Science 1972, 177, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Giralt, E. Three Valuable Peptides from Bee and Wasp Venoms for Therapeutic and Biotechnological Use: Melittin, Apamin and Mastoparan. Toxins 2015, 7, 1126–1150. [Google Scholar] [CrossRef] [Green Version]
- Carpena, M.; Nunez-Estevez, B.; Soria-Lopez, A.; Simal-Gandara, J. Bee Venom: An Updating Review of Its Bioactive Molecules and Its Health Applications. Nutrients 2020, 12, 3360. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Baek, J.H.; Yoon, K.A. Differential Properties of Venom Peptides and Proteins in Solitary vs. Social Hunting Wasps. Toxins 2016, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.; Monge-Fuentes, V.; Gomes, F.; Lopes, K.; dos Anjos, L.; Campos, G.; Arenas, C.; Biolchi, A.; Goncalves, J.; Galante, P.; et al. Pharmacological Alternatives for the Treatment of Neurodegenerative Disorders: Wasp and Bee Venoms and Their Components as New Neuroactive Tools. Toxins 2015, 7, 3179–3209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.B.; Lee, Y.H. The structure and antimicrobial potential of wasp and hornet (Vespidae) mastoparans: A review. Entomological Res. 2020, 50, 369–376. [Google Scholar] [CrossRef]
- Herrera, C.; Leza, M.; Martinez-Lopez, E. Diversity of compounds in Vespa spp. venom and the epidemiology of its sting: A global appraisal. Arch. Toxicol. 2020, 94, 3609–3627. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Wahed, A.; Yosri, N.; Sakr, H.H.; Du, M.; Algethami, A.F.M.; Zhao, C.; Abdelazeem, A.H.; Tahir, H.E.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Wasp Venom Biochemical Components and Their Potential in Biological Applications and Nanotechnological Interventions. Toxins 2021, 13, 206. [Google Scholar] [CrossRef]
- Dongol, Y.; Dhananjaya, B.L.; Shrestha, R.K.; Aryal, G. Wasp Venom Toxins as a Potential Therapeutic Agent. Protein Pept. Lett. 2016, 23, 688–698. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, W.X.; Duan, X.M.; Ni, L.L.; Liu, H.; Zhao, H.R.; Xiao, H.; Zhang, C.G.; Yang, Z.B. Wasp Venom Possesses Potential Therapeutic Effect in Experimental Models of Rheumatoid Arthritis. Evid. Based Complement. Alternat. Med. 2020, 2020, 6394625. [Google Scholar] [CrossRef]
- Im, E.J.; Kim, S.J.; Hong, S.B.; Park, J.K.; Rhee, M.H. Anti-Inflammatory Activity of Bee Venom in BV2 Microglial Cells: Mediation of MyD88-Dependent NF-kappa B Signaling Pathway. Evid. Based Complement. Alternat. Med. 2016, 2016, 3704764. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Jang, K.M.; Park, K.K. Apamin Suppresses LPS-Induced Neuroinflammatory Responses by Regulating SK Channels and TLR4-Mediated Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4319. [Google Scholar] [CrossRef]
- Kaushik, D.K.; Thounaojam, M.C.; Mitra, A.; Basu, A. Vespa tropica venom suppresses lipopolysaccharide-mediated secretion of pro-inflammatory cyto-chemokines by abrogating nuclear factor-kappa B activation in microglia. Inflamm. Res. 2014, 63, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; Yang, H.L.; Yu, H.N.; Li, J.X.; Lai, R. The mastoparanogen from wasp. Peptides 2006, 27, 3053–3057. [Google Scholar] [CrossRef]
- Ziai, M.R.; Russek, S.; Wang, H.C.; Beer, B.; Blume, A.J. Mast cell degranulating peptide: A multi-functional neurotoxin. J. Pharm. Pharmacol. 1990, 42, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.R.; Chen, S.G.; Zhou, Y.; Xie, C.H.; Zhu, B.F.; Zhu, H.M.; Liu, S.P.; Wang, W.; Chen, H.Z.; Ji, Y.H. Deciphering the Venomic Transcriptome of Killer-Wasp Vespa velutina. Sci. Rep. 2015, 5, 9454. [Google Scholar] [CrossRef]
