The Unequal Taxonomic Signal of Mosquito Wing Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Mosquito Colonization
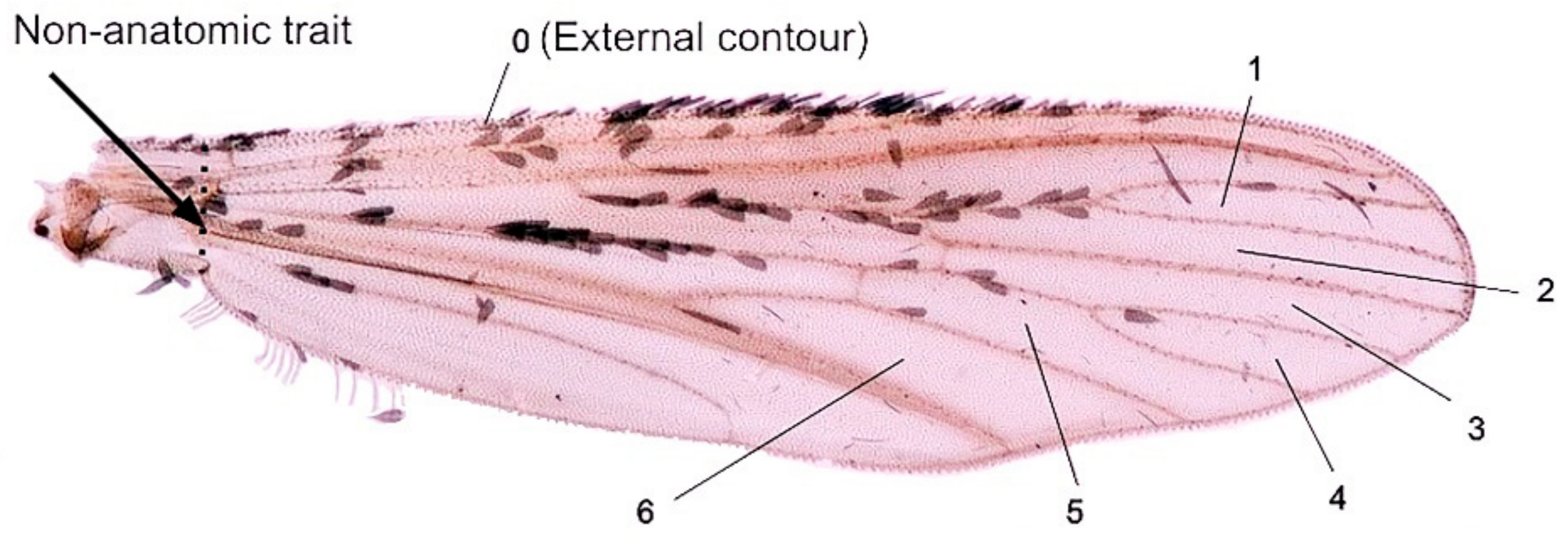
2.3. Wings Preparation for Geometric Morphometric Analysis
2.4. Analyses
2.4.1. Size and Shape
2.4.2. Validated Classification
2.5. Morphometric Software
3. Results
3.1. Wing Size Analysis
3.2. Wing Shape Analysis
3.2.1. Comparing the Taxonomic Information of Different Cells
3.2.2. Reclassifying Four Species
3.2.3. Reclassifying Three Species
3.2.4. Pairwise Reclassifications
4. Discussion
4.1. Wing Size Variation
4.2. Wing Shape Variation
4.2.1. Shape Divergence between Species
4.2.2. Taxonomic Signal among Comparisons
4.2.3. Taxonomic Signal among Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reinert, J.F.; Harbach, R.E.; Kitching, I.J. Phylogeny and classification of Aedini (Diptera: Culicidae), based on morphological characters of all life stages. Zool. J. Linn. Soc. 2004, 142, 289–368. [Google Scholar] [CrossRef] [Green Version]
- Rattanarithikul, R.; Harbach, R.E.; Harrison, B.A.; Panthusiri, P.; Coleman, R.E.; Richardson, J.H. Illustrated keys to the mosquitoes of Thailand VI. Tribe Aedini. Southeast Asian J. Trop. Med. Public Health 2010, 41, 1–225. [Google Scholar] [PubMed]
- Savage, H.M. Classification of mosquitoes in tribe Aedini (Diptera: Culicidae): Paraphylyphobia, and classification versus cladistic analysis. J. Med. Entomol. 2005, 42, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Reinert, J.F. Restoration of Verrallina to generic rank in tribe Aedini (Diptera: Culicidae) and descriptions of the genus and three included subgenera. Contrib. Am. Entomol. Inst. 1999, 31, 1–83. [Google Scholar]
- Potiwat, R.; Komalamisra, N.; Thavara, U.; Tawatsin, A.; Siriyasatien, P. Competitive suppression between chikungunya and dengue virus in Aedes albopictus C6/36 cell line. Southeast Asian J. Trop. Med. Public Health. 2011, 42, 1388–1394. [Google Scholar] [PubMed]
- Sumruayphol, S.; Apiwathnasorn, C.; Ruangsittichai, J.; Sriwichai, P.; Attrapadung, S.; Samung, Y.; Dujardin, J.P. DNA barcoding and wing morphometrics to distinguish three Aedes vectors in Thailand. Acta Trop. 2016, 159, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Puiprom, O.; Morales Vargas, R.E.; Potiwat, R.; Chaichana, P.; Ikuta, K.; Ramasoota, P.; Okabayashi, T. Characterization of chikungunya virus infection of a human keratinocyte cell line: Role of mosquito salivary gland protein in suppressing the host immune response. Infect. Genet. Evol. 2013, 17, 210–215. [Google Scholar] [CrossRef]
