Designing Aedes (Diptera: Culicidae) Mosquito Traps: The Evolution of the Male Aedes Sound Trap by Iterative Evaluation
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Experimental Conditions and Standard Trial Methods
2.2. Standard Trap Design
2.3. Experiment 1. MAST Base Height—Semi-Field Cage Trial
2.4. Experiment 2. MAST Base Height—Single Premises Field Trial
2.5. Experiment 3. MAST Base Height—Multiple Premisses Field Trial
2.6. Experiment 4. MAST Head—Various Entrance Numbers, Sizes, and Shapes Semi-Field Cage Trial
2.7. Experiment 5. MAST Head—Various Entrance Sizes Semi-Field Cage Trial
2.8. Experiment 6. Sound Lure—Continuous vs. Intermittent Tones Semi-Field Cage Trial
2.9. Experiment 7. Sound Lure—Various Volumes Semi-Field Cage Trial
2.10. Experiment 8. Sound Lure—Various Frequencies Tent Trial
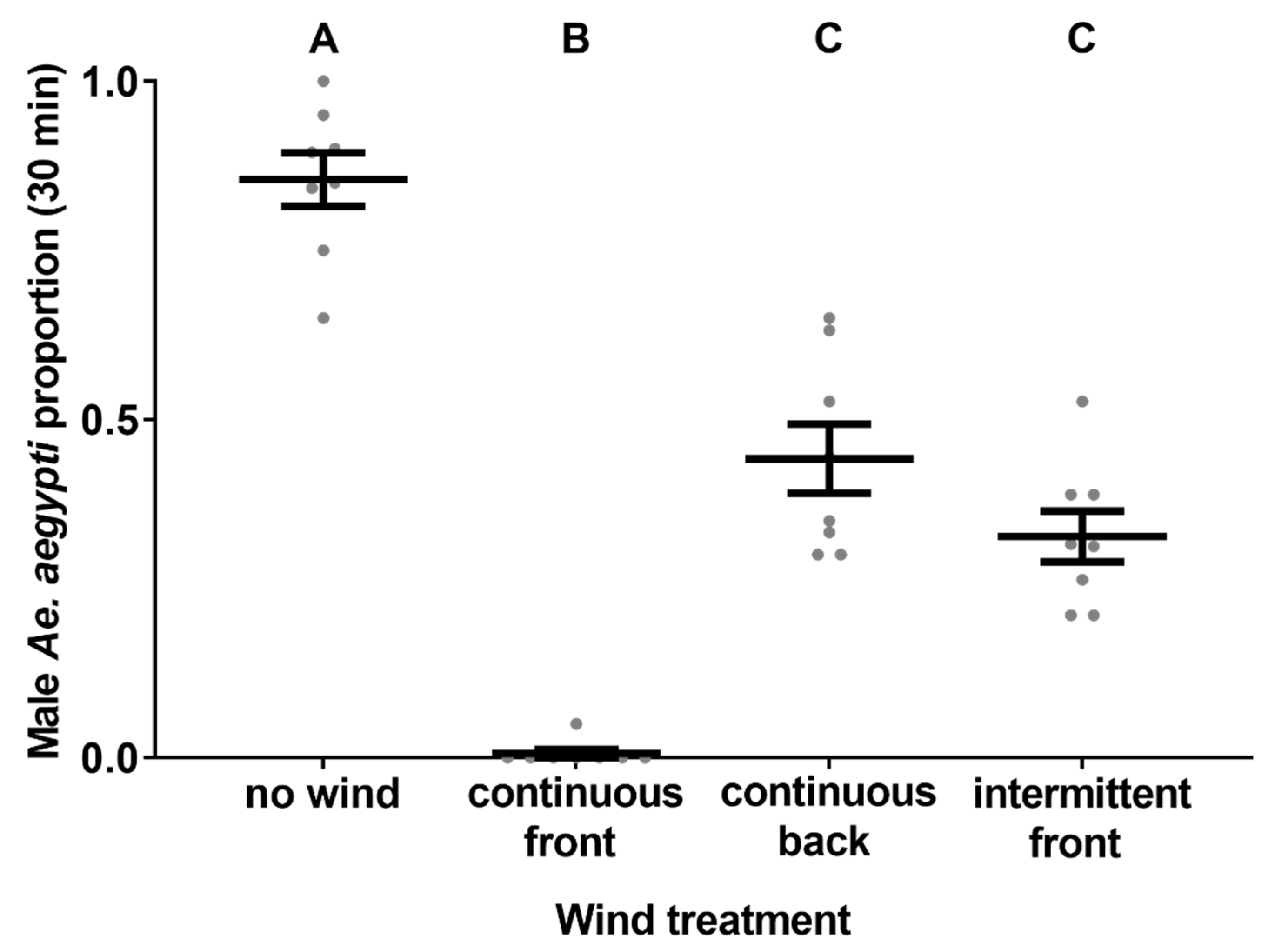
2.11. Experiment 9. Influence of Wind on Catch Rates Tent Trial
2.12. Data Analysis
3. Results
3.1. Experiment 1. MAST Base Height—Flight Cage
3.2. Experiment 2. MAST Base Height—Single Premises Field Trial
3.3. Experiment 3. MAST Base Height—Multiple Premisses
3.4. Experiment 4. MAST Head—Various Entrance Numbers Sizes and Shapes
3.5. Experiment 5. MAST Head—Various Entrance Sizes
3.6. Experiment 6. Sound Lure—Continuous vs. Intermittent Tones
3.7. Experiment 7. Sound Lure—Various Volumes
3.8. Experiment 8. Sound Lure—Various Frequencies
3.9. Experiment 9. Influence of Wind on Catch Rates
4. Discussion
4.1. Base Height
4.2. Entrance Types
4.3. Sound Lure Settings
4.4. Wind Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powell, J.R. Mosquito-borne human viral diseases: Why Aedes aegypti? Am. J. Trop. Med. Hyg. 2018, 98, 1563–1565. [Google Scholar] [CrossRef]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Bowman, L.R.; Runge-Ranzinger, S.; McCall, P.J. Assessing the relationship between vector indices and dengue transmission: A systematic review of the evidence. PLoS Negl. Trop. Dis. 2014, 8, e2848. [Google Scholar] [CrossRef]
- Silver, J.B. Mosquito Ecology: Field Sampling Methods; Springer Science & Business Media: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Day, J.F. Mosquito oviposition behavior and vector control. Insects 2016, 7, 65. [Google Scholar] [CrossRef]
- Muir, L.E.; Thorne, M.J.; Kay, B.H. Aedes aegypti (Diptera: Culicidae) vision: Spectral sensitivity and other perceptual parameters of the female eye. J. Med. Entomol. 1992, 29, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Christophers, S.R. Aedes aegypti (L.) the Yellow Fever Mosquito: Its Life History, Bionomics and Structure; Cambridge University Press: Cambridge, UK, 1960. [Google Scholar]
- Barrera, R.; Mackay, A.J.; Amador, M. An improved trap to capture adult container-inhabiting mosquitoes. J. Am. Mosq. Control 2013, 29, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Muir, L.E.; Kay, B.H.; Thorne, M.J. Aedes aegypti (Diptera: Culicidae) vision: Response to stimuli from the optical environment. J. Med. Entomol. 1992, 29, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Edman, J.; Kittayapong, P.; Linthicum, K.; Scott, T. Attractant resting boxes for rapid collection and surveillance of Aedes aegypti (L.) inside houses. J. Am. Mosq. Control Assoc. 1997, 13, 24–27. [Google Scholar]
