Post-Mortem Interval Estimation Based on Insect Evidence: Current Challenges
Abstract
:Simple Summary
Abstract
1. Introduction
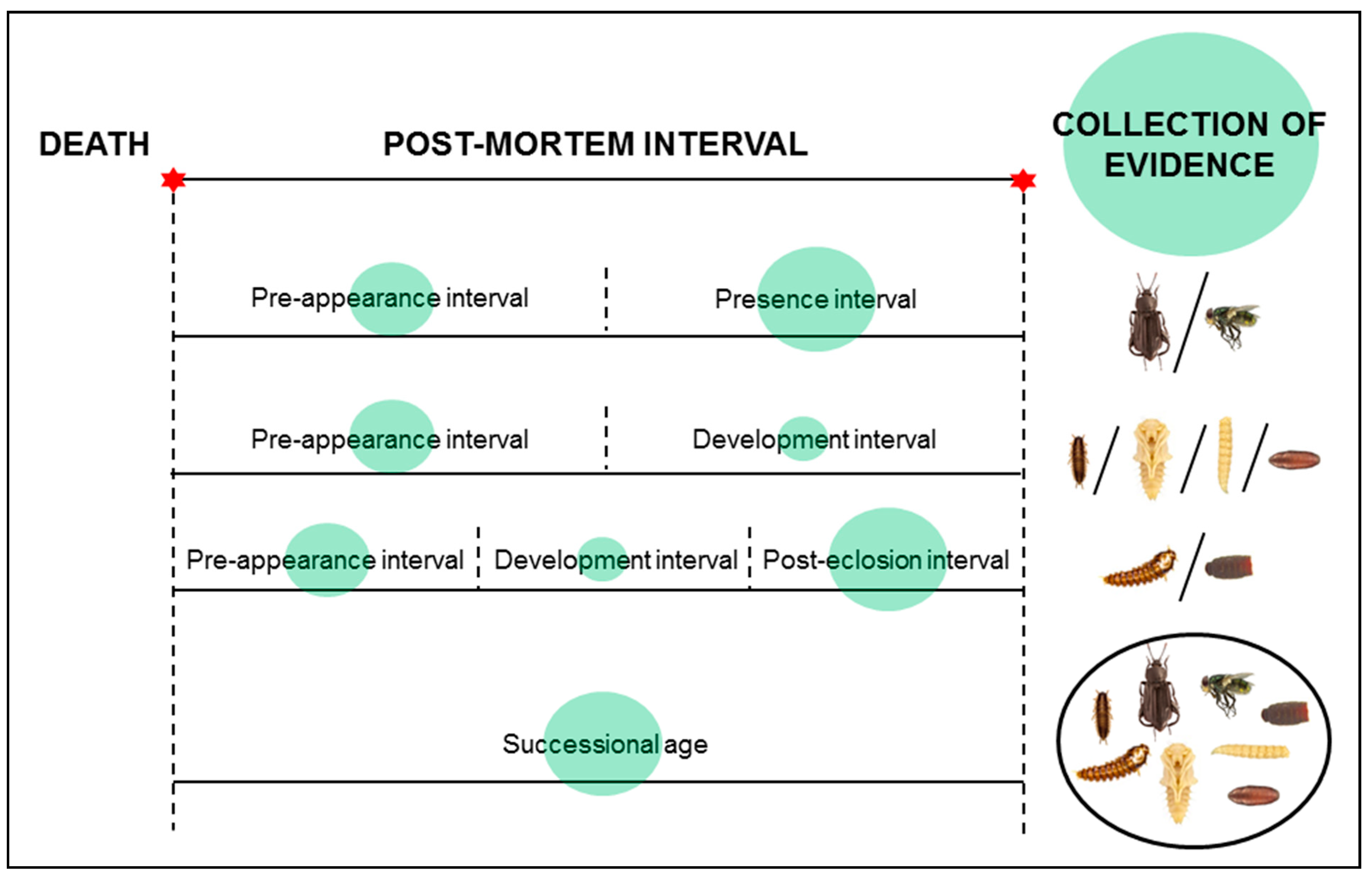
2. Collection of Insect Evidence
3. Insect Development
| Family | Species | Number of Published Datasets | Country of a Population’s Origin | References |
|---|---|---|---|---|
| Calliphoridae | Calliphora vicina | 18 | US,AT,GB,RU,CA,DE,IT,EG | [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] |
| Calliphora vomitoria | 7 | US,GB,RU,DE | [26,29,31,33,37,44,45] | |
| Chrysomya albiceps | 9 | BR,RU,AT,ZA,CO,IR,EG | [14,26,39,46,47,48,49,50,51] | |
| Lucilia caesar | - | - | - | |
| Lucilia sericata | 27 | US,FI,IT,GB,RU,CA,AT,CO,IR,EG,TR,FR,EC,KR,CN | [26,27,28,30,31,33,36,48,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70] | |
| Phormia regina | 7 | US,RU,CA,MX | [26,27,28,31,71,72,73] | |
| Protophormia terraenovae | 7 | US,GB,RU,AT,CA | [26,31,33,44,74,75,76] | |
| Sarcophagidae | Sarcophaga argyrostoma | 3 | AT,DE,TR | [29,77,78] |
| Sarcophaga caerulescens | - | - | - | |
| Muscidae | Hydrotaea dentipes | - | - | - |
| Hydrotaea ignava | - | - | - | |
| Hydrotaea pilipes | - | - | - | |
| Fanniidae | Fannia canicularis | 2 | US,PL | [79,80] |
| Fannia scalaris | - | - | - | |
| Fannia leucosticta | - | - | - | |
| Piophilidae | Stearibia nigriceps | 1 | RU | [26] |
| Silphidae | Necrodes littoralis | 2 | PL | [81,82] |
| Thanatophilus rugosus | 1 | DZ | [83] | |
| Thanatophilus sinuatus | 1 | CZ | [84] | |
| Histeridae | Margarinotus brunneus | - | - | - |
| Saprinus planiusculus | - | - | - | |
| Saprinus semistriatus | - | - | - | |
| Staphylinidae | Aleochara curtula | - | - | - |
| Creophilus maxillosus | 5 | US,CN,PL | [85,86,87,88,89] | |
| Philonthus politus | - | - | - | |
| Dermestidae | Dermestes frischii | 3 | GB,ES,IT | [90,91,92] |
| Dermestes lardarius | 2 | GB | [93,94] | |
| Dermestes murinus | - | - | - | |
| Nitidulidae | Omosita colon | 1 | CN | [95] |
| Cleridae | Necrobia rufipes | 1 | CN | [96] |
| Necrobia violacea | - | - | - | |
| Pteromalidae | Nasonia vitripennis | 5 | AT,US,AU,CN,BR | [97,98,99,100,101] |
4. Insect Succession
| Family | Species | PAI | PI—Seasonal Data (Country/Habitat/Season/Stage) | References | |
|---|---|---|---|---|---|
| Temperature Model | Seasonal Data (Country/Habitat/Season/Stage) | ||||
| Calliphoridae | Calliphora vicina | - | PL/F/S/A,L1 IT/Ou/u/Au,W/A AT/Ou/u/S,Su/A PT/Ou/u/S,Su,Au,W/A PT/Ou/u/S,Au,W/O,L1,P ES/I/S,Su,Au,W/O | PL/F/S/A,L IT/Ou/u/Au,W/A AT/Ou/u/S,Su/A PT/Ou/u/S,Su,Au,W/A PT/Ou/u/S,Au,W/E,L,P ES/I/S,Su,Au,W/E | [22,119,120,121,122] |
| Calliphora vomitoria | - | PL/F/S,Su,Au/A,L1,L3 IT/Ou/u/Au,W/A AT/Ou/u/S/A PT/Ou/u/S,Su,Au,W/A PT/Ou/u/S,W/O,L1,P ES/I/S/O | PL/F/S,Su,Au/A,L IT/Ou/u/Au,W/A AT/Ou/u/S/A PT/Ou/u/S,Su,Au,W/A PT/Ou/u/S,W/E,L,P ES/I/S/E | [22,25,119,120,121,122,123] | |
| Chrysomya albiceps | - | IT/Ou/u/Su,Au/A AT/Ou/u/Su/A PT/Ou/u/Su,Au/A PT/Ou/u/Su,Au/O,L1,P ES/I/S,Su,Au/O | IT/Ou/u/Su,Au/A AT/Ou/u/Su/A PT/Ou/u/Su,Au/A PT/Ou/u/Su,Au/E,L,P ES/I/S,Su,Au/E | [22,120,121,122] | |
| Lucilia caesar | - | PL/Ou/r/S,Su/A,L3 PL/F/S,Su,Au/A,L1,L3 IT/Ou/u/Su,Au,W/A PT/Ou/u/S,Su,Au/A PT/Ou/u/S,Su,Au/O,L1,P | PL/F/S,Su,Au/A,L IT/Ou/u/Su,Au,W/A PT/Ou/u/S,Su,Au/A PT/Ou/u/S,Su,Au/E,L,P | [21,25,119,120,121,123] | |
| Lucilia sericata | - | PL/Ou/r/S,Su/A IT/Ou/u/Su,Au,W/A PT/Ou/u/S,Su,Au/A PT/Ou/u/Au/O,L1,P ES/I/S,Su,Au/O | IT/Ou/u/Su,Au,W/A PT/Ou/u/S,Su,Au/A PT/Ou/u/Au/E,L,P ES/I/S,Su,Au/E | [21,120,121,122] | |
| Phormia regina | A | PL/Ou/r/S,Su/A PL/F/S,Su,Au/A,L1 AT/Ou/u/S,Su/A PL/F/S,Su/L3 | PL/F/S,Su,Au/A,L AT/Ou/u/S,Su/A | [21,22,25,107,119,123] | |
| Protophormia terraenovae | - | AT/Ou/u/S,Su/A | AT/Ou/u/S,Su/A | [22] | |
| Sarcophagidae | Sarcophaga argyrostoma | - | - | - | - |
| Sarcophaga caerulescens | - | - | - | - | |
| Muscidae | Hydrotaea dentipes | A | PL/F/S,Su,Au/A PT/Ou/u/S/A PL/F/S,Su/L1 | PL/F/S,Su,Au/A PT/Ou/u/S/A PL/F/S,Su/L | [25,107,119,121,123] |
| Hydrotaea ignava | A | PL/Ou/r/S,Su/A,L3 PL/F/S,Su,Au/A PT/Ou/u/S,Su,Au/A PL/F/S,Su/L1 PL/F/Su/L3 | PL/F/S,Su,Au/A PT/Ou/u/S,Su,Au/A PL/F/S,Su/L | [21,25,107,119,121] | |
| Hydrotaea pilipes | - | PL/Ou/r/S,Su/A PL/F/S,Su,Au/A | PL/F/Su,Au/A | [21,25,123] | |
| Fanniidae | Fannia canicularis | - | IT/Ou/u/Au,W/A | IT/Ou/u/Au,W/A | [120] |
| Fannia scalaris | - | - | - | - | |
| Fannia leucosticta | - | - | - | - | |
| Piophilidae | Stearibia nigriceps | A,O | PL/Ou/r/S,Su/A,L3 PL/F/S,Su,Au/A,L1 IT/Ou/u/Au/A PT/Ou/u/S,Su,Au/A PT/Ou/u/Su/E PT/Ou/u/S,Su,Au/L1,P PL/F/S,Su/L3 | PL/F/S,Su,Au/A,L IT/Ou/u/Au/A PT/Ou/u/S,Su,Au/A PT/Ou/u/Su/E PT/Ou/u/S,Su,Au/L,P | [21,25,107,119,120,121,123] |
| Silphidae | Necrodes littoralis | A,L1 | PL/Ou/r/S,Su/A,L1 PL/F/S,Su,Au/A,L1 | PL/F/S,Su,Au/A,L | [21,25,103,119,123,124] |
| Thanatophilus rugosus | - | PL/F/S,Su,Au/A IT/F/W/A PL/Ou/r/S/L3 | PL/F/Su,Au/A IT/F/W/A PL/Ou/r/S/L3 | [25,120,123,125] | |
| Thanatophilus sinuatus | A,L1 | PL/Ou/r/S,Su/a PL/F/S,Su,Au/A IT/Ou/u/W/A PT/Ou/u/S,Au,W/A PL/Ou/r/S/L3 | PL/F/S,Su,Au/A IT/Ou/u/W/A PT/Ou/u/S,Au,W/A PL/Ou/r/S/L3 | [21,25,119,120,124,125,126] | |
| Histeridae | Margarinotus brunneus | A | PL/Ou/r/S,Su/A PL/F/S,Su/A PT/Ou/u/S,Su,Au,W/A | PL/F/S,Su/A PT/Ou/u/S,Su,Au,W/A | [21,25,119,124,126] |
| Saprinus planiusculus | A | PL/F/S/A | PL/F/S/A | [119,124] | |
| Saprinus semistriatus | A | PL/Ou/r/S,Su/A PL/F/S,Su,Au/A | PL/F/S,Su,Au/A | [21,25,119,123,124] | |
| Staphylinidae | Aleochara curtula | - | PL/F/S,Su/A IT/Ou/u/W/A | IT/Ou/u/W/A | [25,120] |
| Creophilus maxillosus | A,L1 | PL/Ou/r/S,Su/A,L1 PL/F/S,Su,Au/A,L1 IT/Ou/u/Au,W/A PT/Ou/u/S,Su,Au,W/A | PL/F/S,Su,Au/A,L IT/Ou/u/Au,W/A PT/Ou/u/S,Su,Au,W/A | [21,25,119,120,123,124,126,127] | |
| Philonthus politus | A | PL/F/S,Su,Au/A IT/Ou/u/Au/A | IT/Ou/u/Au/A | [25,120,124] | |
| Dermestidae | Dermestes frischii | - | PL/Ou/r/S,Su/A,L1 PT/Ou/u/S,Su,Au/A | PT/Ou/u/S,Su,Au/A | [21,126] |
| Dermestes lardarius | - | - | - | - | |
| Dermestes murinus | - | PL/F/S,Su,Au/A PL/F/S/Lm | PL/F/Su,Au/A | [25,123] | |
| Nitidulidae | Omosita colon | - | - | - | - |
| Cleridae | Necrobia rufipes | A | IT/Ou/u/Su,W/A PT/Ou/u/Su,Au/A | IT/Ou/u/Su,W/A PT/Ou/u/Su,Au/A | [120,124,126] |
| Necrobia violacea | A | PL/Ou/r/S,Su/A PL/F/S,Su/A PT/Ou/u/S,Su,Au,W/A PL/F/S/L3 | PT/Ou/u/S,Su,Au,W/A PL/F/S/L3 | [21,25,119,124,126] | |
| Pteromalidae | Nasonia vitripennis | - | AT/Ou/u/S/A | AT/Ou/u/S/A | [22] |
5. Temperature Conditions
6. Challenging Evidence
7. Validation of the PMI Estimation Protocols
| Type of the Validation | Aims | Development-Based Protocols | Succession-Based Protocols | ||
|---|---|---|---|---|---|
| Number of Studies | References | Number of Studies | References | ||
| Proof-of-assumptions study 1 | Testing validity of the assumptions that are at the root of the protocol | 26 | [14,17,18,19,27,29,30,34,41,55,56,57,61,144,145,176,187,188,189,190,191,192,193,194,195,196] | 56 | [20,21,24,25,103,107,113,119,122,124,127,128,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240] |
| Proof-of-concept study 1 | Testing validity of the protocol as used in a simplified setting | 12 | [30,73,81,82,87,88,89,241,242,243,244,245] | 3 | [103,127,128] |
| Experimental validation with non-human cadavers | Testing validity of the protocol as used for non-human cadavers in an experimental setting | 6 | [109,241,246,247,248,249] | 6 | [105,106,183,249,250,251] |
| Experimental validation using human cadavers | Testing validity of the protocol as used for human cadavers in an experimental setting | 0 | 1 | [183] | |
| Validation using casework data 1 | Testing validity of the protocol as used in forensic casework | 7 | [46,118,182,185,252,253,254] | 6 | [5,108,118,186,255,256] |
| Reference | N | True PMI 1 (days) | Difference True-Estimated PMI 2 (days) | Error I 3 (%) | Error II 4 (%) | Remarks | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | Mean | Range | |||
| Goff et al., 1988 [252] | 2 | 5.5 | 5–6 | 0.375 | 0.25–0.5 | 6.65 | 5–8.3 | 6.95 | 4.8–9.1 | - |
| Kashyap, Pillay, 1989 [185] | 16 | 4.9 | 0.5–9 | 0.438 | 0–1 | 13.74 | 0–50 | 11.65 | 0–33.3 | No mention of temperature data |
| Grassberger et al., 2003 [46] | 1 | 17 | - | 3 | - | 17.65 | - | 20 | - | - |
| Reibe et al., 2010 [182] | 1 | 4 | - | 0.125 | - | 3.125 | - | 3.03 | - | - |
| Pohjoismäki et al., 2010 [253] | 7 | 10.6 | 5–19 | 4.57 | 2.5–7 | 48.96 | 21.1–83.3 | 144.2 | 26.7–500 | Single average temperature assumed in all cases (24 °C) |
| Bugelli et al., 2015 [254] | 4 | 4.0 | 2–6 | 0.94 | 0.5–1.5 | 23.75 | 20–25 | 31.25 | 25–33 | - |
| Reference | N | True PMI 1 (days) | Estimated PMI (days) | Difference True-Estimated PMI 2 (days) | Error I 3 (%) | Error II 4 (%) | Remarks |
|---|---|---|---|---|---|---|---|
| Goff et al., 1986 [256] | 1 | 20 | 19–20 | 0.5 | 2.5 | 2.6 | - |
| Goff and Odom, 1987 [186] | 1 | 53 | ≥52 | 1 | 1.9 | 1.9 | - |
