UDP-Glycosyltransferases from the UGT344 Family Are Involved in Sulfoxaflor Resistance in Aphis gossypii Glover
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Chemicals
2.3. Toxicity Bioassays
2.4. Synergism Bioassays
2.5. Quantitative Real-Time PCR and Data Analysis
2.6. UGT Genes Silencing and Bioassays
2.7. Data Analysis
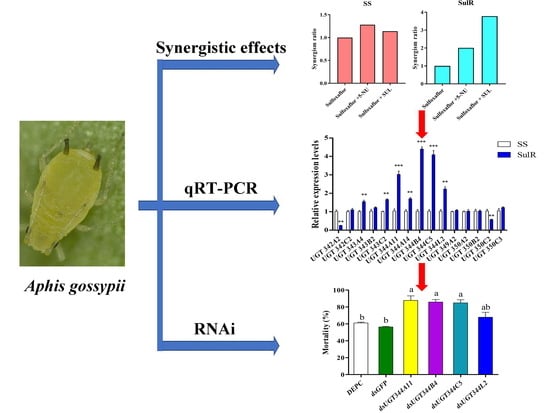
3. Results
3.1. Synergism of Sulfinpyrazone and 5-Nitrouracil
3.2. Expression Profiles of Aphis Gossypii UGT Genes in the Susceptible and Resistant Strains
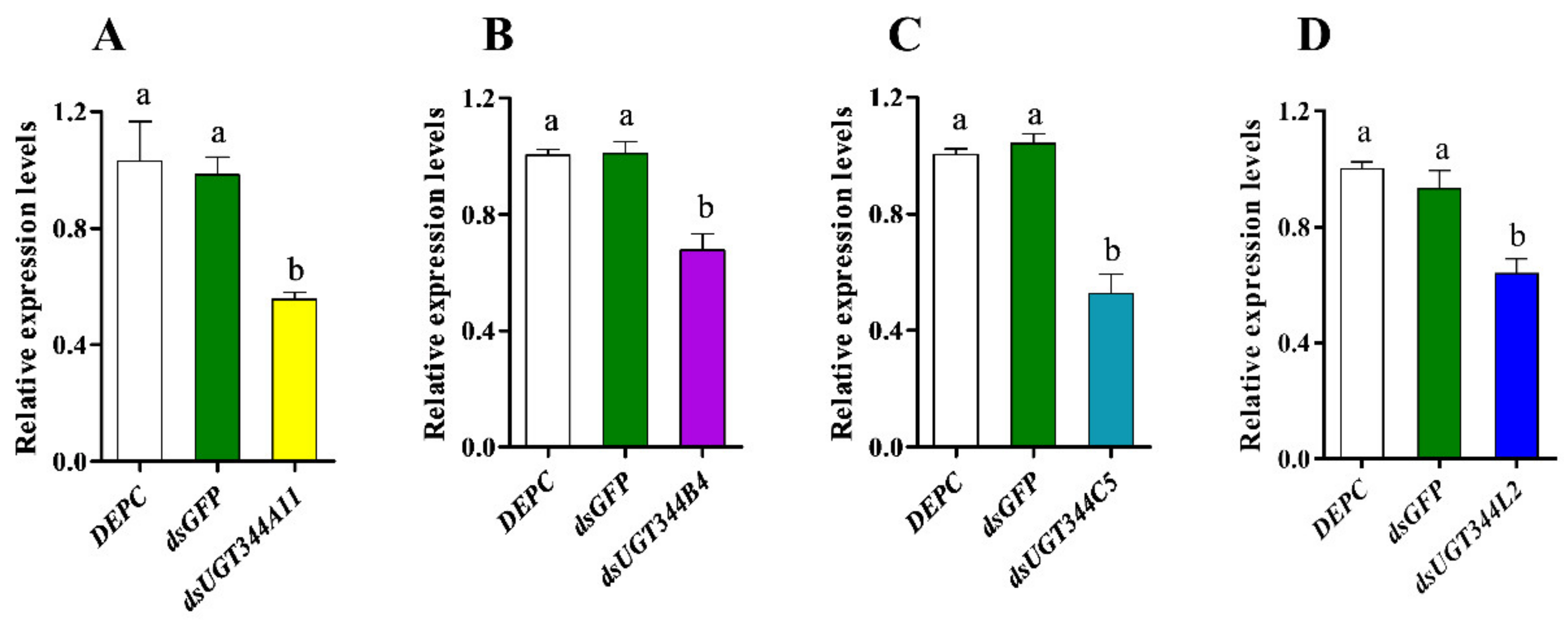
3.3. Effects of UGT Gene Suppression on the Sensitivity of Aphis Gossypii to Sulfoxaflor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops: An Identification Guide; John Wiley and Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Ricupero, M.; Desneux, N.; Zappala, L.; Biondi, A. Target and non-target impact of systemic insecticides on a polyphagous aphid pest and its parasitoid. Chemosphere 2020, 247, 125728. [Google Scholar] [CrossRef]
- Wu, K.M.; Guo, Y.Y. The evolution of cotton pest management practices in China. Annu. Rev. Entomol. 2005, 50, 31–52. [Google Scholar] [CrossRef]
- Ma, K.; Tang, Q.; Zhang, B.; Liang, P.; Wang, B.; Gao, X. Overexpression of multiple cytochrome P450 genes associated with sulfoxaflor resistance in Aphis gossypii Glover. Pestic. Biochem. Physiol. 2019, 157, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.N.; An, J.J.; Park, S.E.; Kim, J.I.; Kim, G.H. Regional susceptibilities to 12 insecticides of melon and cotton aphid, Aphis gossypii (Hemiptera: Aphididae) and a point mutation associated with imidacloprid resistance. Crop Prot. 2014, 55, 91–97. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, X.; Zhang, J.; Gao, X. Polymorphisms in a carboxylesterase gene between organophosphate-resistant and -susceptible Aphis gossypii (Homoptera: Aphididae). J. Econ. Entomol. 2005, 98, 1325–1332. [Google Scholar] [CrossRef]
- Amad, M.; Arif, M.I.; Denholm, I. High resistance of field populations of the cotton aphid Aphis gossypii Glover (Homoptera: Aphididae) to pyrethroid insecticides in Pakistan. J. Econ. Entomol. 2003, 96, 875–878. [Google Scholar] [CrossRef]
- Chen, X.; Tie, M.; Chen, A.; Ma, K.; Li, F.; Liang, P.; Liu, Y.; Song, D.; Gao, X. Pyrethroid resistance associated with M918L mutation and detoxifying metabolism in Aphis gossypii from Bt cotton growing regions of China. Pest Manag. Sci. 2017, 73, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, F.; Chen, A.; Ma, K.; Liang, P.; Liu, Y.; Song, D.; Gao, X. Both point mutations and low expression levels of the nicotinic acetylcholine receptor beta1 subunit are associated with imidacloprid resistance in an Aphis gossypii (Glover) population from a Bt cotton field in China. Pestic. Biochem. Physiol. 