Simple Summary
The seed beetle Acanthoscelides obtectus used in this study is a worldwide pest species that inhabits storage facilities and fields of beans. Knowing that sexual dimorphism is very common among insects, we investigated the level of morphological differences between the sexes. Such an approach allowed us to look into the modular organization of this organism. As expected, the females were larger than the males. The observed two modular organization (thorax and abdomen) was sex specific, indicating that reproductive function has the central role in forming the patterns of modularity. It seems that natural selection is driving force for females, while males are influenced more by sexual selection.
Abstract
Sexual dimorphism and specific patterns of development contribute in a great manner to the direction and degree of the sexual differences in body size and shape in many insects. Using a landmark-based geometric morpohometrics approach, we investigated sex-specific morphological size and shape variation in the seed beetle, Acanthoscelides obtectus. We also tested the functional hypothesis of the two morphological modules—thorax and abdomen in both sexes. Female-biased sexual dimorphism in size was shown, while differences in shape were reflected in the wider thorax and abdomen and shorter abdomen in females in comparison to males. The functional hypothesis of a two-module body was confirmed only in females before correction for size, and in both sexes after the allometry correction. Our results indicate that reproductive function has the central role in forming the patterns of modularity. We hypothesize that high morphological integration of the abdomen in females results from intense stabilizing selection, while the more relaxed integration in males is driven by the higher intensity of sexual selection.
1. Introduction
Seed beetles (Bruchidae) are phytophagous, holometabolous insects with a worldwide distribution and often are major pests of legume plant species [1]. Within bruchine species there is a great variation in body shape and size due to genetic variability, but also as a result of adaptive responses to different ecological factors, i.e., geographical distribution [2], environmental variables such as temperature [3], or insect shift to novel host plant species [4]. In addition, one of the most remarkable sources of morphological variation refers to the direction and degree of sexual differences in body size and shape resulting in sexual dimorphism [5,6,7]. The most common pattern of sex differences among insects is female-biased size dimorphism, in which larger females have adaptive advantages such as greater fecundity, fertility and, in some cases, better parental care [8]. However, different results have been reported in seed beetles from the Chrysomelidae family (i.e., Stator limbatus (Horn, 1873)) [9]. It has been hypothesized that bigger males have large amounts of sperm nutrient which are transferred to females, raising their fecundity. Although the data on the size dimorphism between females and males are abundant and the factors that contribute to the sex size differences are well examined, studies related to coleopteran sexual shape dimorphism are underexplored [5,7,10,11].
Morphological integration refers to functional, developmental and/or evolutionary connection between organism’s morphological traits [12]. More closely, developmental processes that underlie phenotypic variation usually simultaneously encompass several morphological traits that share the same genetic basis, developmental paths or a function, and cause a certain degree of internal integration between them [13]. Such integration between a group of traits leads to the development of a morphological module, which is relatively less linked to other integrated modules [12,14]. This is the concept of modularity which focuses on relative differences in the level of the integration of the parts within and between modules of organism traits and therefore can be applied to address important evolutionary questions [15,16,17,18]. The evolutionary significance of a modular biological organization lays in a potentially enhanced evolvability, that is, the increased ability of organisms to evolve and respond to different selective challenges [19]. In other words, selection is able to act on each of these distinctive entities separately without great interference [20].
The concept of modularity in seed beetles’ body plans could be illustrated by three easily identified separate entities integrated through their function, with a degree of independence between each other: head, thorax and abdomen. The size and shape of the head are adapted to feeding habits, while the thorax is specialized for locomotion [21]. The thorax encompasses muscles for flight and for the movement of legs and body segments, and thus has a major role in locomotion [22]. Finally, the abdomen of beetles is linked to the reproduction, since it contains the reproductive organs and all the nutrients and energy reserves that can be used for producing eggs and ejaculate [23]. Additionally, taking into account that the size and shape of abdomen could be an important trait for mate choice in beetles, abdominal morphological variation is expected to be under sexual selection [24].
In this research we analyzed sexual dimorphism and morphological modularity in one holometabolous, bruchine species—Acanthoscelides obtectus (Say, 1831). Classic morphological analyses pointed out that sexual dimorphism in A. obtectus is related to the last segment of the abdomen (fifth sternite) and the orientation of the pygidium [25,26]. However, these studies have been limited to a few measures. A landmark-based geometric morphometric is a far more powerful tool that allows quantifying and visualizing shape variation, providing precise information on interindividual and intraindividual morphological variability [18,27]. In addition, this approach enables analyzing allometry, which is defined as a relationship between changes in body shape and changes in body size [28]. Exploring allometry shape changes is of great importance in studying sexual shape dimorphism and the detection of modularity, because allometry can have major effects on the patterns of variation and integration [11,18,29]. By applying a geometric morphometric approach we explored and tested: (i) the specific morphological differences in size and shape between A. obtectus females and males; (ii) the influence of size on body shape changes; and (iii) the functional hypothesis of the two modules: thorax and abdomen in females and males separately.
2. Materials and Methods
2.1. Study Species—Seed Beetle (Acanthoscelides obtectus), Laboratory Population and Rearing Conditions
This research was conducted on seed beetles (A. obtectus) from a laboratory population (hereafter referred to as base) maintained for more than 35 years (301 generations) under constant conditions (30 °C ± 0.1 °C, relative humidity 30% ± 1%). Base population was established using beetles that hatched from infected bean seeds from three legume storages. In order to limit the severe effects of inbreeding, at least 600 randomly sampled individuals contributed to the consecutive generation. Individuals from different generations were not mixed, i.e., there was no generation overlap.
According to the laboratory protocol, all insects were reared in the dark incubator set at 30 ± 1 °C. Since A. obtectus is facultative aphagous, food and water was not offered to adults. Food for larvae was pesticide free, organic white bean seeds that were frozen for 48 h on −20 °C before use in order to evade potential contamination.
2.2. Collection of Samples
Immediately upon emergence, a total of 314 adults from base population (157 females and 157 males) were collected and stored on −20 °C in a single day. Adults were set up on plasticine mold glued to a microscope plate and photographed with Nikon Digital Sight Fi2 Camera attached to Nikon SMZ800 against a scale bar 10 mm on ventral side. The distance and magnification were kept constant during photographing.