| Goff and Flynn, 1991 [255] | 1 | 38 | 34–39 | 2.5 | 6.6 | 6.8 | - |
| Schoenly et al., 1996 [118] | 2 | 11 | 10.5–11 | 0.25 | 2.3 | 2.3 | - |
| 36 | 34–36 | 1 | 2.8 | 2.9 | |||
| Archer, 2014 [108] | 1 | 21 | 16–34 | 9 | 42.9 | 36 | - |
| Matuszewski and Mądra-Bielewicz, 2019 [5] | 1 | 72 | 30–64 | 25 | 34.7 | 53.2 | Less reliable true PMI |
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Villet, M.H.; Amendt, J. Advances in entomological methods for death time estimation. In Forensic Pathology Reviews; Turk, E.E., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 213–237. [Google Scholar]
- Catts, E.P.; Goff, M.L. Forensic entomology in criminal investigations. Annu. Rev. Entomol. 1992, 37, 253–272. [Google Scholar] [CrossRef]
- Lei, G.; Liu, F.; Liu, P.; Zhou, Y.; Jiao, T.; Dang, Y.-H. A bibliometric analysis of forensic entomology trends and perspectives worldwide over the last two decades (1998–2017). Forensic Sci. Int. 2019, 295, 72–82. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Benbow, M.E. Forensic Entomology: International Dimensions and Frontiers; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Matuszewski, S.; Mądra-Bielewicz, A. Post-mortem interval estimation based on insect evidence in a quasi-indoor habitat. Sci. Justice 2019, 59, 109–115. [Google Scholar] [CrossRef]
- Sanford, M.R.; Pechal, J.L.; Tomberlin, J.K. Rehydration of forensically important larval Diptera specimens. J. Med. Entomol. 2011, 48, 118–125. [Google Scholar] [CrossRef]
- Mądra-Bielewicz, A.; Frątczak-Łagiewska, K.; Matuszewski, S. Blowfly puparia in a hermetic container: Survival under decreasing oxygen conditions. Forensic Sci. Med. Pathol. 2017, 13, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Sanford, M.R.; Byrd, J.H.; Tomberlin, J.K.; Wallace, J.R. Entomological evidence collection methods: American Board of Forensic Entomology approved protocols. In Forensic Entomology Utility of Arthropods in Legal Investigations; Byrd, J.H., Tomberlin, J.K., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 63–86. [Google Scholar]
- Lord, W.D.; Burger, J.F. Collection and preservation of forensically important entomological materials. J. Forensic Sci. 1983, 28, 936–944. [Google Scholar] [CrossRef]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; LeBlanc, H.N.; Hall, M.J. Best practice in forensic entomology—Standards and guidelines. Int. J. Leg. Med. 2007, 121, 90–104. [Google Scholar] [CrossRef]
- Bajerlein, D.; Taberski, D.; Matuszewski, S. Estimation of postmortem interval (PMI) based on empty puparia of Phormia regina (Meigen) (Diptera: Calliphoridae) and third larval stage of Necrodes littoralis (L.) (Coleoptera: Silphidae)—Advantages of using different PMI indicators. J. Forensic Leg. Med. 2018, 55, 95–98. [Google Scholar] [CrossRef]
- Amendt, J.; Richards, C.S.; Campobasso, C.P.; Zehner, R.; Hall, M.J. Forensic entomology: Applications and limitations. Forensic Sci. Med. Pathol. 2011, 7, 379–392. [Google Scholar] [CrossRef]
- Greenberg, B.; Kunich, J.C. Entomology and the Law: Flies as Forensic Indicators; Cambridge University Press: Cambridge, UK, 2002; p. 306. [Google Scholar]
- Richards, C.S.; Paterson, I.D.; Villet, M.H. Estimating the age of immature Chrysomya albiceps (Diptera: Calliphoridae), correcting for temperature and geographical latitude. Int. J. Leg. Med. 2008, 122, 271–279. [Google Scholar] [CrossRef]
- Owings, C.G.; Spiegelman, C.; Tarone, A.M.; Tomberlin, J.K. Developmental variation among Cochliomyia macellaria Fabricius (Diptera: Calliphoridae) populations from three ecoregions of Texas, USA. Int. J. Leg. Med. 2014, 128, 709–717. [Google Scholar] [CrossRef]
- Midgley, J.M.; Villet, M.H. Effect of the killing method on post-mortem change in length of larvae of Thanatophilus micans (Fabricius 1794) (Coleoptera: Silphidae) stored in 70% ethanol. Int. J. Leg. Med. 2009, 123, 103–108. [Google Scholar] [CrossRef]
- Richards, C.S.; Villet, M.H. Factors affecting accuracy and precision of thermal summation models of insect development used to estimate post-mortem intervals. Int. J. Leg. Med. 2008, 122, 401–408. [Google Scholar] [CrossRef]
- Richards, C.S.; Villet, M.H. Data quality in thermal summation development models for forensically important blowflies. Med. Vet. Entomol. 2009, 23, 269–276. [Google Scholar] [CrossRef]
- Frątczak-Łagiewska, K.; Matuszewski, S. The quality of developmental reference data in forensic entomology: Detrimental effects of multiple, in vivo measurements in Creophilus maxillosus L.(Coleoptera: Staphylinidae). Forensic Sci. Int. 2019, 298, 316–322. [Google Scholar] [CrossRef]
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. Insect succession and carrion decomposition in selected forests of Central Europe. Part 2: Composition and residency patterns of carrion fauna. Forensic Sci. Int. 2010, 195, 42–51. [Google Scholar] [CrossRef]
- Matuszewski, S.; Frątczak, K.; Konwerski, S.; Bajerlein, D.; Szpila, K.; Jarmusz, M.; Szafałowicz, M.; Grzywacz, A.; Mądra, A. Effect of body mass and clothing on carrion entomofauna. Int. J. Leg. Med. 2016, 130, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Grassberger, M.; Frank, C. Initial study of arthropod succession on pig carrion in a central European urban habitat. J. Med. Entomol. 2004, 41, 511–523. [Google Scholar] [CrossRef]
- Anton, E.; Niederegger, S.; Beutel, R.G. Beetles and flies collected on pig carrion in an experimental setting in Thuringia and their forensic implications. Med. Vet. Entomol. 2011, 25, 353–364. [Google Scholar] [CrossRef]
- Feddern, N.; Mitchell, E.A.; Amendt, J.; Szelecz, I.; Seppey, C.V. Decomposition and insect colonization patterns of pig cadavers lying on forest soil and suspended above ground. Forensic Sci. Med. Pathol. 2019, 15, 342–351. [Google Scholar] [CrossRef]
- Jarmusz, M.; Grzywacz, A.; Bajerlein, D. A comparative study of the entomofauna (Coleoptera, Diptera) associated with hanging and ground pig carcasses in a forest habitat of Poland. Forensic Sci. Int. 2020, 309, 110212. [Google Scholar] [CrossRef] [PubMed]
- Marchenko, M.I. Medicolegal relevance of cadaver entomofauna for the determination of the time of death. Forensic Sci. Int. 2001, 120, 89–109. [Google Scholar] [CrossRef]
- Greenberg, B. Flies as forensic indicators. J. Med. Entomol. 1991, 28, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.S. Minimum and maximum development rates of some forensically important Calliphoridae (Diptera). J. Forensic Sci. 2000, 45, 824–832. [Google Scholar] [CrossRef]
- Niederegger, S.; Pastuschek, J.; Mall, G. Preliminary studies of the influence of fluctuating temperatures on the development of various forensically relevant flies. Forensic Sci. Int. 2010, 199, 72–78. [Google Scholar] [CrossRef]
- Martín-Vega, D.; Simonsen, T.J.; Wicklein, M.; Hall, M.J. Age estimation during the blow fly intra-puparial period: A qualitative and quantitative approach using micro-computed tomography. Int. J. Leg. Med. 2017, 131, 1429–1448. [Google Scholar] [CrossRef] [Green Version]
- Kamal, A.S. Comparative study of thirteen species of sarcosaprophagous Calliphoridae and Sarcophagidae (Diptera) I. Bionomics. Ann. Entomol. Soc. Am. 1958, 51, 261–271. [Google Scholar] [CrossRef]
- Donovan, S.; Hall, M.; Turner, B.; Moncrieff, C. Larval growth rates of the blowfly, Calliphora vicina, over a range of temperatures. Med. Vet. Entomol. 2006, 20, 106–114. [Google Scholar] [CrossRef]
- Davies, L.; Ratcliffe, G. Development rates of some pre-adult stages in blowflies with reference to low temperatures. Med. Vet. Entomol. 1994, 8, 245–254. [Google Scholar] [CrossRef]
- Kaneshrajah, G.; Turner, B. Calliphora vicina larvae grow at different rates on different body tissues. Int. J. Leg. Med. 2004, 118, 242–244. [Google Scholar] [CrossRef]
- Brown, K.; Thorne, A.; Harvey, M. Calliphora vicina (Diptera: Calliphoridae) pupae: A timeline of external morphological development and a new age and PMI estimation tool. Int. J. Leg. Med. 2015, 129, 835–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, K.; Harvey, M.L. Internal Morphological Analysis for Age Estimation of Blow Fly Pupae (Diptera: Calliphoridae) in Postmortem Interval Estimation. J. Forensic Sci. 2013, 58, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Niederegger, S.; Wartenberg, N.; Spiess, R.; Mall, G. Influence of food substrates on the development of the blowflies Calliphora vicina and Calliphora vomitoria (Diptera, Calliphoridae). Parasitol. Res. 2013, 112, 2847–2853. [Google Scholar] [CrossRef] [PubMed]
- Baqué, M.; Filmann, N.; Verhoff, M.A.; Amendt, J. Establishment of developmental charts for the larvae of the blow fly Calliphora vicina using quantile regression. Forensic Sci. Int. 2015, 248, 1–9. [Google Scholar] [CrossRef]