2017, 141, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tang, C.; Ma, K.; Xia, J.; Song, D.; Gao, X. Overexpression of UDP-glycosyltransferase potentially involved in insecticide resistance in Aphis gossypii Glover collected from Bt cotton fields in China. Pest Manag. Sci. 2019, 76, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Qi, H.; Yang, D.; Yuan, H.; Rui, C. Cycloxaprid: A novel cis-nitromethylene neonicotinoid insecticide to control imidacloprid-resistant cotton aphid (Aphis gossypii). Pestic. Biochem. Physiol. 2016, 132, 96–101. [Google Scholar] [CrossRef]
- Chen, Y.; Vanlerberghe-Masutti, F.; Wilson, L.J.; Barchia, I.; McLoon, M.O.; Smith, T.; Herron, G.A. Evidence of superclones in Australian cotton aphid Aphis gossypii Glover (Aphididae: Hemiptera). Pest Manag. Sci. 2013, 69, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Mostafiz, M.M.; Hassan, E.; Shim, J.; Lee, K. Insecticidal efficacy of three benzoate derivatives against Aphis gossypii and its predator Chrysoperla carnea. Ecotox. Environ. Safe. 2019, 184, 109653. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwari, D.; Krishna Kumar, N.K.; Manjunatha, H. Multiple mutations on the second acetylcholinesterase gene associated with dimethoate resistance in the melon aphid, Aphis gossypii (Hemiptera: Aphididae). J. Econ. Entomol. 2016, 109, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.B.; Parajulee, M.N. Potential cotton aphid, Aphis gossypii, population suppression by arthropod predators in upland cotton. Insect Sci. 2013, 20, 778–788. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef]
- Grant, C.; Jacobson, R.; Ilias, A.; Berger, M.; Vasakis, E.; Bielza, P.; Zimmer, C.T.; Williamson, M.S.; Ffrench-Constant, R.H.; Vontas, J.; et al. The evolution of multiple-insecticide resistance in UK populations of tomato leafminer, Tuta absoluta. Pest Manag. Sci. 2019, 75, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Hussain, D.; Ghouse, G.; Abbas, M.; Fisher, S.W. Monitoring of insecticide resistance in Spodoptera litura (Lepidoptera: Noctuidae) from four districts of Punjab, Pakistan to conventional and new chemistry insecticides. Crop Prot. 2016, 79, 177–184. [Google Scholar] [CrossRef]
- Umina, P.A.; Lord, A.; Micic, S.; Edwards, O. Discovery and characterisation of field resistance to organophosphorus chemicals in a major mite pest, Halotydeus destructor. Pest Manag. Sci. 2017, 73, 1719–1724. [Google Scholar] [CrossRef]
- Voudouris, C.; Kati, A.N.; Sadikoglou, E.; Williamson, M.; Skouras, P.J.; Dimotsiou, O.; Georgiou, S.; Fenton, B.; Skavdis, G.; Margaritopoulos, J.T. Insecticide resistance status of Myzus persicae in Greece: Long-term surveys and new diagnostics for resistance mechanisms. Pest Manag. Sci. 2016, 72, 671–683. [Google Scholar] [CrossRef]