- Waewwab, P.; Sungvornyothin, S.; Potiwat, R.; Okanurak, K. Impact of dengue-preventive behaviors on Aedes immature production in Bang Kachao, Samut Prakan Province, Thailand: A cross-sectional study. BMC Public Health 2020, 20, 905. [Google Scholar] [CrossRef]
- Rohani, A.; Potiwat, R.; Zamree, I.; Lee, H.L. Refractoriness of Aedes aegypti (Linnaeus) to dual infection with dengue and chikungunya virus. Southeast Asian J. Trop. Med. Public Health 2009, 40, 443–448. [Google Scholar] [PubMed]
- Phumee, A.; Buathong, R.; Boonserm, R.; Intayot, P.; Aungsananta, N.; Jittmittraphap, A.; Joyjinda, Y.; Wacharapluesadee, S.; Siriyasatien, P. Molecular Epidemiology and Genetic Diversity of Zika Virus from Field-Caught Mosquitoes in Various Regions of Thailand. Pathogens 2019, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.M. Contributions to the mosquito fauna of Southeast Asia. XIV. In The Subgenus Stegomyia of Aedes in Southeast Asia I—The Scutellaris Group of Species; Smithsonian Institution: Washington, DC, USA, 1972; Volume 9, pp. 1–109. [Google Scholar]
- Moore, P.R.; Johnson, P.H.; Smith, G.A.; Ritchie, S.A.; Van Den Hurk, A.F. Infection and dissemination of dengue virus type 2 in Aedes aegypti, Aedes albopictus, and Aedes scutellaris from the Torres Strait, Australia. J. Am. Mosq. Control Assoc. 2007, 23, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Yasuno, M.; Tonn, R.J. Colonization of containers by Aedes aegypti, Aedes albopictus and Aedes scutellaris in the environs of Bangkok, Thailand. Bull. World Health Organ. 1969, 160, 19. [Google Scholar]
- Mackerras, I.M. Transmission of dengue fever by Aedes (Stegomyia) Scutellaris Walk in New Guinea. Trans. R. Soc. Trop. Med. Hyg. 1946, 40, 295–312. [Google Scholar] [CrossRef]
- Desowitz, R.S.; Berman, S.J.; Gubler, D.J.; Harinasuta, C.; Guptavanij, P.; Vasuvat, C. The Effects of Herbicides in South Vietnam: Part B, Working Papers, February 1974: Epidemiological—Ecological Effects: Studies on Intact and Deforested Mangrove Ecosystems. Special Collections, USDA National Agricultural Library; 1974. Available online: http://www.nal.usda.gov/exhibits/speccoll/items/show/4799 (accessed on 28 October 2020).