- Kröckel, U.; Rose, A.; Eiras, Á.E.; Geier, M. New tools for surveillance of adult yellow fever mosquitoes: Comparison of trap catches with human landing rates in an urban environment. J. Am. Mosq. Control Assoc. 2006, 22, 229–238. [Google Scholar] [CrossRef]
- Geier, M.; Rose, A.; Grunewald, J.; Jones, O. New mosquito traps improve the monitoring of disease vectors. Int. Pest Control 2006, 48, 124–126. [Google Scholar]
- Eiras, A.E.; Buhagiar, T.S.; Ritchie, S.A. Development of the Gravid Aedes Trap for the capture of adult female container–exploiting mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.J.; Amador, M.; Barrera, R. An improved autocidal gravid ovitrap for the control and surveillance of Aedes aegypti. Parasites Vectors 2013, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Chamberlain, R. A light trap and mechanical aspirator operating on dry cell batteries. Mosq. News 1955, 15, 28–32. [Google Scholar]
- Staunton, K.M.; Crawford, J.E.; Liu, J.; Townsend, M.; Han, Y.; Desnoyer, M.; Howell, P.; Xiang, W.; Burkot, T.R.; Snoad, N.; et al. A low-powered and highly selective trap for male Aedes (Diptera: Culicidae) surveillance: The Male Aedes Sound Trap. J. Med. Entomol. 2021, 58, 408–415. [Google Scholar] [CrossRef]
- Heringer, L.; Johnson, B.J.; Fikrig, K.; Oliveira, B.A.; Silva, R.D.; Townsend, M.; Barrera, R.; Eiras, A.E.; Ritchie, S.A. Evaluation of alternative killing agents for Aedes aegypti (Diptera: Culicidae) in the gravid Aedes trap (GAT). J. Med. Entomol. 2016, 53, 873–879. [Google Scholar] [CrossRef]
- Azil, A.H.; Ritchie, S.A.; Williams, C.R. Field worker evaluation of dengue vector surveillance methods: Factors that determine perceived ease, difficulty, value, and time effectiveness in Australia and Malaysia. Asia Pac. J. Public Health 2015, 27, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Akaratovic, K.I.; Kiser, J.P.; Gordon, S.; Abadam, C.F. Evaluation of the Trapping Performance of Four Biogents AG Traps and Two Lures for the Surveillance of Aedes albopictus and Other Host-Seeking Mosquitoes. J. Am. Mosq. Control Assoc. 2017, 33, 108–115. [Google Scholar] [CrossRef]
- Ross, P.A.; Turelli, M.; Hoffmann, A.A. Evolutionary Ecology of Wolbachia Releases for Disease Control. Annu. Rev. Genet. 2019, 53, 93–116. [Google Scholar] [CrossRef]
- Crawford, J.E.; Clarke, D.W.; Criswell, V.; Desnoyer, M.; Cornel, D.; Deegan, B.; Gong, K.; Hopkins, K.C.; Howell, P.; Hyde, J.S.; et al. Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat. Biotechnol. 2020, 38, 482–492. [Google Scholar] [CrossRef]
- Belton, P. Attraction of male mosquitoes to sound. J. Am. Mosq. Control Assoc. 1994, 10, 297–301. [Google Scholar] [PubMed]