2.3. Landmark Data
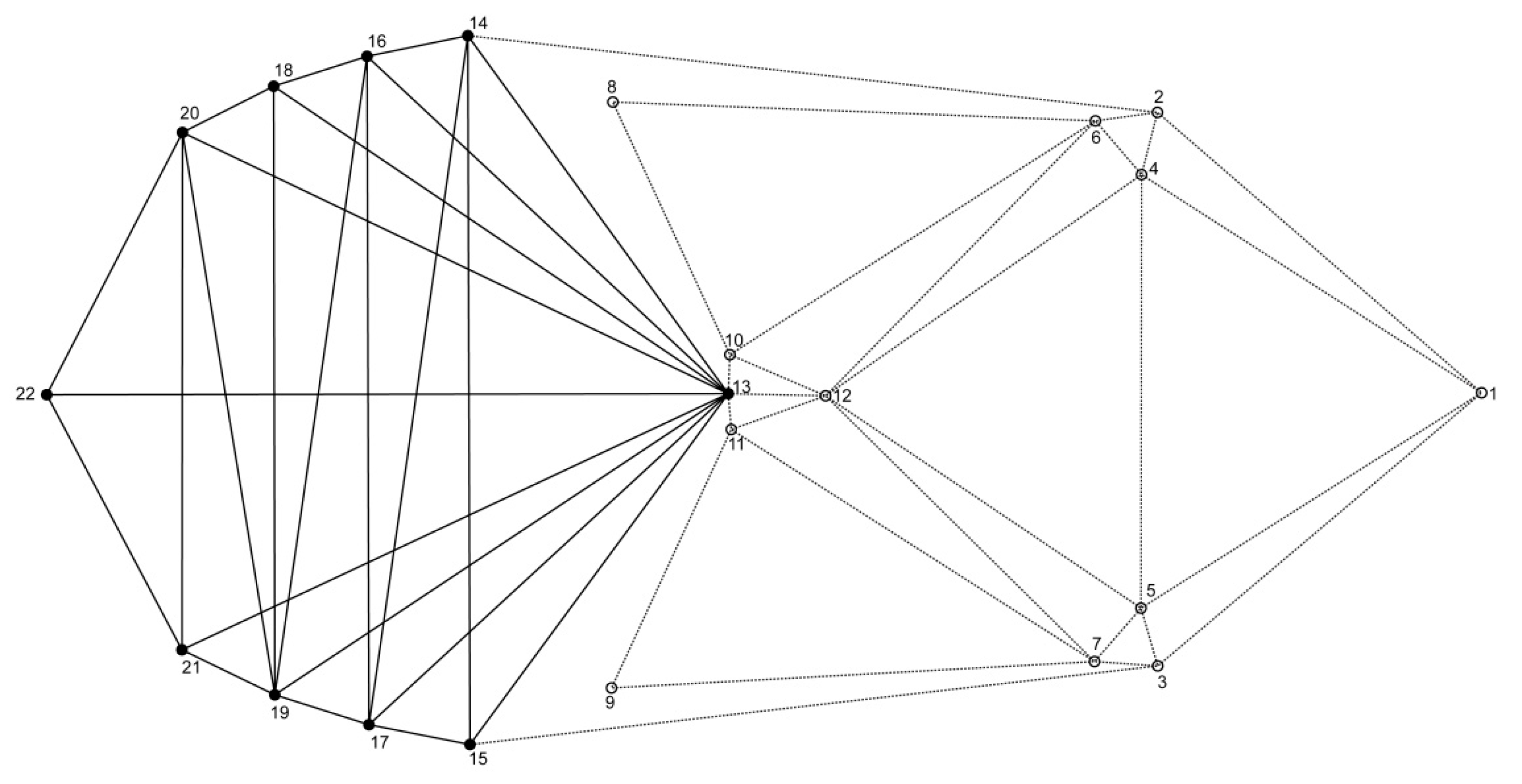
To characterize the shape of body, we applied the methods of geometric morphometrics, which use the relative positions of the set of landmarks to quantify morphological variation [19,30,31]. We selected configurations of 22 landmarks of objects (12 landmarks for the thorax and 10 landmarks for the abdomen) (Figure 1, Table 1). Potential differences in the shape of head and specialized mouth parts (e.g., mandibles) go beyond the scope of this study. Therefore, this part of the body has not been covered with landmarks and included in analyses. The landmarks were digitized by one person in TpsDig2 software [32].
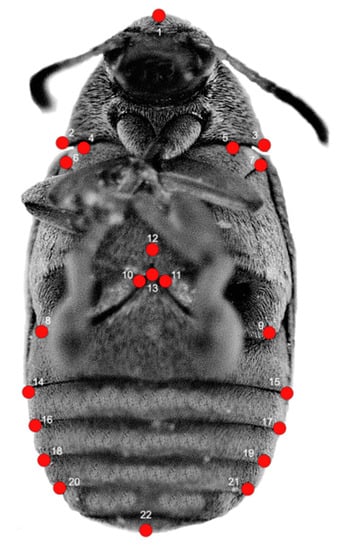
Figure 1.
Landmark configurations on Acanthoscelides obtectus example. See Table 1 for landmark definitions.

Table 1.
Definitions of landmarks on the Acanthoscelides obtectus.
2.4. Geometric Morphometric Analyses
2.4.1. Analyses of Size and Shape Variation Patterns
Centroid size, the square root of the sum of squared distances of all the landmarks from their centroid, was used as a measure of size of the seed beetle’s body [31]. The differences in the body sizes between females and males were tested by one-way ANOVA. Statistical analyses of centroid size were carried out using GLM procedure of SAS statistical software [33].
To extract shape variables from the landmark configuration of beetle’s body, we used Procrustes superimposition to eliminate effects of size, position and orientation [31]. We applied principal component analysis (PCA) on the covariance matrices of shape variables to describe overall shape variation pattern of the seed beetle’s body in females and males [27].
In order to explore and visualize allometric shape changes, we performed multivariate regression analyses of obtained shape variables for females and males [34]. Statistical significance of allometric shape changes were obtained by permutation test (10,000 iterations). Residuals obtained from these regression analyses represent nonallometric component of shape variation.
To quantify differences in shape between females and males we calculated Procrustes distances (PD)—the square root of the squared distances between pairs of corresponding landmarks. This procedure was repeated on nonallometric component of shape variation. The statistical significance of PD was obtained using the permutation test (10,000 iterations).
To estimate the measurement error, we used Procrustes ANOVA with the main effects of: (i) individuals—indicating the interindividual phenotypic differences in shape of females and males; and (ii) residual term representing the measurement error [35]. For this analysis, the whole sample was digitized twice by one person.