- Laffoon, J. The Philippine mosquitoes of the genus Aedes, subgenus Aedes. J. Wash. Acad. Sci. 1946, 36, 228–245. [Google Scholar]
- Harbach, R.E.; Kitching, I.J. Phylogeny and classification of the Culicidae (Diptera). Syst. Entomol. 1998, 23, 327–370. [Google Scholar] [CrossRef]
- Lamche, G.; Whelan, P.I. Variability of larval identification characters of exotic Aedes albopictus (Skuse) intercepted in Darwin, Northern Territory. Commun. Dis. Intell. 2003, 27, 105–109. [Google Scholar]
- Wang, G.; Li, C.; Guo, X.; Xing, D.; Dong, Y.; Wang, Z.; Zhang, Y.; Liu, M.; Zheng, Z.; Zhang, H.; et al. Identifying the main mosquito species in China based on DNA barcoding. PLoS ONE 2012, 7, e47051. [Google Scholar] [CrossRef]
- Absavaran, A.; Mohebali, M.; Moin-Vaziri, V.; Zahraei-Ramazani, A.; Akhavan, A.A.; Mozaffarian, F.; Rafizadeh, S.; Rassi, Y. Morphometric discrimination between females of two isomorphic sand fly species, Phlebotomus caucasicus and Phlebotomus mongolensis (Diptera: Phlebotominae) in endemic and non-endemic foci of zoonotic cutaneous leishmaniasis in Iran. Asian Pac. J. Trop. Med. 2019, 12, 153–162. [Google Scholar]
- Villemant, C.; Simbolotti, G.; Kenis, M. Discrimination of Eubazus (Hymenoptera, Braconidae) sibling species using geometric morphometrics analysis of wing venation. Syst. Entomol. 2007, 32, 625–634. [Google Scholar] [CrossRef]
- Zúñiga-Reinosoa, A.; Benítez, H.A. The overrated use of the morphological cryptic species concept: An example with Nyctelia dark beetles (Coleoptera: Tenebrionidae) using geometric morphometrics. Zool. Anz. J. Comp. Zool. 2015, 255, 47–53. [Google Scholar] [CrossRef]
- Chaiphongpachara, T.; Sriwichai, P.; Samung, Y.; Ruangsittichai, J.; Morales Vargas, R.E.; Cui, L.; Jetsumon, S.; Dujardin, J.P.; Suchada, S. Geometric morphometrics approach towards discrimination of three member species of Maculatus group in Thailand. Acta Trop. 2019, 192, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Lydia, A.; Hill, L.; Davis, J.B.; Hapgood, G.; Whelan, P.I.; Smith, G.A.; Ritchie, S.A.; Cooper, R.D.; van den Hurk, A.F. Rapid Identification of Aedes albopictus, Aedes scutellaris, and Aedes aegypti Life Stages Using Real-time Polymerase Chain Reaction Assays. Am. J. Trop. Med. Hyg. 2008, 79, 866–875. [Google Scholar]
- Dujardin, J.P.; Slice, D. Geometric morphometrics. Contributions to medical entomology—Chapter 25. In Encyclopedia of Infectious Diseases Modern Methodologies; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 435–447. [Google Scholar]
- Dujardin, J.P.; Kaba, D.; Solano, P.; Dupraz, M.; McCoy, K.D.; Jaramillo-O, N. Outline-based morphometrics, an overlooked method in arthropod studies? Infect. Genet. Evol. 2014, 28, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Garros, C.; Dujardin, J.P. Genetic and phenetic approaches to Anopheles systematics. In Anopheles Mosquitoes—New Insights into Malaria Vectors; Manguin, S., Ed.; InTech: Rijeka, Croatia, 2013; pp. 81–105. [Google Scholar]
- Wilke, A.; Christe, R.; Multini, L.; Vidal, P.; Wilk, R.; Carvalho, G.; Marrelli, M. Morphometric Wing Characters as a Tool for Mosquito Identification. PLoS ONE 2016, 11, e0161643. [Google Scholar] [CrossRef] [PubMed]