- Le, T.N.; Da Silva, D.; Colas, C.; Darrouzet, E.; Baril, P.; Leseurre, L.; Maunit, B. Asian hornet Vespa velutina nigrithorax venom: Evaluation and identification of the bioactive compound responsible for human keratinocyte protection against oxidative stress. Toxicon 2020, 176, 1–9. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Perry, V.H.; Nicoll, J.A.R.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201. [Google Scholar] [CrossRef]
- Gonzalez-Scarano, F.; Baltuch, G. Microglia as mediators of inflammatory and degenerative diseases. Annu. Rev. Neurosci. 1999, 22, 219–240. [Google Scholar] [CrossRef]
- Eikelenboom, P.; van Gool, W.A. Neuroinflammatory perspectives on the two faces of Alzheimer’s disease. J. Neural Transm. 2004, 111, 281–294. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [Green Version]
- Blasi, E.; Barluzzi, R.; Bocchini, V.; Mazzolla, R.; Bistoni, F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J. Neuroimmunol. 1990, 27, 229–237. [Google Scholar] [CrossRef]
- Bocchini, V.; Mazzolla, R.; Barluzzi, R.; Blasi, E.; Sick, P.; Kettenmann, H. An immortalized cell line expresses properties of activated microglial cells. J. Neurosci. Res. 1992, 31, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.; Lee, H.; Jeong, Y.S.; Shin, G.Y.; Oh, J.G.; Kim, J.S.; Oh, J. Antioxidant Potential of Selected Korean Edible Plant Extracts. Biomed. Res. Int. 2017, 2017, 7695605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Oh, J.; Jang, C.H.; Lim, J.S.; Lee, J.S.; Kim, J.S. In Vivo Anti-inflammatory Potential of Viscozyme((R))-Treated Jujube Fruit. Foods 2020, 9, 1033. [Google Scholar] [CrossRef]
- Lim, J.S.; Oh, J.; Byeon, S.; Lee, J.S.; Kim, J.S. Protective Effect of Dioscorea batatas Peel Extract Against Intestinal Inflammation. J. Med. Food 2018, 21, 1204–1217. [Google Scholar] [CrossRef]
- Byeon, S.; Oh, J.; Lim, J.S.; Lee, J.S.; Kim, J.S. Protective Effects of Dioscorea batatas Flesh and Peel Extracts against Ethanol-Induced Gastric Ulcer in Mice. Nutrients 2018, 10, 1680. [Google Scholar] [CrossRef] [Green Version]
- Woo, Y.; Oh, J.; Kim, J.S. Suppression of Nrf2 Activity by Chestnut Leaf Extract Increases Chemosensitivity of Breast Cancer Stem Cells to Paclitaxel. Nutrients 2017, 9, 760. [Google Scholar] [CrossRef]
- Moon, D.O.; Park, S.Y.; Lee, K.J.; Heo, M.S.; Kim, K.C.; Kim, M.O.; Lee, J.D.; Choi, Y.H.; Kim, G.Y. Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. Int. Immunopharmacol. 2007, 7, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Kim, S.H.; Yang, S.C.; Lee, S.M.; Choi, S.M. Melittin restores proteasome function in an animal model of ALS. J. Neuroinflamm. 2011, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Han, S.M.; Kim, J.M.; Park, K.K.; Chang, Y.C.; Pak, S.C. Neuroprotective effects of melittin on hydrogen peroxide-induced apoptotic cell death in neuroblastoma SH-SY5Y cells. BMC Complement. Altern. Med. 2014, 14, 286. [Google Scholar] [CrossRef]
- Baek, H.; Lee, C.J.; Choi, D.D.; Kim, N.S.; Kim, Y.S.; Ye, Y.J.; Kim, Y.S.; Kim, J.S.; Shim, I.; Bae, H. Bee venom phospholipase A2 ameliorates Alzheimer’s disease pathology in A beta vaccination treatment without inducing neuro-inflammation in a 3xTg-AD mouse model. Sci. Rep. 2018, 8, 17369. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia development and function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef] [Green Version]