2.4.2. Analyses of Shape Covariation Patterns
To test the two module hypothesis of modularity of beetles’ females and males, we used the covariance matrices of pairways Procrustes distances. As a measure of strength of association between the hypothesized partitions, RV coefficients (ratio that describes the degree of covariation between sets of variables relative to the variation and covariation within sets of variables) were calculated and compared with RV coefficients obtained for all possible alternative partitions [29,36]. Values of the RV coefficient can range from 0 to 1, providing that lower values indicate weaker correlation [36]. If a value of the RV coefficient between hypothesized modules is smaller than for most of all alternative partitions, the modularity hypothesis will be confirmed [29]. RV coefficients for females and males were calculated twice: for allometric and nonallometric component of the shape variation. All statistical analyses and visualizations of shape changes were conducted using MorphoJ software package [37].
3. Results
3.1. Sexual Dimorphism in Size and Shape
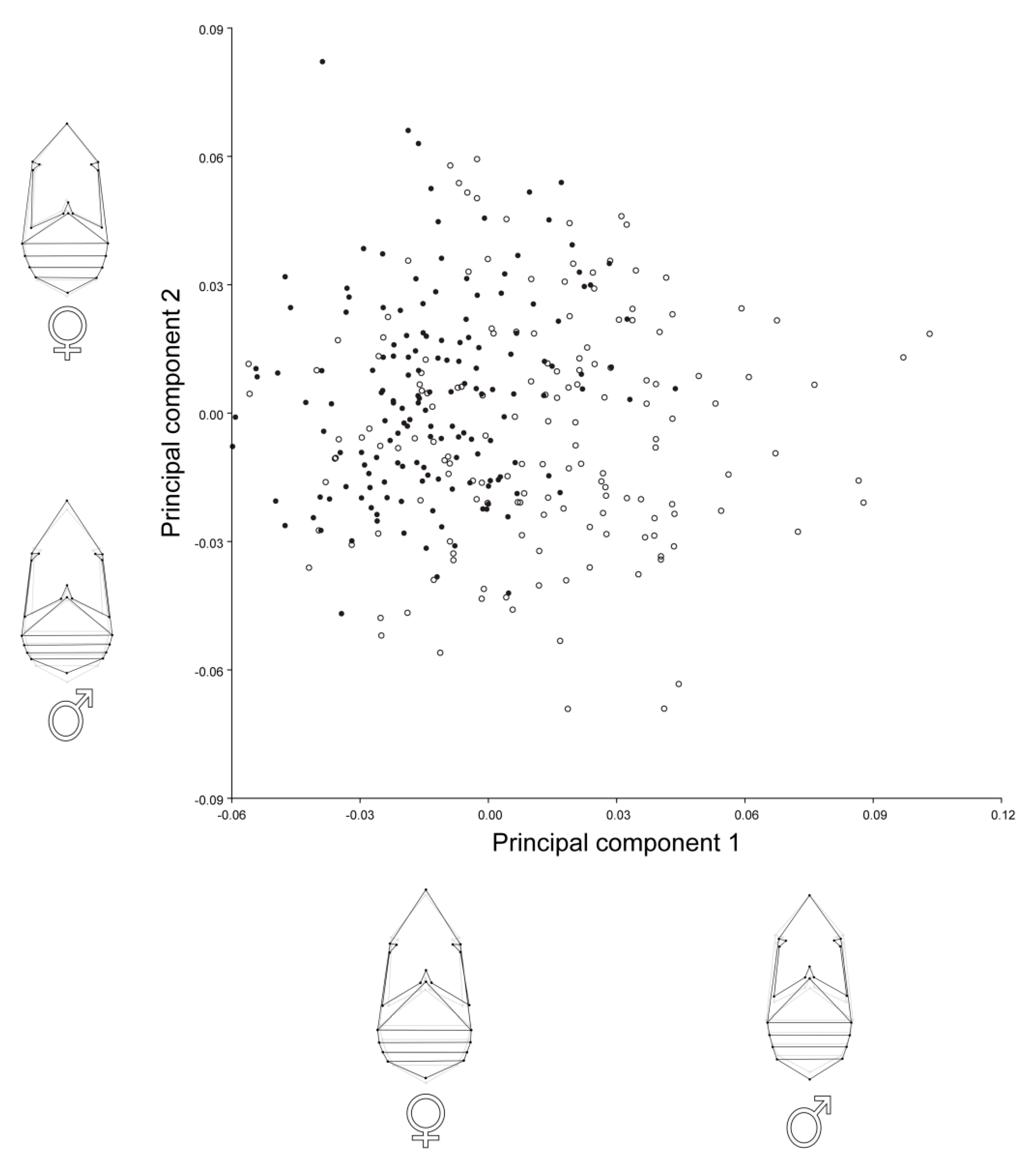
The principal component analysis (PCA) for females and males showed that the first two principal components (PC1 and PC2) described about 55% of the total shape variation (Table 2). The main patterns of the shape variation were changes in the relative length vs. width of the thorax and abdomen (Figure 2). PC1 and PC2 in females were associated with shortening of the abdomen and slight elongation of the thorax. In males, PC1 reflected elongation of abdomen and narrowing and shortening of the thorax, while PC2 was related to a shortening of abdomen and elongation and widening of the thorax. A scatterplot of PC scores revealed clear tendency of differences in body shape between A. obtectus females and males (Figure 2).

Table 2.
Eigenvalues and contribution of principal components (PCs) in the shape variation of Acanthoscelides obtectus females and males.
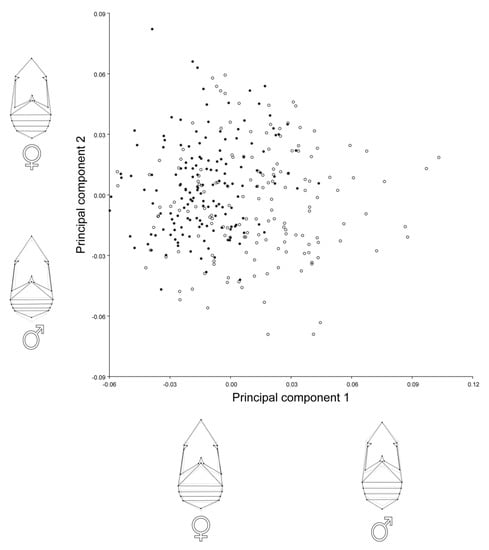
Figure 2.