- Roth, L.M. A study of mosquito behavior. An experimental laboratory study of the sexual behavior of Aedes aegypti (Linnaeus). Am. Midl. Nat. 1948, 40, 265–352. [Google Scholar] [CrossRef]
- Johnson, B.J.; Ritchie, S.A. The siren’s song: Exploitation of female flight tones to passively capture male Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2015, 53, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.J.; Rohde, B.B.; Zeak, N.; Staunton, K.M.; Prachar, T.; Ritchie, S.A. A low-cost, battery-powered acoustic trap for surveilling male Aedes aegypti during rear-and-release operations. PLoS ONE 2018, 13, e0201709. [Google Scholar] [CrossRef]
- Rohde, B.B.; Staunton, K.M.; Zeak, N.C.; Beebe, N.; Snoad, N.; Bondarenco, A.; Liddington, C.; Anderson, J.A.; Xiang, W.; Mankin, R.W.; et al. Waterproof, low-cost, long-battery-life sound trap for surveillance of male Aedes aegypti for rear-and-release mosquito control programmes. Parasites Vectors 2019, 12, 417. [Google Scholar] [CrossRef]
- Swan, T.; Russell, T.L.; Burkot, T.R.; Liu, J.; Ritchie, S.A.; Staunton, K.M. The effect of sound lure frequency and habitat type on male Aedes albopictus (Diptera: Culicidae) capture rates with the Male Aedes Sound Trap. J. Med. Entomol. 2020, 58, 708–716. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Staunton, K.M.; Xiang, W.; Han, Y.; Snoad, N.; Liu, J.; Crawford, J.E.; Desnoyer, M. Insect Trapping Systems. U.S. Patent US20200305406A1, 1 October 2020. [Google Scholar]
- Staunton, K.M.; Leiva, D.; Cruz, A.; Goi, J.; Arisqueta, C.; Liu, J.; Desnoyer, M.; Howell, P.; Espinosa, F.; Mendoza, A.C.; et al. Outcomes from international field trials with Male Aedes Sound Traps: Frequency-dependent effectiveness in capturing target species in relation to bycatch abundance. PLoS Negl. Trop. Dis. 2021, 15, e0009061. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Johnson, P.H.; Freeman, A.J.; Odell, R.G.; Graham, N.; DeJong, P.A.; Standfield, G.W.; Sale, R.W.; O’Neill, S.L. A secure semi-field system for the study of Aedes aegypti. PLoS Negl. Trop. Dis. 2011, 5, e988. [Google Scholar] [CrossRef] [PubMed]
- Darbro, J.M.; Johnson, P.H.; Thomas, M.B.; Ritchie, S.A.; Kay, B.H.; Ryan, P.A. Effects of Beauveria bassiana on survival, blood-feeding success, and fecundity of Aedes aegypti in laboratory and semi-field conditions. Am. J. Trop. Med. Hyg. 2012, 86, 656–664. [Google Scholar] [CrossRef]
- Ryan, P.A.; Turley, A.P.; Wilson, G.; Hurst, T.P.; Retzki, K.; Brown-Kenyon, J.; Hodgson, L.; Kenny, N.; Cook, H.; Montgomery, B.L.; et al. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia [version 2; peer review: 2 approved]. Gates Open Res. 2020, 3, 1547. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 25 April 2021).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 2nd ed.; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. emmeans: Estimated Marginal Means, aka Least-Squares Means. emmeans Package Version 1.4.2. 2019. Available online: https://cran.r-project.org/package=emmeans (accessed on 21 January 2018).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Jakhete, S.S.; Allan, S.A.; Mankin, R.W. Wingbeat frequency-sweep and visual stimuli for trapping male Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2017, 54, 1415–1419. [Google Scholar] [CrossRef]