- Salimi, M.; Rassi, Y.; Oshaghi, M.; Chatrabgoun, O.; Limoee, M.; Rafizadeh, S. Temperature requirements for the growth of immature stages of blowflies species, Chrysomya albiceps and Calliphora vicina, (Diptera: Calliphoridae) under laboratory conditions. Egypt. J. Forensic Sci. 2018, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Defilippo, F.; Bonilauri, P.; Dottori, M. Effect of Temperature on Six Different Developmental Landmarks within the Pupal Stage of the Forensically Important Blowfly Calliphora vicina (Robineau-Desvoidy) (Diptera: Calliphoridae). J. Forensic Sci. 2013, 58, 1554–1557. [Google Scholar] [CrossRef]
- Reiter, C. Zum wachstumsverhalten der maden der blauen schmeißfliege Calliphora vicina. Zeitschrift für Rechtsmedizin 1984, 91, 295–308. [Google Scholar] [CrossRef]
- Hwang, C.; Turner, B. Small-scaled geographical variation in life-history traits of the blowfly Calliphora vicina between rural and urban populations. Entomol. Exp. Appl. 2009, 132, 218–224. [Google Scholar] [CrossRef]
- Limsopatham, K.; Hall, M.J.; Zehner, R.; Zajac, B.K.; Verhoff, M.A.; Sontigun, N.; Sukontason, K.; Sukontason, K.L.; Amendt, J. A molecular, morphological, and physiological comparison of English and German populations of Calliphora vicina (Diptera: Calliphoridae). PLoS ONE 2018, 13, e0207188. [Google Scholar] [CrossRef]
- Greenberg, B.; Tantawi, T.I. Different developmental strategies in two boreal blow flies (Diptera: Calliphoridae). J. Med. Entomol. 1993, 30, 481–484. [Google Scholar] [CrossRef]
- Ireland, S.; Turner, B. The effects of larval crowding and food type on the size and development of the blowfly, Calliphora vomitoria. Forensic Sci. Int. 2006, 159, 175–181. [Google Scholar] [CrossRef]
- Grassberger, M.; Friedrich, E.; Reiter, C. The blowfly Chrysomya albiceps (Wiedemann)(Diptera: Calliphoridae) as a new forensic indicator in Central Europe. Int. J. Leg. Med. 2003, 117, 75–81. [Google Scholar] [CrossRef]
- Vélez, M.C.; Wolff, M. Rearing five species of Diptera (Calliphoridae) of forensic importance in Colombia in semicontrolled field conditions. Papéis Avulsos de Zoologia 2008, 48, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Shiravi, A.; Mostafavi, R.; Akbarzadeh, K.; Oshaghi, M.A. Temperature requirements of some common forensically important blow and flesh flies (Diptera) under laboratory conditions. Iran. J. Arthropod-Borne Dis. 2011, 5, 54. [Google Scholar]
- Beuter, L.; Mendes, J. Development of Chrysomya albiceps (Wiedemann)(Diptera: Calliphoridae) in different pig tissues. Neotrop. Entomol. 2013, 42, 426–430. [Google Scholar] [CrossRef]
- Rashed, S.S.; Yamany, A.S.; El-Basheir, Z.M.; Zaher, E.E. Influence of fluctuated room conditions on the development of the forensically important Chrysomya albiceps (Wiedemann)(Diptera: Calliphoridae). J. Adv. Med. Med. Res. 2015, 5, 1413–1421. [Google Scholar] [CrossRef]
- Queiroz, M.M.d.C. Temperature requirements of Chrysomya albiceps (Wiedemann, 1819)(Diptera, Calliphoridae) under laboratory conditions. Memórias do Instituto Oswaldo Cruz 1996, 91, 785–788. [Google Scholar] [CrossRef] [Green Version]
- Grassberger, M.; Reiter, C. Effect of temperature on Lucilia sericata (Diptera: Calliphoridae) development with special reference to the isomegalen-and isomorphen-diagram. Forensic Sci. Int. 2001, 120, 32–36. [Google Scholar] [CrossRef]
- Tarone, A.; Picard, C.; Spiegelman, C.; Foran, D. Population and temperature effects on Lucilia sericata (Diptera: Calliphoridae) body size and minimum development time. J. Med. Entomol. 2011, 48, 1062–1068. [Google Scholar] [CrossRef]
- Tarone, A.M.; Foran, D.R. Components of developmental plasticity in a Michigan population of Lucilia sericata (Diptera: Calliphoridae). J. Med. Entomol. 2006, 43, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Picard, C.J.; Deblois, K.; Tovar, F.; Bradley, J.L.; Johnston, J.S.; Tarone, A.M. Increasing precision in development-based postmortem interval estimates: What’s sex got to do with it? J. Med. Entomol. 2013, 50, 425–431. [Google Scholar] [CrossRef]
- Introna, F.; Altamura, B.M.; Dell’Erba, A.; Dattoli, V. Time since death definition by experimental reproduction of Lucilia sericata cycles in growth cabinet. J. Forensic Sci. 1989, 34, 478–480. [Google Scholar] [CrossRef]
- Gallagher, M.B.; Sandhu, S.; Kimsey, R. Variation in Developmental Time for Geographically Distinct Populations of the Common Green Bottle Fly, Lucilia sericata (Meigen). J. Forensic Sci. 2010, 55, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Nuorteva, P. Sarcosaprophagous insects as forensic indicators. In Forensic Medicine: A Study in Trauma Environmental Hazards; Tedeschi, C.G., Eckert, W.G., Tedeschi, L.G., Eds.; W.B. Saunders Co.: Philadelphia, PA, USA, 1977; pp. 1072–1095. [Google Scholar]
- Ash, N.; Greenberg, B. Developmental temperature responses of the sibling species Phaenicia sericata and Phaenicia pallescens. Ann. Entomol. Soc. Am. 1975, 68, 197–200. [Google Scholar] [CrossRef]
- Wall, R.; French, N.; Morgan, K. Effects of temperature on the development and abundance of the sheep blowfly Lucilia sericata(Diptera: Calliphoridae). Bull. Entomol. Res. 1992, 82, 125–131. [Google Scholar] [CrossRef]
- Clark, K.; Evans, L.; Wall, R. Growth rates of the blowfly, Lucilia sericata, on different body tissues. Forensic Sci. Int. 2006, 156, 145–149. [Google Scholar] [CrossRef]
- Rueda, L.C.; Ortega, L.G.; Segura, N.A.; Acero, V.M.; Bello, F. Lucilia sericata strain from Colombia: Experimental colonization, life tables and evaluation of two artifcial diets of the blowfy Lucilia sericata (Meigen)(Diptera: Calliphoridae), Bogotá, Colombia Strain. Biol. Res. 2010, 43, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Roe, A.; Higley, L.G. Development modeling of Lucilia sericata (Diptera: Calliphoridae). PeerJ 2015, 3, e803. [Google Scholar] [CrossRef] [Green Version]
- El-Moaty, Z.A.; Abd Elmoneim, M.K. Developmental variation of the blow fly Lucilia sericata (Meigen, 1826)(Diptera: Calliphoridae) by different substrate tissue types. J. Asia-Pac. Entomol. 2013, 16, 297–300. [Google Scholar] [CrossRef]
- Karabey, T.; Sert, O. The analysis of pupal development period in Lucilia sericata (Diptera: Calliphoridae) forensically important insect. Int. J. Leg. Med. 2018, 132, 1185–1196. [Google Scholar] [CrossRef]
- Aubernon, C.; Charabidzé, D.; Devigne, C.; Delannoy, Y.; Gosset, D. Experimental study of Lucilia sericata (Diptera Calliphoridae) larval development on rat cadavers: Effects of climate and chemical contamination. Forensic Sci. Int. 2015, 253, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Cervantès, L.; Dourel, L.; Gaudry, E.; Pasquerault, T.; Vincent, B. Effect of low temperature in the development cycle of Lucilia sericata (Meigen)(Diptera, Calliphoridae): Implications for the minimum postmortem interval estimation. Forensic Sci. Res. 2018, 3, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruna, W.; Guarderas, P.; Donoso, D.A.; Barragán, Á. Life cycle of Lucilia sericata (Meigen 1826) collected from Andean mountains. Neotrop. Biodivers. 2019, 5, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-C.; Kim, S.-J.; Yun, J.-E.; Jo, T.-H.; Choi, B.-R.; Park, C.-G. Development of the greenbottle blowfly, Lucilia sericata, under different temperatures. Korean J. Appl. Entomol. 2007, 46, 141–145. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Hu, G.; Wang, Y.; Xu, W.; Wu, M.; Wang, J. Development of Lucilia sericata (Diptera: Calliphoridae) Under Constant Temperatures and its Significance for the Estimation of Time of Death. J. Med. Entomol. 2020, 57, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.H.; Allen, J.C. The development of the black blow fly, Phormia regina (Meigen). Forensic Sci. Int. 2001, 120, 79–88. [Google Scholar] [CrossRef]
- Nabity, P.; Higley, L.G.; Heng-Moss, T.M. Effects of temperature on development of Phormia regina (Diptera: Calliphoridae) and use of developmental data in determining time intervals in forensic entomology. J. Med. Entomol. 2006, 43, 1276–1286. [Google Scholar] [CrossRef]
- Nunez-Vazquez, C.; Tomberlin, J.K.; Cantu-Sifuentes, M.; Garcia-Martinez, O. Laboratory development and field validation of Phormia regina (Diptera: Calliphoridae). J. Med. Entomol. 2013, 50, 252–260. [Google Scholar] [CrossRef]
- Grassberger, M.; Reiter, C. Effect of temperature on development of the forensically important holarctic blow fly Protophormia terraenovae (Robineau-Desvoidy)(Diptera: Calliphoridae). Forensic Sci. Int. 2002, 128, 177–182. [Google Scholar] [CrossRef]
- Warren, J.A.; Anderson, G.S. The development of Protophormia terraenovae (Robineau-Desvoidy) at constant temperatures and its minimum temperature threshold. Forensic Sci. Int. 2013, 233, 374–379. [Google Scholar] [CrossRef]
- Clarkson, C.; Hobischak, N.; Anderson, G. A comparison of the development rate of Protophormia terraenovae (Robineau-Desvoidy) raised under constant and fluctuating temperature regimes. Can. Soc. Forensic Sci. J. 2004, 37, 95–101. [Google Scholar] [CrossRef]