Principal component analysis (PCA) of body shape of Acanthoscelides obtectus females and males. Scatterplot of the PCs 1 and 2 accounted together for about 55% of the overall shape variation. Filled circles are assigned for females, while open circles are assigned for males. Shape changes of females and males associated with the PCs are represented by wireframe graphs.
Multivariate regression of shape variables on size showed highly statistical significance and explained different portion of the total variation for females (4.18%, p < 0.0001) and males (13.88%, p < 0.0001).
The one-way ANOVA indicated significant sex-specific differences of seed beetles (F = 109.59; d.f. = 1; p < 0.0001). Mean centroid sizes indicated that females (mean CSfemales = 7.711) are larger than males (CSmales = 7.641).
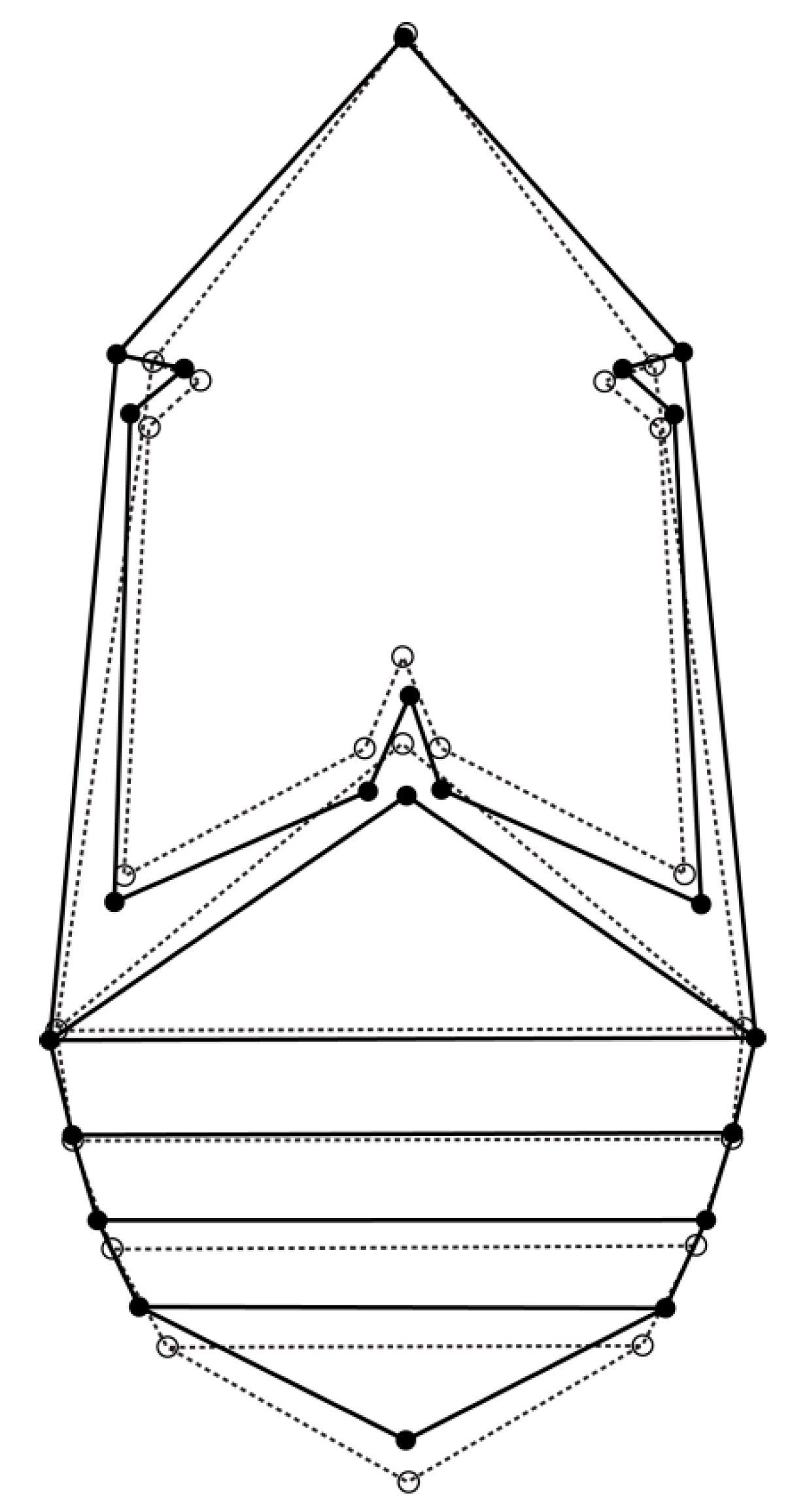
For the allometric and nonallometric component of shape variation, the permutation test revealed high statistical significance (p < 0.0001) for Procrustes distances between females and males (PDallometric = 0.028, PDnon-allometric = 0.014). Visualization of the sexual shape dimorphism is presented by a diagram in Figure 3. The differences in shape between the sexes are characterized by a wider thorax in general, and an elongated metathorax in females in comparison to males. Females’ first abdominal sternite is shortened, while females’ 2nd abdominal sternite is wider than males’. The last abdominal sternite is shorter in females.
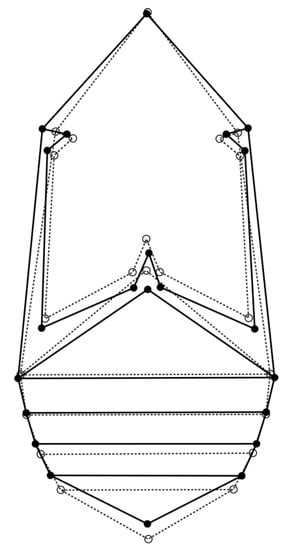
Figure 3.
Discriminant functional analyses of seed beetles. The outline graphs show differences in shape between Acanthoscelides obtectus females (solid line) and males (dashed line).
3.2. Modularity
Procrustes ANOVA revealed that mean squares of interindividual variation have significant higher values than measurement error (Table 3), so Procrustes distances can be used as valid variables for testing hypothesis of modularity.

Table 3.
Procrustes ANOVA of shape in Acanthoscelides obtectus females and males with effects of individual (interindividual variation) and measurement error. SS—sum of squares, MS—mean of the sum of squares, df—degree of freedom, F—value of F test, p—level of statistical significance.