- Menda, G.; Nitzany, E.I.; Shamble, P.S.; Wells, A.; Harrington, L.C.; Miles, R.N.; Hoy, R.R. The long and short of hearing in the mosquito Aedes aegypti. Curr. Biol. 2019, 29, 709–714.e4. [Google Scholar] [CrossRef] [PubMed]
- Wishart, G.; Riordan, D. Flight responses to various sounds by adult males of Aedes aegypti (L.) (Diptera: Culicidae). Can. Entomol. 1959, 91, 181–191. [Google Scholar] [CrossRef]
- Balestrino, F.; Iyaloo, D.P.; Elahee, K.B.; Bheecarry, A.; Campedelli, F.; Carrieri, M.; Bellini, R. A sound trap for Aedes albopictus (Skuse) male surveillance: Response analysis to acoustic and visual stimuli. Acta Trop. 2016, 164, 448–454. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Buhagiar, T.S.; Townsend, M.; Hoffmann, A.; van den Hurk, A.F.; McMahon, J.L.; Eiras, A.E. Field validation of the Gravid Aedes Trap (GAT) for collection of Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Barrera, R.; Amador, M.; Acevedo, V.; Caban, B.; Felix, G.; Mackay, A.J. Use of the CDC autocidal gravid ovitrap to control and prevent outbreaks of Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 145–154. [Google Scholar] [CrossRef]
- Staunton, K.M.; Rohde, B.B.; Townsend, M.; Liu, J.; Desnoyer, M.; Howell, P.; Amos, B.; Crawford, J.; Snoad, N.; Ritchie, S.A. Investigating male Aedes aegypti (Diptera: Culicidae) attraction to different oviposition containers using various configurations of the Sound Gravid Aedes Trap. J. Med. Entomol. 2019, 57, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.B. The Physics of Speech; Cambridge University Press: Cambridge, UK, 1979. [Google Scholar]
- Costello, R.A. Effects of Environmental and Physiological Factors on the Acoustic Behaviour of Aedes aegypti (L.) (Diptera: Culicidae); Simon Fraser University: Burnaby, BC, Canada, 1974. [Google Scholar]
- Brogdon, W.G. Measurement of flight tone differences between female Aedes aegypti and A. albopictus (Diptera: Culicidae). J. Med. Entomol. 1994, 31, 700–703. [Google Scholar] [CrossRef]
- Villarreal, S.M.; Winokur, O.; Harrington, L. The impact of temperature and body size on fundamental flight tone variation in the mosquito vector Aedes aegypti (Diptera: Culicidae): Implications for acoustic lures. J. Med. Entomol. 2017, 54, 1116–1121. [Google Scholar] [CrossRef]
- Tischner, H.; Schief, A. Flight-noise and sound perception of Aedes aegypti (L.) (Culicidae). Ger. Zool. Soc. 1954, 18, 453–460. [Google Scholar]
- Moore, A.; Miller, J.R.; Tabashnik, B.E.; Gage, S.H. Automated identification of flying insects by analysis of wingbeat frequencies. J. Econ. Entomol. 1986, 79, 1703–1706. [Google Scholar] [CrossRef]
- Kennedy, J.S. The visual responses of flying mosquitoes. J. Zool. 1939, 109, 221–242. [Google Scholar] [CrossRef]