- Ziapour, S.P.; Kheiri, S.; Fazeli-Dinan, M.; Sahraei-Rostami, F.; Mohammadpour, R.A.; Aarabi, M.; Nikookar, S.H.; Sarafrazi, M.; Asgarian, F.; Enayati, A.; et al. Pyrethroid resistance in Iranian field populations of Rhipicephalus (Boophilus) annulatus. Pestic. Biochem. Physiol. 2017, 136, 70–79. [Google Scholar] [CrossRef]
- Dawkar, V.V.; Chikate, Y.R.; Lomate, P.R.; Dholakia, B.B.; Gupta, V.S.; Giri, A.P. Molecular insights into resistance mechanisms of Lepidopteran insect pests against toxicants. J. Proteome Res. 2013, 12, 4727–4737. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, J.; Gao, X.; Liang, P.; Guo, H. Overexpression of carboxylesterase gene associated with organophosphorous insecticide resistance in cotton aphids, Aphis gossypii (Glover). Pestic. Biochem. Physiol. 2008, 90, 175–180. [Google Scholar] [CrossRef]
- Field, L.; Williamson, M.; Moores, G.; Devonshire, A. Cloning and analysis of the esterase genes conferring insecticide resistance in the peach-potato aphid, Myzus persicae (sulzer). Biochem. J. 1993, 294, 569–574. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gong, Y.; Ai, G.; Li, M.; Shi, X.; Diao, Q.; Gao, X. Functional characterization of carboxylesterase gene mutations involved in Aphis gossypii resistance to organophosphate insecticides. Insect Mol. Biol. 2017, 26, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Pavlidi, N.; Vontas, J.; Van Leeuwen, T. The role of glutathione S-transferases (GSTs) in insecticide resistance in crop pests and disease vectors. Curr. Opin. Insect Sci. 2018, 27, 97–102. [Google Scholar] [CrossRef]
- Li, X.; Li, R.; Zhu, B.; Gao, X.; Liang, P. Overexpression of cytochrome P450 CYP6BG1 may contribute to chlorantraniliprole resistance in Plutella xylostella (L.). Pest Manag. Sci. 2017, 74, 1386–1393. [Google Scholar] [CrossRef]
- Bass, C.; Carvalho, R.A.; Oliphant, L.; Puinean, A.M.; Field, L.M.; Nauen, R.; Williamson, M.S.; Moores, G.; Gorman, K. Overexpression of a cytochrome P450 monooxygenase, CYP6ER1, is associated with resistance to imidacloprid in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2011, 20, 763–773. [Google Scholar] [CrossRef]
- Ding, Z.; Wen, Y.; Yang, B.; Zhang, Y.; Liu, S.; Liu, Z.; Han, Z. Biochemical mechanisms of imidacloprid resistance in Nilaparvata lugens: Over-expression of cytochrome P450 CYP6AY1. Insect Biochem. Mol. Biol. 2013, 43, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Jin, R.; Zhang, X.; Ali, E.; Mao, K.; Xu, P.; Li, J.; Wan, H. Characterization of sulfoxaflor resistance in the brown planthopper, Nilaparvata lugens (Stal). Pest Manag. Sci. 2018, 75, 1646–1654. [Google Scholar] [CrossRef]