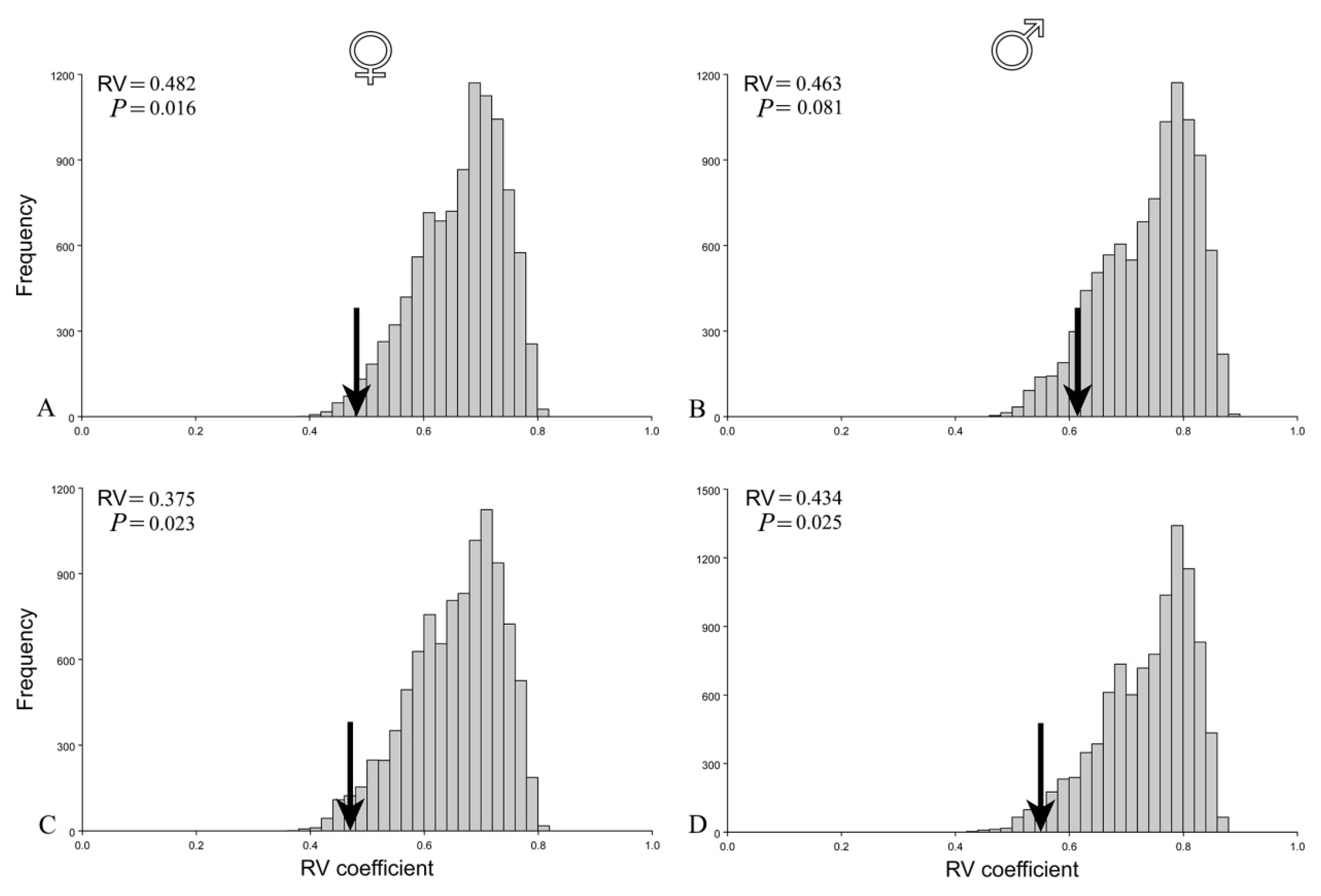
The functional hypothesis of the two modules (thorax and abdomen) (Figure 4) was confirmed for females, but not for males before the correction for size (Figure 5A,B). Covariation between thorax and abdomen in females was among the lowest when compared with covariation for alternative partitions (p = 0.016). On the other hand, in males, the hypothesis of the two modules was not confirmed (p = 0.081). Interestingly, after removing the influence of allometry, the correlation matrices of the residuals were statistically significant and correlated with theoretically derived matrices for both females (p = 0.023) and males (p = 0.025) (Figure 5C,D). Hence, after correction for size, the two module functional hypothesis was confirmed for both sexes.
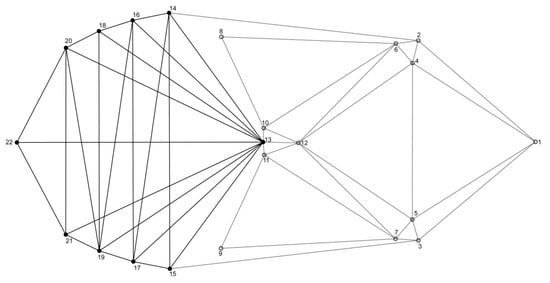
Figure 4.
A priori hypothesis of a two-module (thorax—dashed line, abdomensol—id line) organization of female and male seed beetles.
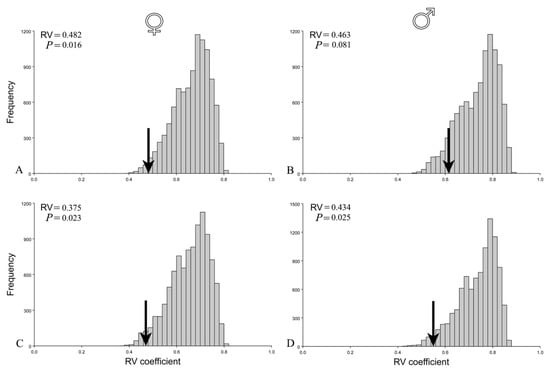
Figure 5.
Evaluation of a two-module hypothesis of female and male seed beetles before (A,B) and after correction for size (C,D). Arrows on histograms of distribution of the RV coefficients for the alternative partitions indicate values of RV coefficients for the hypothesis. Statistical significance of tested hypothesis is presented by p values.