- Wyss-Coray, T.; Mucke, L. Inflammation in neurodegenerative disease—A double-edged sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Block, M.L.; Hong, J.S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.K.; Koppula, S.; Suk, K. Inhibitors of microglial neurotoxicity: Focus on natural products. Molecules 2011, 16, 1021–1043. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.Y.; Wang, X.; Liu, C.; Zhang, H.L. Pharmacological Targeting of Microglial Activation: New Therapeutic Approach. Front. Cell. Neurosci. 2019, 13, 514. [Google Scholar] [CrossRef] [Green Version]
- Biscaro, B.; Lindvall, O.; Tesco, G.; Ekdahl, C.T.; Nitsch, R.M. Inhibition of Microglial Activation Protects Hippocampal Neurogenesis and Improves Cognitive Deficits in a Transgenic Mouse Model for Alzheimer’s Disease. Neurodegener. Dis. 2012, 9, 187–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkhondeh, T.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Folgado, S.L.; Rajabpour-Sanati, A.; Khazdair, M.R.; Samarghandian, S. Green tea catechins inhibit microglial activation which prevents the development of neurological disorders. Neural Regen. Res. 2020, 15, 1792–1798. [Google Scholar]
- Stansley, B.; Post, J.; Hensley, K. A comparative review of cell culture systems for the study of microglial biology in Alzheimer’s disease. J. Neuroinflamm. 2012, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Horvath, R.J.; Nutile-McMenemy, N.; Alkaitis, M.S.; DeLeo, J.A. Differential migration, LPS-induced cytokine, chemokine, and NO expression in immortalized BV-2 and HAPI cell lines and primary microglial cultures. J. Neurochem. 2008, 107, 557–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henn, A.; Lund, S.; Hedtjarn, M.; Schrattenholz, A.; Porzgen, P.; Leist, M. The Suitability of BV2 Cells as Alternative Model System for Primary Microglia Cultures or for Animal Experiments Examining Brain Inflammation. Altex-Alternativen Zu Tierexperimenten 2009, 26, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Kai, S.; Matsuyama, T.; Adachi, T.; Fukuda, K.; Hirota, K. General Anesthetics Inhibit LPS-Induced IL-1 beta Expression in Glial Cells. PLoS ONE 2013, 8, e82930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.M.; Pei, L.; Hu, L.S.; Pan, S.W.; Xiong, W.; Liu, M.; Wu, Y.; Shang, Y.; Yao, S.L. Death-associated protein kinase 1 mediates interleukin-1 beta production through regulating inlfammasome activation in Bv2 microglial cells and mice. Sci. Rep. 2018, 8, 9930. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hwang, S.Y.; Oh, E.S.; Oh, S.; Han, I.O. IL-1 beta, an immediate early protein secreted by activated microglia, induces iNOS/NO in C6 astrocytoma cells through p38 MAPK and NF-kappa B pathways. J. Neurosci. Res. 2006, 84, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Araujo, G.W.; Trajkovic, K.; Herrmann, M.M.; Merkler, D.; Mandelkow, E.M.; Weissert, R.; Simons, M. Hyperphosphorylation and aggregation of tau in experimental autoimmune encephalomyelitis. J. Biol. Chem. 2004, 279, 55833–55839. [Google Scholar] [CrossRef] [Green Version]
- Gotzsche, P.C.; Johansen, H.K. Short-term low-dose corticosteroids vs placebo and nonsteroidal antiinflammatory drugs in rheumatoid arthritis. Cochrane Database Syst. Rev. 2004, 2005, CD000189. [Google Scholar]
- Wyss-Coray, T. Inflammation in Alzheimer disease: Driving force, bystander or beneficial response? Nat. Med. 2006, 12, 1005–1015. [Google Scholar]