- Sun, X.; Gong, Y.; Ali, S.; Hou, M. Mechanisms of resistance to thiamethoxam and dinotefuran compared to imidacloprid in the brown planthopper: Roles of cytochrome P450 monooxygenase and a P450 gene CYP6ER1. Pestic. Biochem. Physiol. 2018, 150, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.; Sun, H.; Liu, Z. Metabolic imidacloprid resistance in the brown planthopper, Nilaparvata lugens, relies on multiple P450 enzymes. Insect Biochem. Mol. Biol. 2016, 79, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Mao, K.; Liao, X.; Xu, P.; Li, Z.; Ali, E.; Wan, H.; Li, J. Overexpression of CYP6ER1 associated with clothianidin resistance in Nilaparvata lugens (Stal). Pestic. Biochem. Physiol. 2019, 154, 39–45. [Google Scholar] [CrossRef]
- Field, L.M.; Blackman, R.L.; Tyler-Smith, C.; Devonshire, A.L. Relationship between amount of esterase and gene copy number in insecticide-resistant Myzus persicae (Sulzer). Biochem. J. 1999, 339, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Field, L.M.; Devonshire, A.L. Evidence that the E4 and FE4 esterase genes responsible for insecticide resistance in the aphid Myzus persicae (Sulzer) are part of a gene family. Biochem. J. 1998, 330, 169–173. [Google Scholar] [CrossRef]
- Tang, Q.; Ma, K.; Hou, Y.; Gao, X. Monitoring insecticide resistance and diagnostics of resistance mechanisms in the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae) in China. Pestic. Biochem. Physiol. 2017, 143, 39–47. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, P.; Zeng, X.; Liu, X.; Shang, Q. Characterization of UDP-glucuronosyltransferases and the potential contribution to nicotine tolerance in Myzus persicae. Int. J. Mol. Sci. 2019, 20, 3637. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xia, J.; Shang, Q.; Song, D.; Gao, X. UDP-glucosyltransferases potentially contribute to imidacloprid resistance in Aphis gossypii glover based on transcriptomic and proteomic analyses. Pestic. Biochem. Physiol. 2019, 159, 98–106. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Shi, L.; Liu, J.; Shen, G.; Zhang, P.; Lu, W.; He, L. Functional analysis of UGT201D3 associated with abamectin resistance in Tetranychus cinnabarinus (Boisduval). Insect Sci. 2020, 27, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, H.; Gao, X.; Liang, P. Characterization of UDP-glucuronosyltransferase genes and their possible roles in multi-insecticide resistance in Plutella xylostella (L.). Pest Manag. Sci. 2018, 74, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, B.; Gao, X.; Liang, P. Over-expression of UDP-glycosyltransferase gene UGT2B17 is involved in chlorantraniliprole resistance in Plutella xylostella (L.). Pest Manag. Sci. 2017, 73, 1402–1409. [Google Scholar] [CrossRef]