- Amos, B.A.; Ritchie, S.A.; Cardé, R.T. Attraction versus capture II: Efficiency of the BG-Sentinel trap under semifield conditions and characterizing response behaviors of male Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2020, 57, 1539–1549. [Google Scholar] [CrossRef] [PubMed]




| Title | Development and validation of a male Aedes sound trap | |
| Category | Vector surveillance | |
| Disease | Dengue, Zika, chikungunya, yellow fever | |
| Grantee Organization | Verily Life Sciences | |
| Product Class | New vector surveillance tool | |
| Product Description | A sound trap targeting male adult Aedes aegypti mosquitoes | |
| Item | Desired Target | Minimally Acceptable Standard |
| Indication | Develop novel entomological surveillance tool to trap male Aedes mosquitoes (especially Ae. aegypti) comparable to the catch rates in adult traps in use (e.g., Biogents Sentinel trap 2) | Develop novel entomological surveillance tool to trap male Aedes mosquitoes, especially Ae. aegypti. |
| Target Human Population (s) | All age groups and populations in vector borne disease endemic countries in key space | All age groups and populations in vector borne disease endemic countries in key space |
| Application Method | For use in a variety of surveillance programs | For use in a male release programs throughout urban landscapes |
| Safety | Trapping device safe for use in or near human dwellings | Is not an electrical or fire hazard, does not include the use of non-WHO recommended insecticides |
| Expected Performance: | Male Ae. aegypti catch rates exceed other adult traps in use (e.g., Biogents Sentinel trap 2, Sound BG-GAT) | Male Ae. aegypti catch rates are comparable to other adult traps in use (e.g., Biogents Sentinel trap 2, Sound-GAT) |
| Trap uses small (e.g., AA) batteries which last long periods of time (e.g., 1–2 months) | Trap uses small (e.g., AA) batteries which last one week | |
| Specimens are retained in suitable condition for morphological identification and/or genetic analysis after 1–2 months of deployment | Specimens are retained in suitable condition for morphological identification and/or genetic analysis after 1 week of deployment | |
| Trap design is able to support sensors and protect them from environmental conditions even in unsheltered locations | Trap design is able to support sensors, but must be placed in sheltered location to protect sensors from environmental conditions | |
| Trap design supports varying audio levels, frequencies (single and sweeps) and programmable for timed operations to enable targeting of different Aedes species | Trap design supports varying audio levels and frequencies to enable targeting of different Aedes species | |
| Nontarget organisms and environmental risk Assessment | The proportion of nontarget organisms captured in this trap is <1% and are able to be identified by sensor equipment | The proportion of nontarget organisms captured in this trap is <5% and are able to be identified by sensor equipment |