4. Discussion
4.1. Morphological Variation and Sexual Dimorphism in Size and Shape
In the present work, we quantified and compared female and male morphological variation in A. obtectus. Our results revealed the female-biased size dimorphism that is a common pattern in insects. It is expected for sexual dimorphism in insects to be affected by both life-history evolution and sexual selection. Larger female body size is usually related to the fitness, that is, to the higher number of viable offspring and an increase in mating success [38,39,40]. In many insect species, as well as in A. obtectus [41,42], it has been confirmed that large females are able to convert higher portions of their accumulated resources into fecundity [43,44,45]. Evolutionary changes of body size in insects could also be achieved via selection on development time, being that the time needed for the development of an adult is positively correlated with its body size [42,46]. Namely, in protandrous insects, the slower development of female sex leads to larger adults and their higher fecundity, whereas faster male development, although it results in smaller individuals, increases the chances for males to copulate with newly hatched females and therefore could increase male reproductive success [42].
The evolution of the female-bias size dimorphism in A. obtectus could also be related to the behavior during copulation. It has been shown that in this species courtship activities are simple; they do not include specific rituals or acoustic signals, and the most important activity for the males is the level of their aggressiveness and persistence in chasing females [47]. In general, copulation in insects often has harmful effects in females, resulting in reduced fitness and even death [48]. If smaller males could potentially do less harm to females during copulation, then sexual selection could influence the general size and shape of males as well as to select for females that are better in recognizing the least harmful males [49].
Sexual dimorphism in the shape of abdomen has been confirmed in different groups of insects, such as Scathophagidae [49], and, recently, the Carabidae family [5,7]. The most pronounced sex difference in shape that we observed in A. obtectus was divergence in the abdomen, which was wider and shorter in females than in males. Such specific shape can be related to the ability of females to accumulate and transport more eggs [39,50]. Hence, the shape of the female’s abdomen and fecundity can be positively correlated through natural selection [24,51]. Again, the specific shape of the abdomen in this species could be the result of specific patterns of sexual selection, especially related to its reproductive behavior. Unlike many insect species, contact between females and males during copulation is loosed in A. obtectus enabling females to avoid injuries relatively easily [52]. The particular size and shape of female and male abdomens allow the copulation to be efficient enough for potentially a short period of time. Accordingly, the more elongated body of A. obtectus males can assist in forced copulation with females [53] and more accurate positioning above females just before copulation [52].
4.2. Modularity
Research on morphological shape variation in holometabolous adults, integration and modularity are mostly limited to Carabidae and Hymenoptera [5,54,55]. The origin of morphological integration and modularity has been analyzed in different insect species on various body parts: mouthparts in Pterostichus thunbergi (Morawitz, 1862) [56], hind wings in Diabrotica virgifera virgifera (LeConte, 1868) [57], wings in bumblebees [58], dragonflies [59] and in adult ants [54]. In our study, the hypothesis of the two functional modules (thorax and abdomen) was confirmed for A. obtectus females before correction for size, and for both sexes after size correction.
One common explanation for the evolution of tight integration of traits within a module refers to a strong stabilizing selection acting on the functionality of the module [60]. Although the existence of distinctive entities enables their evolution independently from other body parts, this also constrains fast diversification of a module in order to maintain conserved modes of function. Genetically and/or environmentally induced perturbations during development, which could alter the basic functional structure, have to be limited in their effects on a module. It seems that the female abdomen in A. obtectus is under strong selective pressure due to its importance in reproductive function and it is independent from body size [61]. On the other hand, the shape of male abdomen is significantly associated with body size, indicating lower integration of male modules. Thus, we hypothesize that male abdominal parts could have greater potential for the evolution of diverse shapes and structures because they are more driven by sexual selection. Being that the reproductive successes of males is highly dependent on their ability to mount females, it could be suggested that different abdominal sizes need different abdominal shapes in order to achieve copulation.
Our results on A. obtectus lead to conclusion that evolution of modules of body parts with reproductive function are under the influence of both natural and sexual selection, but the courses and intensity of these mechanisms are different in females and males. In support of that is the recent morphological study on green-belly stink bug (Dichelops melacanthus (Dallas, 1851) (Heteroptra: Pentatomidae)) genitalia which strongly indicates that the reproductive organs are subjected to sex specific selection, although male and female genitalia are functionally associated [62]. The authors hypothesized that integration of reproductive organs in females constrains diversification via stabilizing selection, while in males directional selection is more responsible for their maintenance or for the improvement of copulatory performance. It seems that sex specific modular patterns may be of greater importance in the evolution of different insect species than previously thought.
5. Conclusions
- Female-biased size dimorphism in seed beetle (Acanthoscelides obtectus) laboratory population is affected by both life-history and sexual selection.
- Females have shorter and wider abdomens compared to more elongated abdomens in males.
- By testing the modularity hypothesis it was confirmed that female and male body is compartmentalized into two functional modules: thorax and abdomen. The integration of the abdomen in males is dependent on their size, indicating the more prominent role of sexual selection. On the other hand, strong modularity in females, regardless of size, is the result of strong natural selection on reproductive function.
Author Contributions
Conceptualization, S.B. and B.S.; methodology, S.B. and U.S.; formal analysis, S.B.; investigation, S.B. and U.S.; writing—original draft preparation, S.B.; writing—review and editing, S.B., U.S., M.Đ., L.V. and B.S; supervision, B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Serbian Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant numbers 451-03-9/2021-14/200007).
Institutional Review Board Statement
The study was conducted according to the Serbian and European ethical normative (Directive 2010/63/EU) on the protection of animals used for experimental and other scientific purposes. Among invertebrates, ethical protection is granted to cephalopodes by the EU and Serbian legislatives. The national ethical legislative also grants protection to endangered species. However, Acanthoscelides obtectus does not fall into one of these categories and this study is in concordance with current state of ethical legislative in the EU and the Republic of Serbia.