- Ahn, S.; Vogel, H.; Heckel, D. Comparative analysis of the UDP-glycosyltransferase multigene family in insects. Insect Biochem. Mol. Biol. 2012, 42, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Badenes-Perez, F.; Reichelt, M.; Svatos, A.; Schneider, B.; Gershenzon, J.; Heckel, D. Metabolic detoxification of capsaicin by UDP-glycosyltransferase in three Helicoverpa species. Arch. Insect Biochem. Physiol. 2011, 78, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Bock, K. The UDP-glycosyltransferase (UGT) superfamily expressed in humans, insects and plants: Animal-plant arms-race and co-evolution. Biochem. Pharmacol. 2016, 99, 11–17. [Google Scholar] [CrossRef]
- Bock, K. Vertebrate UDP-glucuronosyltransferases: Functional and evolutionary aspects. Biochem. Pharmacol. 2003, 66, 691–696. [Google Scholar] [CrossRef]
- Meech, R.; Hu, D.G.; McKinnon, R.A.; Mubarokah, S.; Haines, A.; Nair, P.; Rowland, A.; Mackenzie, P. The UDP-glycosyltransferase (UGT) superfamily: New members, new functions, and novel paradigms. Physiol. Rev. 2019, 99, 1153–1222. [Google Scholar] [CrossRef]
- Ahmad, S.; Hopkins, T. Phenol β-glucosyltransferase and bglucosidase activities in the tobacco hornworm larva Manduca sexta (L.): Properties and tissue localization. Arch. Insect Biochem. 1992, 21, 207–224. [Google Scholar] [CrossRef]
- Kojima, W.; Fujii, T.; Suwa, M.; Miyazawa, M.; Ishikawa, Y. Physiological adaptation of the Asian corn borer Ostrinia furnacalis to chemical defenses of its host plant, maize. J. Insect Physiol. 2010, 56, 1349–1355. [Google Scholar] [CrossRef]
- Krempl, C.; Sporer, T.; Reichelt, M.; Ahn, S.; Heidel-Fischer, H.; Vogel, H.; Heckel, D.G.; Joussen, N. Potential detoxification of gossypol by UDP-glycosyltransferases in the two Heliothine moth species Helicoverpa armigera and Heliothis virescens. Insect Biochem. Mol. Biol. 2016, 71, 49–57. [Google Scholar] [CrossRef]
- Wouters, F.; Reichelt, M.; Glauser, G.; Bauer, E.; Erb, M.; Gershenzon, J.; Vassao, D. Reglucosylation of the benzoxazinoid DIMBOA with inversion of stereochemical configuration is a detoxification strategy in Lepidopteran herbivores. Angew. Chem. Int. Ed. Engl. 2014, 126, 11502–11506. [Google Scholar] [CrossRef]
- Kaplanoglu, E.; Chapman, P.; Scott, I.M.; Donly, C. Overexpression of a cytochrome P450 and a UDP-glycosyltransferase is associated with imidacloprid resistance in the Colorado potato beetle, Leptinotarsa decemlineata. Sci. Rep. 2017, 7, 1762. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, W.; Si, F.; Yan, Z.; Zhang, Y.; He, Q.; Chen, B. UDP-glycosyltransferase genes and their association and mutations associated with pyrethroid resistance in Anopheles sinensis (Diptera: Culicidae). Malaria J. 2019, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, L.; Sun, Y.; Song, P.; Han, Z. UDP-glycosyltransferase genes in the striped rice stem borer, Chilo suppressalis (Walker), and their contribution to chlorantraniliprole resistance. Int. J. Mol. Sci. 2019, 20, 1064. [Google Scholar] [CrossRef]