| The abundance of nontarget organisms killed in this trap is significantly less than other traps using fan capture systems | Acceptable risk to non-target organisms and environment when product is used according to directions | |
| Trap will not act as larval habitat | Trap able to be treated so that it remains as a non-productive larval habitat | |
| Freedom to operate | Commercialisation controlled by Verily Life Sciences | Patented by Verily Life Sciences |
| Shelf life/storage stability | Product able to be stored for years without reduction in performance | Product able to be stored for duration comparable with other mosquito traps |
| User acceptability | Trap design facilitates reasonable ‘ease of use’ so that it is deployable and easily serviceable by a range of general surveillance staff | Trap design facilitates reasonable ‘ease of use’ so that it is deployable and serviceable so that is suitable to male release programs |
| Very quiet, but effective noise (sound lure not irritable to occupants) | Acceptable noise (sound lure not irritable to occupants) | |
| No smell (odour from lures not irritable to occupants) | Acceptable smell (odour from lures not irritable to occupants) | |
| Target price | Significantly less expensive than other mosquito traps, especially when deployed at scale | Commercially competitive with other mosquito traps, especially when deployed at scale |
| Challenges/Risks | Trap components unattractive to community and unlikely to be tampered with. | Trap components are still attractive to community and therefore may be tampered with, but are easily replaceable. |
| Effective on limited spectrum of Aedes spp. | Effective collection method on key regional Aedes spp. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staunton, K.M.; Liu, J.; Townsend, M.; Desnoyer, M.; Howell, P.; Crawford, J.E.; Xiang, W.; Snoad, N.; Burkot, T.R.; Ritchie, S.A. Designing Aedes (Diptera: Culicidae) Mosquito Traps: The Evolution of the Male Aedes Sound Trap by Iterative Evaluation. Insects 2021, 12, 388. https://doi.org/10.3390/insects12050388
Staunton KM, Liu J, Townsend M, Desnoyer M, Howell P, Crawford JE, Xiang W, Snoad N, Burkot TR, Ritchie SA. Designing Aedes (Diptera: Culicidae) Mosquito Traps: The Evolution of the Male Aedes Sound Trap by Iterative Evaluation. Insects. 2021; 12(5):388. https://doi.org/10.3390/insects12050388
Chicago/Turabian StyleStaunton, Kyran M., Jianyi Liu, Michael Townsend, Mark Desnoyer, Paul Howell, Jacob E. Crawford, Wei Xiang, Nigel Snoad, Thomas R. Burkot, and Scott A. Ritchie. 2021. "Designing Aedes (Diptera: Culicidae) Mosquito Traps: The Evolution of the Male Aedes Sound Trap by Iterative Evaluation" Insects 12, no. 5: 388. https://doi.org/10.3390/insects12050388
APA StyleStaunton, K. M., Liu, J., Townsend, M., Desnoyer, M., Howell, P., Crawford, J. E., Xiang, W., Snoad, N., Burkot, T. R., & Ritchie, S. A. (2021). Designing Aedes (Diptera: Culicidae) Mosquito Traps: The Evolution of the Male Aedes Sound Trap by Iterative Evaluation. Insects, 12(5), 388. https://doi.org/10.3390/insects12050388