- Grassberger, M.; Reiter, C. Effect of temperature on development of Liopygia (= Sarcophaga) argyrostoma (Robineau-Desvoidy) (Diptera: Sarcophagidae) and its forensic implications. J. Forensic Sci. 2002, 47, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Sert, O.; Örsel, G.M.; Şabanoğlu, B.; Özdemir, S. A Study of the pupal developments of Sarcophaga argyrostoma (Robineau-Desvoidy, 1830). Forensic Sci. Med. Pathol. 2020, 16, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Grzywacz, A. Thermal requirements for the development of immature stages of Fannia canicularis (Linnaeus)(Diptera: Fanniidae). Forensic Sci. Int. 2019, 297, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.A.; Mullens, B.A. Development of immature Fannia spp. (Diptera: Muscidae) at constant laboratory temperatures. J. Med. Entomol. 1988, 25, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, J.; Matuszewski, S. Estimation of physiological age at emergence based on traits of the forensically useful adult carrion beetle Necrodes littoralis L.(Silphidae). Forensic Sci. Int. 2020, 314, 110407. [Google Scholar] [CrossRef] [PubMed]
- Novák, M.; Frątczak-Łagiewska, K.; Mądra-Bielewicz, A.; Matuszewski, S. Eye-background contrast as a quantitative marker for pupal age in a forensically important carrion beetle Necrodes littoralis L. (Silphidae). Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Guerroudj, F.Z.; Berchi, S. Effect of temperature on the development of carrion beetle Silpha rugosa (Linnaeus, 1758) (Coleoptera: Silphidae) in Algeria. J. Entomol. Zool. Stud. 2016, 4, 920–922. [Google Scholar]
- Montoya-Molina, S.; Jakubec, P.; Qubaiová, J.; Novák, M.; Šuláková, H.; Růžička, J. Developmental Models of the Forensically Important Carrion Beetle, Thanatophilus sinuatus (Coleoptera: Silphidae). J. Med. Entomol. 2020, in press. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.B.; Wang, J.F.; Li, L.L.; Wang, M.; Yang, L.J.; Tao, L.Y.; Chu, J.; Hou, Y.D. Development of the forensically important beetle Creophilus maxillosus (Coleoptera: Staphylinidae) at constant temperatures. J. Med. Entomol. 2017, 54, 281–289. [Google Scholar] [CrossRef]
- Watson-Horzelski, E.J. Survival and time of development for Creophilus maxillosus (L.) (Coleoptera: Staphylinidae) at three constant temperatures. Coleopt. Bull. 2012, 66, 365–370. [Google Scholar] [CrossRef]
- Frątczak-Łagiewska, K.; Grzywacz, A.; Matuszewski, S. Development and validation of forensically useful growth models for Central European population of Creophilus maxillosus L. (Coleoptera: Staphylinidae). Int. J. Leg. Med. 2020, 134, 1–15. [Google Scholar]
- Matuszewski, S.; Frątczak-Łagiewska, K. Size at emergence improves accuracy of age estimates in forensically-useful beetle Creophilus maxillosus L. (Staphylinidae). Sci. Rep. 2018, 8, 2390. [Google Scholar] [CrossRef]
- Frątczak-Łagiewska, K.; Matuszewski, S. Sex-specific developmental models for Creophilus maxillosus (L.) (Coleoptera: Staphylinidae): Searching for larger accuracy of insect age estimates. Int. J. Leg. Med. 2018, 132, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Martín-Vega, D.; Díaz-Aranda, L.M.; Baz, A.; Cifrián, B. Effect of temperature on the survival and development of three forensically relevant Dermestes species (Coleoptera: Dermestidae). J. Med. Entomol. 2017, 54, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Amos, T. Some laboratory observations on the rates of development, mortality and oviposition of Dermestes frischii (Kug.)(Col., Dermestidae). J. Stored Prod. Res. 1968, 4, 103–117. [Google Scholar] [CrossRef]
- Lambiase, S.; Murgia, G.; Sacchi, R.; Ghitti, M.; Di Lucia, V. Effects of different temperatures on the development of Dermestes Frischii and Dermestes undulatus (Coleoptera, Dermestidae): Comparison between species. J. Forensic Sci. 2018, 63, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Coombs, C. The effect of temperature and relative humidity upon the development and fecundity of Dermestes lardarius L.(Coleoptera, Dermestidae). J. Stored Prod. Res. 1978, 14, 111–119. [Google Scholar] [CrossRef]
- Fleming, D.; Jacob, T. The influence of temperature and relative humidity upon the number and duration of larval instars in Dermestes lardarius L.(Col., Dermestidae). Entomol. Mon. Mag. 1986, 122, 43–50. [Google Scholar]
- Wang, Y.; Wang, M.; Hu, G.; Xu, W.; Wang, Y.; Wang, J. Temperature-dependent development of Omosita colon at constant temperature and its implication for PMImin estimation. J. Forensic Leg. Med. 2020, 72, 101946. [Google Scholar] [CrossRef]
- Hu, G.; Wang, M.; Wang, Y.; Tang, H.; Chen, R.; Zhang, Y.; Zhao, Y.; Jin, J.; Wang, Y.; Wu, M. Development of Necrobia rufipes (De Geer, 1775)(Coleoptera: Cleridae) under Constant Temperature and Its Implication in Forensic Entomology. Forensic Sci. Int. 2020, 311, 110275. [Google Scholar] [CrossRef] [PubMed]
- Grassberger, M.; Frank, C. Temperature-related development of the parasitoid wasp Nasonia vitripennis as forensic indicator. Med. Vet. Entomol. 2003, 17, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Rivers, D.B.; Losinger, M. Development of the gregarious ectoparasitoid N asonia vitripennis using five species of necrophagous flies as hosts and at various developmental temperatures. Entomol. Exp. Appl. 2014, 151, 160–169. [Google Scholar] [CrossRef]
- Voss, S.C.; Spafford, H.; Dadour, I.R. Temperature-dependant development of Nasonia vitripennis on five forensically important carrion fly species. Entomol. Exp. Appl. 2010, 135, 37–47. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Liu, C.; Wang, J.; Hu, G.; Wang, M.; Yang, L.; Chu, J. Development of Nasonia vitripennis (Hymenoptera: Pteromalidae) at constant temperatures in China. J. Med. Entomol. 2019, 56, 368–377. [Google Scholar] [CrossRef]
- da Silva Mello, R.; Aguiar-Coelho, V.M. Durations of immature stage development period of Nasonia vitripennis (Walker)(Hymenoptera: Pteromalidae) under laboratory conditions: Implications for forensic entomology. Parasitol. Res. 2009, 104, 411–418. [Google Scholar] [CrossRef]
- Matuszewski, S.; Hall, M.J.; Moreau, G.; Schoenly, K.G.; Tarone, A.M.; Villet, M.H. Pigs vs people: The use of pigs as analogues for humans in forensic entomology and taphonomy research. Int. J. Leg. Med. 2020, 134, 793–810. [Google Scholar] [CrossRef] [Green Version]
- Matuszewski, S. Estimating the pre-appearance interval from temperature in Necrodes littoralis L. (Coleoptera: Silphidae). Forensic Sci. Int. 2011, 212, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.D. A forensic entomological analysis can yield an estimate of postmortem interval, and not just a minimum postmortem interval: An explanation and illustration using a case. J. Forensic Sci. 2019, 64, 634–637. [Google Scholar] [CrossRef]
- Matuszewski, S. A general approach for postmortem interval based on uniformly distributed and interconnected qualitative indicators. Int. J. Leg. Med. 2017, 131, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Matuszewski, S.; Mądra-Bielewicz, A. Validation of temperature methods for the estimation of pre-appearance interval in carrion insects. Forensic Sci. Med. Pathol. 2016, 12, 50–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matuszewski, S.; Szafałowicz, M.; Grzywacz, A. Temperature-dependent appearance of forensically useful flies on carcasses. Int. J. Leg. Med. 2014, 128, 1013–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archer, M. Comparative Analysis of Insect Succession Data from Victoria (Australia) Using Summary Statistics versus Preceding Mean Ambient Temperature Models. J. Forensic Sci. 2014, 59, 404–412. [Google Scholar] [CrossRef] [PubMed]
- VanLaerhoven, S. Blind validation of postmortem interval estimates using developmental rates of blow flies. Forensic Sci. Int. 2008, 180, 76–80. [Google Scholar] [CrossRef]
- Peters, F.T.; Drummer, O.H.; Musshoff, F. Validation of new methods. Forensic Sci. Int. 2007, 165, 216–224. [Google Scholar] [CrossRef]
- Goff, M.L. Early postmortem changes and stages of decomposition. In Current Concepts in Forensic Entomology; Amendt, J., Goff, M.L., Campobasso, C.P., Grassberger, M., Eds.; Springer: Dordrecht, Germany, 2010. [Google Scholar]
- Schoenly, K.G.; Michaud, J.P.; Moreau, G. Design and analysis of field studies in carrion ecology. In Carrion Ecology, Evolution, and Their Applications; Benbow, M.E., Tomberlin, J.K., Tarone, A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 129–148. [Google Scholar]
- Schoenly, K.G.; Haskell, N.H.; Hall, R.D.; Gbur, J.R. Comparative performance and complementarity of four sampling methods and arthropod preference tests from human and porcine remains at the Forensic Anthropology Center in Knoxville, Tennessee. J. Med. Entomol. 2007, 44, 881–894. [Google Scholar] [CrossRef]