- Ruangsittichai, J.; Apiwathnasorn, C.; Dujardin, J.P. Interspecific and sexual shape variation in the filariasis vectors Mansonia dives and Ma. bonneae. J. Med. Entomol. 2011, 11, 2089–2094. [Google Scholar] [CrossRef]
- Lorenz, C.; Marques, T.C.; Sallum, M.; Suesdek, L. Morphometrical diagnosis of the malaria vectors Anopheles cruzii, An. homunculus and An. bellator. Parasit Vectors 2012, 5, 257. [Google Scholar] [CrossRef] [Green Version]
- Vidal, P.O.; Suesdek, L. Comparison of wing geometry data and genetic data for assessing the population structure of Aedes aegypti. Infect. Genet. Evol. 2012, 12, 591–596. [Google Scholar] [CrossRef]
- Morales Vargas, R.E.; Phumala-Morales, N.; Tsunoda, T.; Apiwathnasorn, C.; Dujardin, J.P. The phenetic structure of Aedes albopictus. Infect. Genet. Evol. 2013, 13, 242–251. [Google Scholar] [CrossRef]
- Virginio, F.; Vidal, P.O.; Suesdek, L. Wing sexual dimorphism of pathogen-vector culicids. Parasit Vectors 2015, 8, 159. [Google Scholar] [CrossRef] [Green Version]
- Sumruayphol, S.; Siribat, P.; Dujardin, J.P.; Dujardin, S.; Komalamisra, C.; Thaenkham, U. Fasciola gigantica, F. hepatica and Fasciola intermediate forms: Geometric morphometrics and an artificial neural network to help morphological identification. PeerJ 2020, 8, e8597. [Google Scholar] [CrossRef] [Green Version]
- Sungvornyothin, S.; Kumlert, R.; Paris, D.H.; Prasartvit, A.; Sonthayanon, P.; Apiwathnasorn, C.; Morand, S.; Stekolnikov, A.A.; Sumruayphol, S. Geometric morphometrics of the scutum for differentiation of trombiculid mites within the genus Walchia (Acariformes: Prostigmata: Trombiculidae), a probable vector of scrub typhus. Ticks Tick Borne Dis. 2019, 10, 495–503. [Google Scholar] [CrossRef]
- Kuhl, F.P.; Giardina, C.R. Elliptic Fourier features of a closed contour. Comput. Gr. Image Process. 1982, 18, 236–258. [Google Scholar] [CrossRef]
- Arnqvist, G.; Mårtensson, T. Measurement error in geometric morphometrics: Empirical strategies to assess and reduce its impact on measures of shape. Acta Zool. Acad. Sci. Hung. 1998, 44, 73–96. [Google Scholar]
- Manly, B.F.J. Multivariate Statistical Methods: A Primer, 3rd ed.; Chapman & Hall/CRC Press: Boca Raton, FL, USA, 2004; p. 214. [Google Scholar]
- Dujardin, S.; Dujardin, J.P. Geometric morphometrics in the cloud. Infect. Genet. Evol. 2019, 70, 189–196. [Google Scholar] [CrossRef]
- Waewwab, P.; Sungvornyothin, S.; Okanurak, K.; Soonthornworasiri, N.; Raksakoon, C.; Potiwat, R. Characteristics of water containers influencing the presence of Aedes immatures in an ecotourism area of Bang Kachao Riverbend, Thailand. J. Health Res. 2019, 33, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Jirakanjanakit, N.; Dujardin, J.P. Discrimination of Aedes aegypti (Diptera; Culicidae) laboratory lines based on wing geometry. Southeast Asian J. Trop. Med. Public Health 2005, 36, 1–4. [Google Scholar]
- Jirakanjanakit, N.; Leemingsawat, S.; Thongrungkiat, S.; Apiwathnasorn, C.; Singhaniyom, S.; Bellec, C.; Dujardin, J.P. Influence of larval density or food variation on the geometry of the wing of Aedes (Stegomyia) aegypti. Trop. Med. Int. Health 2007, 12, 1354–1360. [Google Scholar] [CrossRef]