- Cho, J.Y.; Yoo, E.S.; Baik, K.U.; Park, M.H.; Han, B.H. In vitro inhibitory effect of protopanaxadiol ginsenosides on tumor necrosis factor (TNF)-alpha production and its modulation by known TNF-alpha antagonists. Planta Med. 2001, 67, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.K.; Chaduvula, M.; Atkinson, S.E.; Khanolkar-Young, S.; Jain, S.; Suneetha, L.; Suneetha, S.; Lockwood, D.N. Effects of prednisolone treatment on cytokine expression in patients with leprosy type 1 reactions. Infect. Immun. 2005, 73, 3725–3733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, H.; Shigemori, N.; Hisada, Y.; Ishizuka, T.; Kawashima, K.; Sugita, T. Suppression of NF-kappa B and AP-1 activation by glucocorticoids in experimental glomerulonephritis in rats: Molecular mechanisms of anti-nephritic action. Biochim. Biophys. Acta 1997, 1362, 252–262. [Google Scholar] [CrossRef] [Green Version]
- De Kruif, M.D.; Lemaire, L.C.; Giebelen, I.A.; van Zoelen, M.A.; Pater, J.M.; van den Pangaart, P.S.; Groot, A.P.; de Vos, A.F.; Elliott, P.J.; Meijers, J.C.; et al. Prednisolone dose-dependently influences inflammation and coagulation during human endotoxemia. J. Immunol. 2007, 178, 1845–1851. [Google Scholar] [CrossRef]
- Han, S.; Lee, K.; Yeo, J.; Kweon, H.; Woo, S.; Lee, M.; Baek, H.; Kim, S.; Park, K. Effect of honey bee venom on microglial cells nitric oxide and tumor necrosis factor-alpha production stimulated by LPS. J. Ethnopharmacol. 2007, 111, 176–181. [Google Scholar] [CrossRef]
- Rodriguez-Gomez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.R.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS Pro-Inflammatory Response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef] [PubMed]
- Catorce, M.N.; Gevorkian, G. LPS-induced Murine Neuroinflammation Model: Main Features and Suitability for Pre-clinical Assessment of Nutraceuticals. Curr. Neuropharmacol. 2016, 14, 155–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.H.; Jalabi, W.; Shpargel, K.B.; Farabaugh, K.T.; Dutta, R.; Yin, X.H.; Kidd, G.J.; Bergmann, C.C.; Stohlman, S.A.; Trapp, B.D. Lipopolysaccharide-Induced Microglial Activation and Neuroprotection against Experimental Brain Injury Is Independent of Hematogenous TLR4. J. Neurosci. 2012, 32, 11706–11715. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Dong, H.; Zhang, S.; Lu, S.; Sun, J.; Qian, Y. Enhancement of LPS-induced microglial inflammation response via TLR4 under high glucose conditions. Cell. Physiol. Biochem. 2015, 35, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Guijarro-Munoz, I.; Compte, M.; Alvarez-Cienfuegos, A.; Alvarez-Vallina, L.; Sanz, L. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-kappaB signaling pathway and proinflammatory response in human pericytes. J. Biol. Chem. 2014, 289, 2457–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zusso, M.; Lunardi, V.; Franceschini, D.; Pagetta, A.; Lo, R.; Stifani, S.; Frigo, A.C.; Giusti, P.; Moro, S. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J. Neuroinflamm. 2019, 16, 148. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, H.S.; Oh, J.; Lim, J.S.; Kim, H.J.; Kim, J.-S. Anti-Inflammatory Effect of Wasp Venom in BV-2 Microglial Cells in Comparison with Bee Venom. Insects 2021, 12, 297. https://doi.org/10.3390/insects12040297
Yun HS, Oh J, Lim JS, Kim HJ, Kim J-S. Anti-Inflammatory Effect of Wasp Venom in BV-2 Microglial Cells in Comparison with Bee Venom. Insects. 2021; 12(4):297. https://doi.org/10.3390/insects12040297
Chicago/Turabian StyleYun, Hyun Seok, Jisun Oh, Ji Sun Lim, Hyo Jung Kim, and Jong-Sang Kim. 2021. "Anti-Inflammatory Effect of Wasp Venom in BV-2 Microglial Cells in Comparison with Bee Venom" Insects 12, no. 4: 297. https://doi.org/10.3390/insects12040297
APA StyleYun, H. S., Oh, J., Lim, J. S., Kim, H. J., & Kim, J.-S. (2021). Anti-Inflammatory Effect of Wasp Venom in BV-2 Microglial Cells in Comparison with Bee Venom. Insects, 12(4), 297. https://doi.org/10.3390/insects12040297