- Schoenly, K.G.; Haskell, N.H.; Mills, D.K.; Bieme-Ndi, C.; Larsen, K.; Lee, Y. Recreating death’s acre in the school yard: using pig carcasses as model corpses to teach concepts of forensic entomology & ecological succession. Am. Biol. Teach. 2006, 68, 402–410. [Google Scholar] [CrossRef]
- Schoenly, K.; Griest, K.; Rhine, S. An experimental field protocol for investigating the postmortem interval using multidisciplinary indicators. J. Forensic Sci. 1991, 36, 1395–1415. [Google Scholar] [CrossRef]
- Tomberlin, J.; Byrd, J.; Wallace, J.; Benbow, M. Assessment of decomposition studies indicates need for standardized and repeatable research methods in forensic entomology. J. Forensic Res. 2012, 3, 147. [Google Scholar] [CrossRef] [Green Version]
- Schoenly, K.; Goff, M.L.; Early, M. A BASIC algorithm for calculating the postmortem interval from arthropod successional data. J. Forensic Sci. 1992, 37, 808–823. [Google Scholar] [CrossRef]
- Schoenly, K.; Goff, M.L.; Wells, J.D.; Lord, W.D. Quantifying statistical uncertainty in succession-based entomological estimates of the postmortem interval in death scene investigations: A simulation study. Am. Entomol. 1996, 42, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. Insect succession and carrion decomposition in selected forests of Central Europe. Part 3: Succession of carrion fauna. Forensic Sci. Int. 2011, 207, 150–163. [Google Scholar] [CrossRef]
- Bonacci, T.; Brandmayr, P.; Greco, S.; Tersaruolo, C.; Vercillo, V.; Brandmayr, T.Z.B. A preliminary investigation of insect succession on carrion in Calabria (southern Italy). Terr. Arthropod Rev. 2010, 3, 97–110. [Google Scholar]
- Prado e Castro, C.; Serrano, A.; Martins Da Silva, P.; García, M.D. Carrion flies of forensic interest: A study of seasonal community composition and succession in Lisbon, Portugal. Med. Vet. Entomol. 2012, 26, 417–431. [Google Scholar] [CrossRef]
- Martín-Vega, D.; Nieto, C.M.; Cifrián, B.; Baz, A.; Díaz-Aranda, L.M. Early colonisation of urban indoor carcasses by blow flies (Diptera: Calliphoridae): An experimental study from central Spain. Forensic Sci. Int. 2017, 278, 87–94. [Google Scholar] [CrossRef]
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. An initial study of insect succession and carrion decomposition in various forest habitats of Central Europe. Forensic Sci. Int. 2008, 180, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, S.; Szafałowicz, M. Temperature-dependent appearance of forensically useful beetles on carcasses. Forensic Sci. Int. 2013, 229, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Frątczak-Łagiewska, K.; Matuszewski, S. Resource partitioning between closely related carrion beetles: Thanatophilus sinuatus (F.) and Thanatophilus rugosus (L.)(Coleoptera: Silphidae). Entomol. Gen. 2018, 37, 143–156. [Google Scholar] [CrossRef]
- Prado e Castro, C.; García, M.D.; Martins da Silva, P.; Faria e Silva, I.; Serrano, A. Coleoptera of forensic interest: A study of seasonal community composition and succession in Lisbon, Portugal. Forensic Sci. Int. 2013, 232, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, S. Estimating the Preappearance Interval from Temperature in Creophilus maxillosus L. (Coleoptera: Staphylinidae). J. Forensic Sci. 2012, 57, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Michaud, J.P.; Moreau, G. Predicting the visitation of carcasses by carrion-related insects under different rates of degree-day accumulation. Forensic Sci. Int. 2009, 185, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Higley, L.G.; Haskell, N.H. Insect development and forensic entomology. In Forensic Entomology: Utility of Arthropods in Legal Investigations; Byrd, J.H., Castner, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 287–302. [Google Scholar]
- Archer, M.S. The effect of time after body discovery on the accuracy of retrospective weather station ambient temperature corrections in forensic entomology. J. Forensic Sci. 2004, 49, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Hofer, I.M.; Hart, A.J.; Martín-Vega, D.; Hall, M.J. Optimising crime scene temperature collection for forensic entomology casework. Forensic Sci. Int. 2017, 270, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.P.; Wallman, J.F.; Archer, M.S. Experimental and casework validation of ambient temperature corrections in forensic entomology. J. Forensic Sci. 2012, 57, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Hofer, I.M.; Hart, A.J.; Martín-Vega, D.; Hall, M.J. Estimating crime scene temperatures from nearby meteorological station data. Forensic Sci. Int. 2020, 306, 110028. [Google Scholar] [CrossRef]
- Lutz, L.; Amendt, J. Stay cool or get hot? An applied primer for using temperature in forensic entomological case work. Sci. Justice 2020, 60, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Charabidze, D.; Hedouin, V. Temperature: The weak point of forensic entomology. Int. J. Leg. Med. 2019, 133, 633–639. [Google Scholar] [CrossRef]
- Dourel, L.; Pasquerault, T.; Gaudry, E.; Benoît, V. Using Estimated On-Site Ambient Temperature Has Uncertain Benefit When Estimating Postmortem Interval. Psyche 2010, 2010, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Dadour, I.; Almanjahie, I.; Fowkes, N.; Keady, G.; Vijayan, K. Temperature variations in a parked vehicle. Forensic Sci. Int. 2011, 207, 205–211. [Google Scholar] [CrossRef]
- Moreau, G.; Lutz, L.; Amendt, J. Honey, can you take out the garbage can? modeling weather data for cadavers found within containers. Pure Appl. Geophys. 2019, 1–12, in press. [Google Scholar] [CrossRef]
- Michalski, M.; Nadolski, J. Thermal conditions in selected urban and semi-natural habitats, important for the forensic entomology. Forensic Sci. Int. 2018, 287, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Calla, L.; Bohun, C.; LeBlanc, H. Advancing the Forensic Estimation of Time Since Death. Pure Appl. Geophys. 2021, 1–11, in press. [Google Scholar]
- Podhorna, J.; Aubernon, C.; Borkovcova, M.; Boulay, J.; Hedouin, V.; Charabidze, D. To eat or get heat: Behavioral trade-offs between thermoregulation and feeding in gregarious necrophagous larvae. Insect Sci. 2018, 25, 883–893. [Google Scholar] [CrossRef]
- Aubernon, C.; Hedouin, V.; Charabidze, D. The maggot, the ethologist and the forensic entomologist: Sociality and thermoregulation in necrophagous larvae. J. Adv. Res. 2018, 16, 67–73. [Google Scholar] [CrossRef]
- Aubernon, C.; Boulay, J.; Hédouin, V.; Charabidzé, D. Thermoregulation in gregarious dipteran larvae: Evidence of species-specific temperature selection. Entomol. Exp. Appl. 2016, 160, 101–108. [Google Scholar] [CrossRef]
- Charabidze, D.; Bourel, B.; Gosset, D. Larval-mass effect: Characterisation of heat emission by necrophageous blowflies (Diptera: Calliphoridae) larval aggregates. Forensic Sci. Int. 2011, 211, 61–66. [Google Scholar] [CrossRef]
- Gruner, S.V.; Slone, D.H.; Capinera, J.L.; Turco, M.P. Volume of larvae is the most important single predictor of mass temperatures in the forensically important calliphorid, Chrysomya megacephala (Diptera: Calliphoridae). J. Med. Entomol. 2016, 54, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Slone, D.H.; Gruner, S.V. Thermoregulation in larval aggregations of carrion-feeding blow flies (Diptera: Calliphoridae). J. Med. Entomol. 2007, 44, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Girard, M. Etudes sur la Chaleur Libre Degagee par les Animaux Invertebres et Specialement les Insectes; Victor Masson: Paris, France, 1869. [Google Scholar]
- Turner, B.; Howard, T. Metabolic heat generation in dipteran larval aggregations: A consideration for forensic entomology. Med. Vet. Entomol. 1992, 6, 179–181. [Google Scholar] [CrossRef]
- Rivers, D.B.; Thompson, C.; Brogan, R. Physiological trade-offs of forming maggot masses by necrophagous flies on vertebrate carrion. Bull. Entomol. Res. 2011, 101, 599–611. [Google Scholar] [CrossRef]
- Heaton, V.; Moffatt, C.; Simmons, T. Quantifying the temperature of maggot masses and its relationship to decomposition. J. Forensic Sci. 2014, 59, 676–682. [Google Scholar] [CrossRef]
- Johnson, A.P.; Wallman, J.F. Effect of massing on larval growth rate. Forensic Sci. Int. 2014, 241, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Kotzé, Z.; Villet, M.H.; Weldon, C.W. Heat accumulation and development rate of massed maggots of the sheep blowfly, Lucilia cuprina (Diptera: Calliphoridae). J. Insect Physiol. 2016, 95, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, S.; Mądra-Bielewicz, A. Heat production in a feeding matrix formed on carrion by communally breeding beetles. Front. Zool. 2021, 18, 5. [Google Scholar] [CrossRef]
- Johnson, A.P.; Wighton, S.J.; Wallman, J.F. Tracking movement and temperature selection of larvae of two forensically important blow fly species within a “maggot mass”. J. Forensic Sci. 2014, 59, 1586–1591. [Google Scholar] [CrossRef]