- Jirakanjanakit, N.; Leemingsawat, S.; Dujardin, J.P. The geometry of the wing of Aedes (Stegomyia) aegypti in isofemale lines through successive generations. Infect. Genet. Evol. 2008, 8, 414–421. [Google Scholar] [CrossRef]
- Phanitchat, T.; Apiwathnasorn, C.; Sungvornyothin, S.; Samung, Y.; Dujardin, S.; Dujardin, J.P.; Sumruayphol, S. Geometric morphometric analysis of the effect of temperature on wing size and shape in Aedes albopictus. Med. Vet. Entomol. 2019, 33, 476–484. [Google Scholar] [CrossRef]
- Morales Vargas, R.E.; Ya-Umphan, P.; Phumala-Morales, N.; Komalamisra, N.; Dujardin, J.P. Climate associated size and shape changes in Aedes aegypti (Diptera: Culicidae) populations from Thailand. Infect. Genet. Evol. 2010, 10, 580–585. [Google Scholar] [CrossRef]
- Perrard, A.; Baylac, M.; Carpenter, J.M.; Villemant, C. Evolution of wing shape in hornets: Why is the wing venation efficient for species identification? J. Evol. Biol. 2014, 27, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, R.A. An introduction to ordination, principal components analysis and discriminant analysis. In Ordination in the Study of Morphology, Evolution and Systematics of Insects: Applications and Quantitative Genetic Rationales; Foottit, R.G., Sorensen, J.T., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; pp. 11–28, 418. [Google Scholar]
- Francoy, T.M.; Franco, F.F.; Roubik, D.W. Integrated landmark and outline-based morphometric methods efficiently distinguish species of Euglossa (Hymenoptera, Apidae, Euglossini). Apidologie 2012, 43, 609–617. [Google Scholar] [CrossRef] [Green Version]









| Species | Locality | Province | Latitude | Longitude | Year | F |
|---|---|---|---|---|---|---|
| Ae. aegypti | Bangkhae | Bangkok | 13°41′43.6″ N | 100°23′05.1″ E | 2019 | F3 |
| Ae. albopictus | Lum Sum | Kanchanaburi | 14°12′16.2″ N | 99°07′58.5″ E | 2009 | F49 |
| Ae. scutellaris | Phasi charoen | Bangkok | 13°43′19.8″ N | 100°26′09.2″ E | 2011 | F33 |
| Ve. dux | Bang Pakong | Chachoengsao | 13°28′25.0″ N | 100°52′19.9″ E | 2019 | F3 |
| Species | N | Mean (mm) | S.D. | S.E. |
|---|---|---|---|---|
| Cell 0 | ||||
| Ae. aegypti | 30 | 5.60 (4.78–6.45) a | 0.45 | 0.08 |
| Ae. albopictus | 30 | 6.10 (5.60–6.54) b | 0.27 | 0.04 |
| Ae. scutellaris | 30 | 5.43 (4.87–5.72) a | 0.16 | 0.03 |
| Ve. dux | 30 | 3.59 (3.09–3.83) c | 0.19 | 0.03 |
| Cell 1 | ||||
| Ae. aegypti | 30 | 1.55 (1.30–1.90) a | 0.14 | 0.02 |
| Ae. albopictus | 30 | 1.58 (1.37–1.80) a,b | 0.11 | 0.02 |
| Ae. scutellaris | 30 | 1.48 (1.24–1.59) a,c | 0.07 | 0.01 |
| Ve. dux | 30 | 0.99 (0.80–1.12) d | 0.07 | 0.01 |
| Cell 2 | ||||
| Ae. aegypti | 30 | 2.19 (1.86–2.52) a | 0.16 | 0.02 |
| Ae. albopictus | 30 | 2.46 (2.23–2.65) b | 0.12 | 0.02 |
| Ae. scutellaris | 30 | 2.20 (1.95–2.30) a | 0.07 | 0.01 |
| Ve. dux | 30 | 1.46 (1.24–1.61) c | 0.08 | 0.01 |
| Cell 3 | ||||
| Ae. aegypti | 30 | 2.10 (1.81–2.36) a | 0.14 | 0.02 |
| Ae. albopictus | 30 | 2.37 (2.12–2.57) b | 0.11 | 0.02 |
| Ae. scutellaris | 30 | 2.08 (1.88–2.20) a | 0.07 | 0.01 |
| Ve. dux | 30 | 1.41 (1.21–1.55) c | 0.08 | 0.01 |
| Cell 4 | ||||
| Ae. aegypti | 30 | 1.22 (1.04–1.46) a | 0.10 | 0.01 |
| Ae. albopictus | 30 | 1.27 (1.09–1.45) a | 0.09 | 0.01 |
| Ae. scutellaris | 30 | 1.25 (1.06–1.40) a | 0.06 | 0.01 |