- Tian, F.; Wang, Z.; Li, C.; Liu, J.; Zeng, X. UDP-Glycosyltransferases are involved in imidacloprid resistance in the Asian citrus psyllid, Diaphorina citri (Hemiptera: Lividae). Pestic. Biochem. Physiol. 2019, 154, 23–31. [Google Scholar] [CrossRef]
- Pan, Y.; Tian, F.; Wei, X.; Wu, Y.; Gao, X.; Xi, J.; Shang, Q. Thiamethoxam resistance in Aphis gossypii Glover relies on multiple UDP-glucuronosyltransferases. Front. Physiol. 2018, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Tian, Y.; Yan, W.; Chen, J.; Zhang, Z.; Xu, H. Drip chemigation of flonicamid effectively controls cotton aphid (Aphis gossypii) and is benign to lady beetle (Coccinella septempunctata) and lacewing larva (Chrysoperla sinica). Crop Prot. 2020, 129, 105039. [Google Scholar] [CrossRef]
- Ma, K.; Tang, Q.; Xia, J.; Lv, N.; Gao, X. Fitness costs of sulfoxaflor resistance in the cotton aphid, Aphis gossypii Glover. Pestic. Biochem. Physiol. 2019, 158, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Moores, G.; Gao, X.; Denholm, I.; Devonshire, A. Characterisation of insensitive acetylcholinesterase in insecticide-resistant cotton aphids, Aphis gossypii Glover (Homoptera: Aphididae). Pestic. Biochem. Physiol. 1996, 56, 102–110. [Google Scholar] [CrossRef]
- Ma, K.; Li, F.; Liang, P.; Chen, X.; Liu, Y.; Tang, Q.; Gao, X. RNA interference of Dicer-1 and Argonaute-1 increasing the sensitivity of Aphis gossypii Glover (Hemiptera: Aphididae) to plant allelochemical. Pestic. Biochem. Physiol. 2017, 138, 71–75. [Google Scholar] [CrossRef]
- Ma, K.; Li, F.; Liang, P.; Chen, X.; Liu, Y.; Gao, X. Identification and validation of reference genes for the normalization of gene expression data in qRT-PCR analysis in Aphis gossypii (Hemiptera: Aphididae). J. Insect Sci. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhu, Y.; Loso, M.; Watson, G.; Sparks, T.; Rogers, R.; Huang, J.; Gerwick, B.; Babcock, J.; Kelley, D.; Hegde, V.; et al. Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J. Agric. Food Chem. 2011, 59, 2950–2957. [Google Scholar] [CrossRef] [PubMed]
- Babcock, J.; Gerwick, C.; Huang, J.; Loso, M.; Nakamura, G.; Nolting, S.; Rogers, R.; Sparks, T.; Thomas, J.; Watson, G.; et al. Biological characterization of sulfoxaflor, a novel insecticide. Pest Manag. Sci. 2011, 67, 328–334. [Google Scholar] [CrossRef] [PubMed]


| Primer Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| EF1α | GAAGCCTGGTATGGTTGTCGT | GGGTGGGTTGTTCTTTGTG |
| β-Actin | GGGAGTCATGGTTGGTATGG | TCCATATCGTCCCAGTTGGT |
| UGT344C5 | GCCGAATCCAGCAACAGTAT | TTCATGAACACCAACGACGG |
| UGT344B4 | GGTTCGTGGGTCACTACTCC | TTGCCCATCTAGTATCTTCTCA |