- Heaton, V.; Moffatt, C.; Simmons, T. The movement of fly (Diptera) larvae within a feeding aggregation. Can. Entomol. 2018, 150, 326–333. [Google Scholar] [CrossRef]
- Gruszka, J.; Krystkowiak-Kowalska, M.; Frątczak-Łagiewska, K.; Mądra-Bielewicz, A.; Charabidze, D.; Matuszewski, S. Patterns and mechanisms for larval aggregation in carrion beetle Necrodes littoralis (Coleoptera: Silphidae). Anim. Behav. 2020, 162, 1–10. [Google Scholar] [CrossRef]
- Szpila, K. Key for identification of European and Mediterranean blowflies (Diptera, Calliphoridae) of medical and veterinary importance-adult flies. In Forensic Entomology, an Introduction; Gennard, D., Ed.; Willey-Blackwell: Chichester, UK, 2012; pp. 77–81. [Google Scholar]
- Akbarzadeh, K.; Wallman, J.F.; Sulakova, H.; Szpila, K. Species identification of Middle Eastern blowflies (Diptera: Calliphoridae) of forensic importance. Parasitol. Res. 2015, 114, 1463–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, L.; Williams, K.A.; Villet, M.H.; Ekanem, M.; Szpila, K. Species identification of adult African blowflies (Diptera: Calliphoridae) of forensic importance. Int. J. Leg. Med. 2018, 132, 831–842. [Google Scholar] [CrossRef] [Green Version]
- Prado e Castro, C.; Szpila, K.; Martínez-Sánchez, A.I.; Rego, C.; Silva, I.; Serrano, A.R.M.; Boieiro, M. The blowflies of the Madeira Archipelago: Species diversity, distribution and identification (Diptera, Calliphoridae sl). ZooKeys 2016, 634, 101–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallman, J. A key to the adults of species of blowflies in southern Australia known or suspected to breed in carrion. Med. Vet. Entomol. 2001, 15, 433–437. [Google Scholar] [CrossRef] [Green Version]
- Szpila, K. Key for the identification of third instars of European blowflies (Diptera: Calliphoridae) of forensic importance. In Current Concepts in Forensic Entomology; Amendt, J., Campobasso, C.P., Goff, M.L., Grassberger, M., Eds.; Springer: Dordrecht, Germany, 2010; pp. 43–56. [Google Scholar]
- Szpila, K.; Richet, R.; Pape, T. Third instar larvae of flesh flies (Diptera: Sarcophagidae) of forensic importance—Critical review of characters and key for European species. Parasitol. Res. 2015, 114, 2279–2289. [Google Scholar] [CrossRef] [Green Version]
- Grzywacz, A.; Hall, M.J.; Pape, T.; Szpila, K. Muscidae (Diptera) of forensic importance—An identification key to third instar larvae of the western Palaearctic region and a catalogue of the muscid carrion community. Int. J. Leg. Med. 2017, 131, 855–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordani, G.; Grzywacz, A.; Vanin, S. Characterization and identification of puparia of Hydrotaea Robineau-Desvoidy, 1830 (Diptera: Muscidae) from forensic and archaeological contexts. J. Med. Entomol. 2019, 56, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Aranda, L.M.; Martín-Vega, D.; Baz, A.; Cifrián, B. Larval identification key to necrophagous Coleoptera of medico-legal importance in the western Palaearctic. Int. J. Leg. Med. 2018, 132, 1795–1804. [Google Scholar] [CrossRef]
- Park, S.-H.; Moon, T.-Y. Carrion Beetles (Coleoptera, Silphidae) of Potential Forensic Importance and Their Pictorial Identification Key by User-Friendly Characters in Korea. Korean J. Leg. Med. 2020, 44, 143–149. [Google Scholar] [CrossRef]
- Novák, M.; Jakubec, P.; Qubaiová, J.; Šuláková, H.; Růžička, J. Revisited larval morphology of Thanatophilus rugosus (Coleoptera: Silphidae). Int. J. Leg. Med. 2018, 132, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Jakubec, P.; Novák, M.; Qubaiová, J.; Šuláková, H.; Růžička, J. Description of immature stages of Thanatophilus sinuatus (Coleoptera: Silphidae). Int. J. Leg. Med. 2019, 133, 1549–1565. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Huang, J.; Wang, J.F. Study on the pupal morphogenesis of Chrysomya rufifacies (Macquart) (Diptera: Calliphoridae) for postmortem interval estimation. Forensic Sci. Int. 2015, 253, 88–93. [Google Scholar] [CrossRef]
- Flissak, J.; Moura, M. Intrapuparial development of Sarconesia chlorogaster (Diptera: Calliphoridae) for postmortem interval estimation (PMI). J. Med. Entomol. 2018, 55, 277–284. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.M.; Moura, M.O. Intrapuparial development of Hemilucilia semidiaphana (Diptera: Calliphoridae) and its use in forensic entomology. J. Med. Entomol. 2019, 56, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Pastrana, Y.; Londoño, C.A.; Wolff, M. Intra-puparial development of Lucilia eximia (Diptera, Calliphoridae). Acta Amazon. 2017, 47, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gu, Z.-y.; Xia, S.-x.; Wang, J.-f.; Zhang, Y.-n.; Tao, L.-y. Estimating the age of Lucilia illustris during the intrapuparial period using two approaches: Morphological changes and differential gene expression. Forensic Sci. Int. 2018, 287, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Souza, M.; Couri, M.S.; Aguiar, V.M. Chronology of the intrapuparial development of the blowfly Chrysomya albiceps (Diptera: Calliphoridae): Application in forensic entomology. J. Med. Entomol. 2018, 55, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Wydra, J.; Matuszewski, S. The optimal post-eclosion interval while estimating the post-mortem interval based on an empty puparium. Forensic Sci. Med. Pathol. 2020, 1–7, in press. [Google Scholar] [CrossRef] [PubMed]
- Frere, B.; Suchaud, F.; Bernier, G.; Cottin, F.; Vincent, B.; Dourel, L.; Lelong, A.; Arpino, P. GC-MS analysis of cuticular lipids in recent and older scavenger insect puparia. An approach to estimate the postmortem interval (PMI). Anal. Bioanal. Chem. 2014, 406, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.E.; Pechal, J.L.; Benbow, M.E.; Drijfhout, F.P. The potential use of cuticular hydrocarbons and multivariate analysis to age empty puparial cases of Calliphora vicina and Lucilia sericata. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.-H.; Jia, Z.-J.; Yu, X.-J.; Wu, K.-S.; Chen, L.-S.; Lv, J.-Y.; Benbow, M.E. Predictable weathering of puparial hydrocarbons of necrophagous flies for determining the postmortem interval: A field experiment using Chrysomya rufifacies. Int. J. Leg. Med. 2017, 131, 885–894. [Google Scholar] [CrossRef]
- Wells, J.D.; LaMotte, N.L. Estimating the postmortem interval. In Forensic Entomology: The Utility of Arthropods in Legal Investigations, 2nd ed.; Byrd, J.H., Castner, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 367–388. [Google Scholar]
- Wells, J.; LaMotte, L.R. Estimating maggot age from weight using inverse prediction. J. Forensic Sci. 1995, 40, 585–590. [Google Scholar] [CrossRef]
- Reibe, S.; Doetinchem, P.v.; Madea, B. A new simulation-based model for calculating post-mortem intervals using developmental data for Lucilia sericata (Dipt.: Calliphoridae). Parasitol. Res. 2010, 107, 9–16. [Google Scholar] [CrossRef]
- Mohr, R.M.; Tomberlin, J.K. Development and validation of a new technique for estimating a minimum postmortem interval using adult blow fly (Diptera: Calliphoridae) carcass attendance. Int. J. Leg. Med. 2015, 129, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Villet, M.H.; Richards, C.S.; Midgley, J.M. Contemporary precision, bias and accuracy of minimum post-mortem intervals estimated using development of carrion-feeding insects. In Current Concepts in Forensic Entomology; Amendt, J., Campobasso, C.P., Goff, M.L., Grassberger, M., Eds.; Springer: Dordrecht, Germany, 2010; pp. 109–138. [Google Scholar]
- Kashyap, V.; Pillay, V. Efficacy of entomological method in estimation of postmortem interval: A comparative analysis. Forensic Sci. Int. 1989, 40, 245–250. [Google Scholar] [CrossRef]
- Goff, M.L.; Odom, C.B. Forensic entomology in the Hawaiian Islands. Three case studies. Am. J. Forensic Med. Pathol. 1987, 8, 45–50. [Google Scholar] [CrossRef]
- Lutz, L.; Amendt, J. Precocious egg development in wild Calliphora vicina (Diptera: Calliphoridae)—An issue of relevance in forensic entomology? Forensic Sci. Int. 2020, 306, 110075. [Google Scholar] [CrossRef]
- Richards, C.S.; Simonsen, T.J.; Abel, R.L.; Hall, M.J.; Schwyn, D.A.; Wicklein, M. Virtual forensic entomology: Improving estimates of minimum post-mortem interval with 3D micro-computed tomography. Forensic Sci. Int. 2012, 220, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Richards, C.; Rowlinson, C.; Cuttiford, L.; Grimsley, R.; Hall, M.R. Decomposed liver has a significantly adverse affect on the development rate of the blowfly Calliphora vicina. Int. J. Leg. Med. 2013, 127, 259–262. [Google Scholar] [CrossRef] [Green Version]
- Richards, C.; Rowlinson, C.; Hall, M.R. Effects of storage temperature on the change in size of Calliphora vicina larvae during preservation in 80% ethanol. Int. J. Leg. Med. 2013, 127, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Myskowiak, J.-B.; Doums, C. Effects of refrigeration on the biometry and development of Protophormia terraenovae (Robineau–Desvoidy)(Diptera: Calliphoridae) and its consequences in estimating post-mortem interval in forensic investigations. Forensic Sci. Int. 2002, 125, 254–261. [Google Scholar] [CrossRef]