| Ve. dux | 30 | 0.83 (0.67–0.96) b | 0.06 | 0.01 |
| Cell 5 | ||||
| Ae. aegypti | 30 | 1.84 (1.54–2.14) a | 0.14 | 0.02 |
| Ae. albopictus | 30 | 2.12 (1.92–2.34) b | 0.10 | 0.01 |
| Ae. scutellaris | 30 | 1.89 (1.69–1.98) a | 0.06 | 0.01 |
| Ve. dux | 30 | 1.25 (1.06–1.37) c | 0.07 | 0.01 |
| Cell 6 | ||||
| Ae. aegypti | 30 | 2.13 (1.75–2.54) a | 0.17 | 0.03 |
| Ae. albopictus | 30 | 2.37 (2.13–2.62) b | 0.14 | 0.02 |
| Ae. scutellaris | 30 | 2.14 (1.97–2.34) a | 0.09 | 0.01 |
| Ve. dux | 30 | 1.42 (1.22–1.58) c | 0.09 | 0.01 |
| Ae. aegypti | Ae. albopictus | Ae. scutellaris | Ve. dux | Total | |
|---|---|---|---|---|---|
| Cell 0 | 80% (24/30) | 80% (24/30) | 93% (28/30) | 77% (23/30) | 82% (99/120) |
| Cell 1 | 43% (16/30) | 40% (12/30) | 70% (21/30) | 80% (24/30) | 61% (73/120) |
| Cell 2 | 83% (25/30) | 77% (23/30) | 90% (27/30) | 83% (25/30) | 83% (100/120) |
| Cell 3 | 77% (23/30) | 83% (25/30) | 70% (21/30) | 80% (24/30) | 77% (93/120) |
| Cell 4 | 70% (21/30) | 60% (18/30) | 80% (24/30) | 100% (30/30) | 77% (93/100) |
| Cell 5 | 73% (22/30) | 83% (25/30) | 87% (26/30) | 93% (28/30) | 84% (101/120) |
| Cell 6 | 3% (1/30) | 53% (16/30) | 7% (2/30) | 60% (18/30) | 31% (37/120) |
| Ae. aegypti | Ae. albopictus | Ae. scutellaris | Total | |
|---|---|---|---|---|
| Cell 0 | 80% (24/30) | 77% (23/30) | 93% (28/30) | 83% (75/90) |
| Cell 1 | 57% (17/30) | 53% (16/30) | 67% (20/30) | 59% (53/90) |
| Cell 2 | 90% (27/30) | 83% (25/30) | 90% (27/30) | 88% (79/90) |
| Cell 3 | 77% (23/30) | 87% (26/30) | 77% (23/30) | 80% (72/90) |
| Cell 4 | 70% (21/30) | 63% (19/30) | 83% (25/30) | 72% (65/90) |
| Cell 5 | 80% (24/30) | 83% (25/30) | 83% (25/30) | 82% (74/90) |
| Cell 6 | 43% (13/30) | 57% (17/30) | 7% (2/30) | 36% (32/90) |
| Contours | Aedes spp. | aeg | aeg | aeg | alb | alb | scu | Average |
|---|---|---|---|---|---|---|---|---|
| Ve. dux | alb | scu | dux | scu | dux | dux | ||
| cell0 | 90% | 77% | 77% | 85% | 93% | 83% | 90% | 85% |
| cell1 | 82% | 67% | 82% | 75% | 77% | 87% | 95% | 81% |
| cell2 | 84% | 92% | 98% | 85% | 87% | 85% | 85% | 88% |
| cell3 | 86% | 95% | 83% | 83% | 92% | 90% | 97% | 89% |
| cell4 | 94% | 63% | 95% | 92% | 78% | 90% | 98% | 87% |
| cell5 | 96% | 92% | 88% | 98% | 97% | 92% | 93% | 94% |
| cell6 | 58% | 83% | 80% | 87% | 77% | 92% | 82% | 80% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chonephetsarath, S.; Raksakoon, C.; Sumruayphol, S.; Dujardin, J.-P.; Potiwat, R. The Unequal Taxonomic Signal of Mosquito Wing Cells. Insects 2021, 12, 376. https://doi.org/10.3390/insects12050376
Chonephetsarath S, Raksakoon C, Sumruayphol S, Dujardin J-P, Potiwat R. The Unequal Taxonomic Signal of Mosquito Wing Cells. Insects. 2021; 12(5):376. https://doi.org/10.3390/insects12050376
Chicago/Turabian StyleChonephetsarath, Somsanith, Chadchalerm Raksakoon, Suchada Sumruayphol, Jean-Pierre Dujardin, and Rutcharin Potiwat. 2021. "The Unequal Taxonomic Signal of Mosquito Wing Cells" Insects 12, no. 5: 376. https://doi.org/10.3390/insects12050376
APA StyleChonephetsarath, S., Raksakoon, C., Sumruayphol, S., Dujardin, J.-P., & Potiwat, R. (2021). The Unequal Taxonomic Signal of Mosquito Wing Cells. Insects, 12(5), 376. https://doi.org/10.3390/insects12050376