Acknowledgments
We thank three anonymous reviewers and the deciding editor for their comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ofuya, T.I.; Credland, P.F. Differences in the susceptibility of seeds of selected varieties of cowpea to Bruchidius atrolineatus (Coleoptera: Bruchidae). Bull. Entomol. Res. 1995, 85, 259–265. [Google Scholar] [CrossRef]
- Stillwell, R.C.; Fox, C.W. Environmental effects on sexual size dimorphism of a seed-feeding beetle. Oecologia 2007, 153, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Stillwell, R.C.; Moya-Laraño, J.; Fox, C.W. Selection does not favor larger body size at lower temperature in a seed—Feeding beetle. Evol. Int. J. Org. Evol. 2008, 62, 2534–2544. [Google Scholar] [CrossRef]
- Messina, F.J. Predictable modification of body size and competitive ability following a host shift by a seed beetle. Evolution 2004, 58, 2788–2797. [Google Scholar] [CrossRef] [PubMed]
- Benítez, H.A.; Avaria-Llautureo, J.; Canales-Aguirre, C.B.; Jerez, V.; Parra, L.E.; Hernandez, C.E. Evolution of sexual size dimorphism and its relationship with sex ratio in carabid beetles of Genus Ceroglossus Solier. Curr. Zool. 2013, 59, 769–777. [Google Scholar] [CrossRef]
- Colgoni, A.; Vamosi, S.M. Sexual dimorphism and allometry in two seed beetles (Coleoptera: Bruchidae). Entomol. Sci. 2006, 9, 171–179. [Google Scholar] [CrossRef]
- Vesović, N.; Ivanović, A.; Ćurčić, S. Sexual size and shape dimorphism in two ground beetle taxa, Carabus (Procrustes) coriaceus cerisyi and C. (Morphocarabus) kollari praecellens (Coleoptera: Carabidae)—A geometric morphometric approach. Arthropod Struct. Dev. 2019, 49, 1–9. [Google Scholar] [CrossRef]
- Honěk, A. Intraspecific variation in body size and fecundity in insects: A general relationship. Oikos 1993, 66, 483–492. [Google Scholar] [CrossRef]
- Fox, C.W.; Czesak, M.E. Selection on body size and sexual size dimorphism differs between host species in a seed—Feeding beetle. J. Evol. Biol. 2006, 19, 1167–1174. [Google Scholar] [CrossRef]
- Benítez, H.A.; Sukhodolskaya, R.A.; Órdenes-Clavería, R.; Avtaeva, T.A.; Kushalieva, S.A.; Saveliev, A.A. Measuring the inter and intraspecific sexual shape dimorphism and body shape variation in generalist ground geetles in Russia. Insects 2020, 11, 361. [Google Scholar] [CrossRef]
- Espinoza-Donoso, S.; Angulo-Bedoya, M.; Lemic, D.; Benítez, H.A. Assessing the influence of allometry on sexual and non-sexual traits: An example in Cicindelidia trifasciata (Coleoptera: Cicindelinae) using geometric morphometrics. Zool. Anz. 2020, 5, 9. [Google Scholar] [CrossRef]
- Olson, E.C.; Miller, R.L. Morphological Integration; University of Chicago Press: Chicago, IL, USA, 1999. [Google Scholar]
- Klingenberg, C.P. Developmental constraints, modules, and evolvability. In Variation; Hallgrímsson, H., Hall, B., Eds.; Academic Press: Cambridge, MA, USA, 2005; pp. 219–247. [Google Scholar]
- Klingenberg, C.P. Morphological integration and developmental modularity. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 115–132. [Google Scholar] [CrossRef]
- Martínez-Abadías, N.; Esparza, M.; Sjøvold, T.; González-José, R.; Santos, M.; Hernández, M.; Klingenberg, C.P. Pervasive genetic integration directs the evolution of human skull shape. Evol. Int. J. Org. Evol. 2012, 66, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P.; Marugán-Lobón, J. Evolutionary covariation in geometric morphometric data: Analyzing integration, modularity, and allometry in a phylogenetic context. Syst. Biol. 2013, 62, 591–610. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Bookstein, F. The conceptual and statistical relationship between modularity and morphological integration. Syst. Biol. 2007, 56, 818–836. [Google Scholar] [CrossRef] [PubMed]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D. Geometric Morphometrics for Biologists: A Primer; Academic Press: Cambridge, MA USA, 2012. [Google Scholar]
- Hansen, T.F. Is modularity necessary for evolvability? Remarks on the relationship between pleiotropy and evolvability. Biosystems 2003, 69, 83–94. [Google Scholar] [CrossRef]
- Griswold, C.K. Pleiotropic mutation, modularity and evolvability. Evol. Dev. 2006, 8, 81–93. [Google Scholar] [CrossRef]
- Talarico, F.; Brandmayr, P.; Giglio, A.; Massolo, A.; Brandmayr, T.Z. Morphometry of eyes, antennae and wings in three species of Siagona (Coleoptera, Carabidae). Zookeys 2011, 203. [Google Scholar] [CrossRef] [PubMed]
- Frantsevich, L.; Gorb, S.; Radchenko, V.; Gladun, D.; Polilov, A. Lehr’s fields of campaniform sensilla in beetles (Coleoptera): Functional morphology. I. General part and allometry. Arthropod Struct. Dev. 2014, 43, 523–535. [Google Scholar] [CrossRef]
- Boggs, C.L. Selection pressures affecting male nutrient investment at mating in heliconiine butterflies. Evolution 1981, 35, 931–940. [Google Scholar] [CrossRef]
- Moya-Laraño, J.; Fox, C.W. Ejaculate size, second male size, and moderate polyandry increase female fecundity in a seed beetle. Behav. Ecol. 2006, 17, 940–946. [Google Scholar] [CrossRef][Green Version]
- Haines, C.P. Insects and Arachnids of Tropical Stored Products: Their Biology and Identification (a Training Manual), 2nd ed.; Natural Resources Inst.: Chatham, Medway, UK, 1991. [Google Scholar]
- Halstead, D.G.H. External sex differences in stored-products Coleoptera. Bull. Entomol. Res. 1963, 54, 119–134. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Barluenga, M.; Meyer, A. Shape analysis of symmetric structures: Quantifying variation among individuals and asymmetry. Evolution 2002, 56, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J. Allometry and size in ontogeny and phylogeny. Biol. Rev. 1966, 41, 587–638. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. Morphometric integration and modularity in configurations of landmarks: Tools for evaluating a priori hypotheses. Evol. Dev. 2009, 11, 405–421. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Evolution and development of shape: Integrating quantitative approaches. Nat. Rev. Genet. 2010, 11, 623–635. [Google Scholar] [CrossRef]
- Dryden, I.L.; Mardia, K.V. Statistical Shape Analysis: With Applications in R; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Rohlf, F.J. tpsDig, Version 2.10; Informer Technologies Inc.: Los Angeles, CA, USA, 2006. [Google Scholar]