- Holmes, L.; Vanlaerhoven, S.; Tomberlin, J. Relative humidity effects on the life history of Hermetia illucens (Diptera: Stratiomyidae). Environ. Entomol. 2012, 41, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Bauer, A.; Bauer, A.M.; Tomberlin, J.K. Impact of diet moisture on the development of the forensically important blow fly Cochliomyia macellaria (Fabricius)(Diptera: Calliphoridae). Forensic Sci. Int. 2020, 312, 110333. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, C.; Heaton, V.; De Haan, D. The distribution of blow fly (Diptera: Calliphoridae) larval lengths and its implications for estimating post mortem intervals. Int. J. Leg. Med. 2016, 130, 287–297. [Google Scholar] [CrossRef]
- Wells, J.D.; Lecheta, M.C.; Moura, M.O.; LaMotte, L.R. An evaluation of sampling methods used to produce insect growth models for postmortem interval estimation. Int. J. Leg. Med. 2015, 129, 405–410. [Google Scholar] [CrossRef]
- Bernhardt, V.; Schomerus, C.; Verhoff, M.; Amendt, J. Of pigs and men—Comparing the development of Calliphora vicina (Diptera: Calliphoridae) on human and porcine tissue. Int. J. Leg. Med. 2017, 131, 847–853. [Google Scholar] [CrossRef]
- Avila, F.W.; Goff, M.L. Arthropod succession patterns onto burnt carrion in two contrasting habitats in the Hawaiian Islands. J. Forensic Sci. 1998, 43, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Hewadikaram, K.A.; Goff, M.L. Effect of carcass size on rate of decomposition and arthropod succession patterns. Am. J. Forensic Med. Pathol. 1991, 12, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Shean, B.S.; Messinger, L.; Papworth, M. Observations of differential decomposition on sun exposed v. shaded pig carrion in coastal Washington State. J. Forensic Sci. 1993, 38, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Komar, D.; Beattie, O. Effects of Carcass Size on Decay Rates of Shade and Sun Exposed Carrion. Can. Soc. Forensic Sci. J. 1998, 31, 35–43. [Google Scholar] [CrossRef]
- Shalaby, O.A.; deCarvalho, L.M.; Goff, M.L. Comparison of patterns of decomposition in a hanging carcass and a carcass in contact with soil in a xerophytic habitat on the Island of Oahu, Hawaii. J. Forensic Sci. 2000, 45, 1267–1273. [Google Scholar] [CrossRef]
- VanLaerhoven, S.L.; Anderson, G.S. Insect succession on buried carrion in two biogeoclimatic zones of British Columbia. J. Forensic Sci. 1999, 44, 32–43. [Google Scholar] [CrossRef]
- Schoenly, K.G.; Shahid, S.A.; Haskell, N.H.; Hall, R.D. Does carcass enrichment alter community structure of predaceous and parasitic arthropods? A second test of the arthropod saturation hypothesis at the Anthropology Research Facility in Knoxville, Tennessee. J. Forensic Sci. 2005, 50, 134–142. [Google Scholar] [CrossRef]
- Shahid, S.A.; Schoenly, K.; Haskell, N.H.; Hall, R.D.; Zhang, W. Carcass enrichment does not alter decay rates or arthropod community structure: A test of the arthropod saturation hypothesis at the anthropology research facility in Knoxville, Tennessee. J. Med. Entomol. 2003, 40, 559–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archer, M.S. Annual variation in arrival and departure times of carrion insects at carcasses: Implications for succession studies in forensic entomology. Aust. J. Zool. 2004, 51, 569–576. [Google Scholar] [CrossRef]
- Archer, M.S.; Elgar, M.A. Effects of decomposition on carcass attendance in a guild of carrion-breeding flies. Med. Vet. Entomol. 2003, 17, 263–271. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Chen, Y.; Chen, Q.; Yin, X. The succession and development of insects on pig carcasses and their significances in estimating PMI in south China. Forensic Sci. Int. 2008, 179, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Sharanowski, B.J.; Walker, E.G.; Anderson, G.S. Insect succession and decomposition patterns on shaded and sunlit carrion in Saskatchewan in three different seasons. Forensic Sci. Int. 2008, 179, 219–240. [Google Scholar] [CrossRef]
- McIntosh, C.S.; Dadour, I.R.; Voss, S.C. A comparison of carcass decomposition and associated insect succession onto burnt and unburnt pig carcasses. Int. J. Leg. Med. 2016. [Google Scholar] [CrossRef]
- Voss, S.C.; Cook, D.F.; Dadour, I.R. Decomposition and insect succession of clothed and unclothed carcasses in Western Australia. Forensic Sci. Int. 2011, 211, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Voss, S.C.; Spafford, H.; Dadour, I.R. Annual and seasonal patterns of insect succession on decomposing remains at two locations in Western Australia. Forensic Sci. Int. 2009, 193, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Voss, S.C.; Forbes, S.L.; Dadour, I.R. Decomposition and insect succession on cadavers inside a vehicle environment. Forensic Sci. Med. Pathol. 2008, 4, 22–32. [Google Scholar] [CrossRef]
- Michaud, J.P.; Moreau, G. Effect of variable rates of daily sampling of fly larvae on decomposition and carrion insect community assembly: Implications for forensic entomology field study protocols. J. Med. Entomol. 2013, 50, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Michaud, J.P.; Moreau, G. A statistical approach based on accumulated degree-days to predict decomposition-related processes in forensic studies. J. Forensic Sci. 2011, 56, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Michaud, J.P.; Majka, C.G.; Prive, J.P.; Moreau, G. Natural and anthropogenic changes in the insect fauna associated with carcasses in the North American Maritime lowlands. Forensic Sci. Int. 2010, 202, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. Insect succession and carrion decomposition in selected forests of Central Europe. Part 1: Pattern and rate of decomposition. Forensic Sci. Int. 2010, 194, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Reibe, S.; Madea, B. How promptly do blowflies colonise fresh carcasses? A study comparing indoor with outdoor locations. Forensic Sci. Int. 2010, 195, 52–57. [Google Scholar] [CrossRef]
- Bugajski, K.N.; Seddon, C.C.; Williams, R.E. A comparison of blow fly (Diptera: Calliphoridae) and beetle (Coleoptera) activity on refrigerated only versus frozen-thawed pig carcasses in Indiana. J. Med. Entomol. 2011, 48, 1231–1235. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Ahmad, A.H.; Dieng, H.; Satho, T.; Ahmad, H.; Aziz, A.T.; Boots, M. Cadaver wrapping and arrival performance of adult flies in an oil palm plantation in northern peninsular Malaysia. J. Med. Entomol. 2011, 48, 1236–1246. [Google Scholar] [CrossRef]
- Anderson, G.S. Comparison of decomposition rates and faunal colonization of carrion in indoor and outdoor environments. J. Forensic Sci. 2011, 56, 136–142. [Google Scholar] [CrossRef]
- Kelly, J.A.; van der Linde, T.C.; Anderson, G.S. The influence of wounds, severe trauma, and clothing, on carcass decomposition and arthropod succession in South Africa. Can. Soc. Forensic Sci. J. 2011, 44, 144–157. [Google Scholar] [CrossRef]
- Kelly, J.A.; van der Linde, T.C.; Anderson, G.S. The influence of clothing and wrapping on carcass decomposition and arthropod succession during the warmer seasons in central South Africa. J. Forensic Sci. 2009, 54, 1105–1112. [Google Scholar] [CrossRef]
- Gunn, A.; Bird, J. The ability of the blowflies Calliphora vomitoria (Linnaeus), Calliphora vicina (Rob-Desvoidy) and Lucilia sericata (Meigen) (Diptera: Calliphoridae) and the muscid flies Muscina stabulans (Fallen) and Muscina prolapsa (Harris) (Diptera: Muscidae) to colonise buried remains. Forensic Sci. Int. 2011, 207, 198–204. [Google Scholar] [CrossRef]
- Sutherland, A.; Myburgh, J.; Steyn, M.; Becker, P.J. The effect of body size on the rate of decomposition in a temperate region of South Africa. Forensic Sci. Int. 2013, 231, 257–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, A.E.; Haskell, N.H.; Wells, J.D. Commonly Used Intercarcass Distances Appear to Be Sufficient to Ensure Independence of Carrion Insect Succession Pattern. Ann. Entomol. Soc. Am. 2016, 109, 72–80. [Google Scholar] [CrossRef]
- Perez, A.E.; Haskell, N.H.; Wells, J.D. Evaluating the utility of hexapod species for calculating a confidence interval about a succession based postmortem interval estimate. Forensic Sci. Int. 2014, 241, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, S.; Konwerski, S.; Fratczak, K.; Szafalowicz, M. Effect of body mass and clothing on decomposition of pig carcasses. Int. J. Leg. Med. 2014, 128, 1039–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cammack, J.A.; Cohen, A.C.; Kreitlow, K.L.; Roe, R.M.; Watson, D.W. Decomposition of Concealed and Exposed Porcine Remains in the North Carolina Piedmont. J. Med. Entomol. 2016, 53, 67–75. [Google Scholar] [CrossRef]