| UGT344D6 | GTCAGCCCATCTATTATCTTCC | GGCGGGTTTCAGGTGTAT |
| UGT344L2 | TCCGCCGTTCCCAAGAC | CCACCGACACTAACAACATTCG |
| UGT344A11 | GCCAAGCACGGAAGTCA | ACGCACTCGGACACCAG |
| UGT344A14 | GGGACTTGAAGGTTAGGG | ATCGGTGACGGAATGAC |
| UGT343A4 | TCATAACTCACGGAGGATTG | GCACTTCTTTGACGGCATT |
| UGT343B2 | CCGTCAATGGTCTGGGTC | TGAGCGTTCATCAGCGTTA |
| UGT343C2 | ATCCGTCCACTTTACCA | TGAATCCCACTTCCACA |
| UGT342A2 | CAAAGCCACTGTTGCCTAAT | AATACGCTGGTGCTGTTTC |
| UGT342C2 | AAACGACGCTCAACTAACCA | GGAGCCGAGCAATTCTGT |
| UGT349A2 | CGGTGGACTGTTAGGGGTA | CGCATTTATAGCGTAACTGTCA |
| UGT350A2 | CACAGTGTTGAAGAGGCAGT | AGCAGCTCCTCTAGATTCCA |
| UGT350B2 | CATCTATTCCAAATGCTGGTG | TGACGGTCGTGTCTCCC |
| UGT350C2 | AAAATGCCCAAGGAAACAG | GGGAACTCCGTGATAGACG |
| UGT350C3 | GTGTCGCAGTGGCAAGG | CGTTCTGGAGCATCGTCT |
| 344C5-RNAi | taatacgactcactatagggAGCACAAGTACCTCAGAGAGT | taatacgactcactatagggACAACTGATTCTGCTGGTGAC |
| 344B4-RNAi | taatacgactcactatagggACGATGAGTAGAATGCTGTGC | taatacgactcactatagggGACTTGCCGGTTCGATTGTA |
| 344L2-RNAi | taatacgactcactatagggTATGAGTGCTGTGCTTCGAG | taatacgactcactatagggATTGTTGACACCGTTGCTGG |
| 344A11-RNAi | taatacgactcactatagggTGGACATGAACGGATGGTGA | taatacgactcactatagggCGTGCCGATTCAGTGATGAA |
| Strains | Insecticide + Synergist | Slope ± SE a | LC50 (95% CL) (mg L−1) b | χ2 | df | SR c |
|---|---|---|---|---|---|---|
| SS | Sulfoxaflor | 1.18 ± 0.15 | 0.40 (0.24–0.57) | 9.37 | 16 | |
| Sulfoxaflor +5-NU | 0.93 ± 0.14 | 0.32 (0.14–0.51) | 4.84 | 16 | 1.28 | |
| Sulfoxaflor + SUL | 0.96 ± 0.14 | 0.35 (0.17–0.58) | 18.36 | 19 | 1.14 | |
| SulR | Sulfoxaflor | 1.05 ± 0.16 | 131.30 (90.59–209.20) | 11.38 | 16 | |
| Sulfoxaflor +5-NU | 1.09 ± 0.14 | 65.40 (47.34–90.29) | 13.63 | 16 | 2.01 | |
| Sulfoxaflor + SUL | 1.75 ± 0.33 | 34.74 (13.58–52.39) | 23.69 | 16 | 3.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, K.; Tang, Q.; Liang, P.; Li, J.; Gao, X. UDP-Glycosyltransferases from the UGT344 Family Are Involved in Sulfoxaflor Resistance in Aphis gossypii Glover. Insects 2021, 12, 356. https://doi.org/10.3390/insects12040356
Ma K, Tang Q, Liang P, Li J, Gao X. UDP-Glycosyltransferases from the UGT344 Family Are Involved in Sulfoxaflor Resistance in Aphis gossypii Glover. Insects. 2021; 12(4):356. https://doi.org/10.3390/insects12040356
Chicago/Turabian StyleMa, Kangsheng, Qiuling Tang, Pingzhuo Liang, Jianhong Li, and Xiwu Gao. 2021. "UDP-Glycosyltransferases from the UGT344 Family Are Involved in Sulfoxaflor Resistance in Aphis gossypii Glover" Insects 12, no. 4: 356. https://doi.org/10.3390/insects12040356
APA StyleMa, K., Tang, Q., Liang, P., Li, J., & Gao, X. (2021). UDP-Glycosyltransferases from the UGT344 Family Are Involved in Sulfoxaflor Resistance in Aphis gossypii Glover. Insects, 12(4), 356. https://doi.org/10.3390/insects12040356