- SAS Institute Inc. The SAS System for Windows; Release 9.9; SAS Institute: Cary, NC, USA, 2010. [Google Scholar]
- Monteiro, L.R. Multivariate regression models and geometric morphometrics: The search for causal factors in the analysis of shape. Syst. Biol. 1999, 48, 192–199. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; McIntyre, G.S. Geometric morphometrics of developmental instability: Analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution 1998, 52, 1363–1375. [Google Scholar] [CrossRef]
- Robert, P.; Escoufier, Y. A unifying tool for linear multivariate statistical methods: The RV-coefficient. J. R. Stat. Soc. Ser. C 1976, 25, 257–265. [Google Scholar] [CrossRef]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Steiger, S. Bigger mothers are better mothers: Disentangling size-related prenatal and postnatal maternal effects. Proc. R. Soc. B Biol. Sci. 2013, 280, 1225. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M. Sexual Selection; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Bonsignore, C.P.; Jones, T.M. Aggregation and mating success of Capnodis tenebrionis (Coleoptera: Buprestidae). Insect Sci. 2013, 21, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Šešlija, D.; Tucić, N. Selection for developmental time in bean weevil (Acanthoscelides obtectus): Correlated responses for other life history traits and genetic architecture of line differentiation. Entomol. Exp. Appl. 2003, 106, 19–35. [Google Scholar] [CrossRef]
- Đorđević, M.; Savković, U.; Lazarević, J.; Tucić, N.; Stojković, B. Intergenomic interactions in hybrids between short-lived and long-lived lines of a seed beetle: Analyses of life history traits. Evol. Biol. 2015, 42, 461–472. [Google Scholar] [CrossRef]
- Rutowski, R.L. Epigamic selection by males as evidenced by courtship partner preferences in the checkered white butterfly (Pieris protodice). Anim. Behav. 1982, 30, 108–112. [Google Scholar] [CrossRef]
- Sigurjonsdottir, H.; Snorrason, S.S. Distribution of male yellow dungflies around ovipasition sites: The effect of body size. Ecol. Entomol. 1995, 20, 84–90. [Google Scholar] [CrossRef]
- Uhl, G. Mating behaviour in the cellar spider, Pholcus phalangioides, indicates sperm mixing. Anim. Behav. 1998, 56, 1155–1159. [Google Scholar] [CrossRef]
- Zonneveld, C. Being big or emerging early? Polyandry and the trade-off between size and emergence in male butterflies. Am. Nat. 1996, 147, 946–965. [Google Scholar] [CrossRef]
- Stojković, B.; Jovanović, D.Š.; Perovanović, J.; Tucić, N. Sexual activity and reproductive isolation between age-specific selected populations of seed beetle. Ethology 2011, 117, 812–821. [Google Scholar] [CrossRef]
- Rönn, J.; Katvala, M.; Arnqvist, G. Coevolution between harmful male genitalia and female resistance in seed beetles. Proc. Natl. Acad. Sci. USA 2007, 104, 10921–10925. [Google Scholar] [CrossRef]
- Maklakov, A.A.; Kremer, N.; Arnquist, G. The effects of age at mating on female life-history traits in a seed beetle. Behav. Ecol. 2007, 18, 551–555. [Google Scholar] [CrossRef]
- Cepeda-Pizarro, J.; Vásquez, H.; Veas, H.; Colon, G.O. Relaciones entre tamaño corporal y biomasa en adultos de Tenebrionidae (Coleoptera) de la estepa costera del margen meridional del desierto chileno. Rev. Chil. Hist. Nat. 1996, 69, 67–76. [Google Scholar]
- Alibert, P.; Moureau, B.; Dommergues, J.; David, B. Differentiation at a microgeographical scale within two species of ground beetle, Carabus auronitens and C. nemoralis (Coleoptera, Carabidae): A geometrical morphometric approach. Zool. Scr. 2001, 30, 299–311. [Google Scholar] [CrossRef]
- Düngelhoef, S.; Schmitt, M. Genital feelers: The putative role of parameres and aedeagal sensilla in Coleoptera Phytophaga (Insecta). Genetica 2010, 138, 45. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U.; Kraushaar, U.; Reim, C. Sexual selection on morphological and physiological traits and fluctuating asymmetry in the yellow dung fly. J. Evol. Biol. 2003, 16, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Molet, M.; Wheeler, D.E.; Peeters, C. Evolution of novel mosaic castes in ants: Modularity, phenotypic plasticity, and colonial buffering. Am. Nat. 2012, 180, 328–341. [Google Scholar] [CrossRef]
- Benítez, H.A.; Vidal, M.; Briones, R.; Jerez, V. Sexual dimorphism and morphological variation in populations of Ceroglossus chilensis (Eschscholtz, 1829) (Coleoptera, Carabidae). J. Entomol. Res. Soc. 2010, 12, 87–95. [Google Scholar]
- Sasakawa, K. Utility of geometric morphometrics for inferring feeding habit from mouthpart morphology in insects: Tests with larval Carabidae (Insecta: Coleoptera). Biol. J. Linn. Soc. 2016, 118, 394–409. [Google Scholar] [CrossRef]
- Benítez, H.A.; Lemic, D.; Bažok, R.; Gallardo-Araya, C.M.; Mikac, K.M. Evolutionary directional asymmetry and shape variation in Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae): An example using hind wings. Biol. J. Linn. Soc. 2014, 111, 110–118. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Badyaev, A.V.; Sowry, S.M.; Beckwith, N.J. Inferring developmental modularity from morphological integration: Analysis of individual variation and asymmetry in bumblebee wings. Am. Nat. 2001, 157, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Blanke, A. Analysis of modularity and integration suggests evolution of dragonfly wing venation mainly in response to functional demands. J. R. Soc. Interface 2018, 15, 0277. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.F.; Houle, D. Evolvability, stabilizing selection, and problem of stasis. In Phenotypic Integration: Studying the Ecology and Evolution of Complex Phenotypes; Pigliucci, M., Preston, K., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 130–150. [Google Scholar]
- Muto, L.; Kamimura, Y.; Tanaka, K.M.; Takahashi, A. An innovative ovipositor for niche exploitation impacts genital coevolution between sexes in a fruit-damaging Drosophila. Proc. R. Soc. B Biol. Sci. 2018, 285, 1635. [Google Scholar] [CrossRef] [PubMed]
- Genevcius, B.C.; Simon, M.N.; Moraes, T.; Schwertner, C.F. Copulatory function and development shape modular architecture of genitalia differently in males and females. Evolution 2020, 74, 1048–1062. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).