- Charabidze, D.; Hedouin, V.; Gosset, D. An experimental investigation into the colonization of concealed cadavers by necrophagous blowflies. J. Insect Sci. 2015, 15, 149. [Google Scholar] [CrossRef] [Green Version]
- Matuszewski, S.; Mądra, A. Factors affecting quality of temperature models for the pre-appearance interval of forensically useful insects. Forensic Sci. Int. 2015, 247, 28–35. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wang, Z.; Tao, L. Insect succession on pig carcasses using different exposure time-A preliminary study in Guangzhou, China. J. Forensic Leg. Med. 2017, 52, 24–29. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.Y.; Jiang, X.Y.; Wang, J.F.; Li, L.L.; Yin, X.J.; Wang, M.; Lai, Y.; Tao, L.Y. Insect succession on remains of human and animals in Shenzhen, China. Forensic Sci. Int. 2017, 271, 75–86. [Google Scholar] [CrossRef]
- Mądra-Bielewicz, A.; Frątczak-Łagiewska, K.; Matuszewski, S. Sex-and Size-Related Patterns of Carrion Visitation in Necrodes littoralis (Coleoptera: Silphidae) and Creophilus maxillosus (Coleoptera: Staphylinidae). J. Forensic Sci. 2017, 62, 1229–1233. [Google Scholar] [CrossRef]
- Cruise, A.; Hatano, E.; Watson, D.W.; Schal, C. Comparison of techniques for sampling adult necrophilous insects from pig carcasses. J. Med. Entomol. 2018, 55, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Knobel, Z.; Ueland, M.; Nizio, K.D.; Patel, D.; Forbes, S.L. A comparison of human and pig decomposition rates and odour profiles in an Australian environment. Aust. J. Forensic Sci. 2019, 51, 557–572. [Google Scholar] [CrossRef]
- Steadman, D.W.; Dautartas, A.; Kenyhercz, M.W.; Jantz, L.M.; Mundorff, A.; Vidoli, G.M. Differential Scavenging among Pig, Rabbit, and Human Subjects. J. Forensic Sci. 2018. [Google Scholar] [CrossRef]
- Dautartas, A.; Kenyhercz, M.W.; Vidoli, G.M.; Meadows Jantz, L.; Mundorff, A.; Steadman, D.W. Differential Decomposition Among Pig, Rabbit, and Human Remains. J. Forensic Sci. 2018. [Google Scholar] [CrossRef]
- Connor, M.; Baigent, C.; Hansen, E.S. Testing the Use of Pigs as Human Proxies in Decomposition Studies. J. Forensic Sci. 2017. [Google Scholar] [CrossRef]
- Dawson, B.M.; Barton, P.S.; Wallman, J.F. Contrasting insect activity and decomposition of pigs and humans in an Australian environment: A preliminary study. Forensic Sci. Int. 2020, 316, 110515. [Google Scholar] [CrossRef] [PubMed]
- Jarmusz, M.; Bajerlein, D. Decomposition of hanging pig carcasses in a forest habitat of Poland. Forensic Sci. Int. 2019, 300, 32–42. [Google Scholar] [CrossRef]
- Bourel, B.; Callet, B.; Hedouin, V.; Gosset, D. Flies eggs: A new method for the estimation of short-term post-mortem interval? Forensic Sci. Int. 2003, 135, 27–34. [Google Scholar] [CrossRef]
- Faris, A.; West, W.; Tomberlin, J.; Tarone, A. Field validation of a development data set for Cochliomyia macellaria (Diptera: Calliphoridae): Estimating insect age based on development stage. J. Med. Entomol. 2020, 57, 39–49. [Google Scholar] [CrossRef]
- Faris, A.; Wang, H.-H.; Tarone, A.; Grant, W. Forensic entomology: Evaluating uncertainty associated with postmortem interval (PMI) estimates with ecological models. J. Med. Entomol. 2016, 53, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Harnden, L.M.; Tomberlin, J.K. Effects of temperature and diet on black soldier fly, Hermetia illucens (L.)(Diptera: Stratiomyidae), development. Forensic Sci. Int. 2016, 266, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Voss, S.C.; Magni, P.; Dadour, I.; Nansen, C. Reflectance-based determination of age and species of blowfly puparia. Int. J. Leg. Med. 2017, 131, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Tarone, A.M.; Foran, D.R. Generalized additive models and Lucilia sericata growth: Assessing confidence intervals and error rates in forensic entomology. J. Forensic Sci. 2008, 53, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Reibe-Pal, S.; Madea, B. Calculating time since death in a mock crime case comparing a new computational method (ExLAC) with the ADH method. Forensic Sci. Int. 2015, 248, 78–81. [Google Scholar] [CrossRef]
- Weatherbee, C.R.; Pechal, J.L.; Stamper, T.; Benbow, M.E. Post-Colonization Interval Estimates Using Multi-Species Calliphoridae Larval Masses and Spatially Distinct Temperature Data Sets: A Case Study. Insects 2017, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Pittner, S.; Bugelli, V.; Weitgasser, K.; Zissler, A.; Sanit, S.; Lutz, L.; Monticelli, F.; Campobasso, C.P.; Steinbacher, P.; Amendt, J. A field study to evaluate PMI estimation methods for advanced decomposition stages. Int. J. Leg. Med. 2020, 134, 1361–1373. [Google Scholar] [CrossRef] [Green Version]
- Turner, B.; Wiltshire, P. Experimental validation of forensic evidence: A study of the decomposition of buried pigs in a heavy clay soil. Forensic Sci. Int. 1999, 101, 113–122. [Google Scholar] [CrossRef]
- Bhadra, P.; Hart, A.; Hall, M. Factors affecting accessibility to blowflies of bodies disposed in suitcases. Forensic Sci. Int. 2014, 239, 62–72. [Google Scholar] [CrossRef]
- Goff, M.L.; Omori, A.I.; Gunatilake, K. Estimation of postmortem interval by arthropod succession. Three case studies from the Hawaiian Islands. Am. J. Forensic Med. Pathol. 1988, 9, 220–225. [Google Scholar] [CrossRef]
- Pohjoismäki, J.L.; Karhunen, P.J.; Goebeler, S.; Saukko, P.; Sääksjärvi, I.E. Indoors forensic entomology: Colonization of human remains in closed environments by specific species of sarcosaprophagous flies. Forensic Sci. Int. 2010, 199, 38–42. [Google Scholar] [CrossRef]
- Bugelli, V.; Forni, D.; Bassi, L.A.; Di Paolo, M.; Marra, D.; Lenzi, S.; Toni, C.; Giusiani, M.; Domenici, R.; Gherardi, M. Forensic entomology and the estimation of the minimum time since death in indoor cases. J. Forensic Sci. 2015, 60, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Goff, M.L.; Flynn, M.M. Determination of postmortem interval by arthropod succession: A case study from the Hawaiian Islands. J. Forensic Sci. 1991, 36, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Goff, M.; Odom, C.; Early, M. Estimation of post-mortem interval by entomological techniques: A case study from Oahu, Hawaii. Bull. Soc. Vector Ecol. 1986, 11, 242–246. [Google Scholar]

| State of a Cadaver | Insect Evidence | |
|---|---|---|
| What to Look For | Where to Look For | |
| Relatively fresh | Eggs or larvae of flies | Natural orifices (particularly of the head), wounds |
| Signs of putrefaction (bloating, marbling, etc.) | Larvae of flies | Natural orifices, wounds, interface cadaver/ground |
| Signs of active decay (large masses of insect larvae, stench of decay, leakage of decomposition fluids, etc.) | 1. Larvae (particularly post-feeding) of flies 2. Larvae of beetles | 1. Larval masses, the surface of soil (outdoor scenarios) or the floor (indoor scenarios) in the vicinity of a cadaver 2. Larval masses, clothes and cadaver surface, the soil in the vicinity of a cadaver (outdoor scenarios, soil samples are recommended), the floor in the vicinity of a cadaver (indoor scenarios) |
| Signs of advanced decay (exposed bones; greasy by-products of active decay, darkening of the remaining skin, etc.) | 1. Puparia (full and empty) of flies 2. Larvae and pupae of beetles 3. Larvae of late-colonizing flies (e.g., skipper flies) | 1. The soil in the vicinity of a cadaver (outdoor scenarios, soil samples are recommended), the floor (under carpets or furniture) in the vicinity of a cadaver (indoor scenarios), pockets and foldings of clothes, cadaver surface (all scenarios) 2. Larval masses, clothes and cadaver surface, the soil in the vicinity of a cadaver (outdoor scenarios, soil samples are recommended), the floor in the vicinity of a cadaver (indoor scenarios) 3. Larval masses, the surface of soil (outdoor scenarios) or the floor (indoor scenarios) in the vicinity of a cadaver |
| Signs of minimal insect infestation (e.g., massive putrefaction or mummification) | All types of insect evidence | Natural orifices, wounds, clothes and cadaver surface, the soil (outdoor scenarios) or the floor (indoor scenarios) in the vicinity of a cadaver |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matuszewski, S. Post-Mortem Interval Estimation Based on Insect Evidence: Current Challenges. Insects 2021, 12, 314. https://doi.org/10.3390/insects12040314
Matuszewski S. Post-Mortem Interval Estimation Based on Insect Evidence: Current Challenges. Insects. 2021; 12(4):314. https://doi.org/10.3390/insects12040314
Chicago/Turabian StyleMatuszewski, Szymon. 2021. "Post-Mortem Interval Estimation Based on Insect Evidence: Current Challenges" Insects 12, no. 4: 314. https://doi.org/10.3390/insects12040314
APA StyleMatuszewski, S. (2021). Post-Mortem Interval Estimation Based on Insect Evidence: Current Challenges. Insects, 12(4), 314. https://doi.org/10.3390/insects12040314






