Possible Epigenetic Origin of a Recurrent Gynandromorph Pattern in Megachile Wild Bees
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
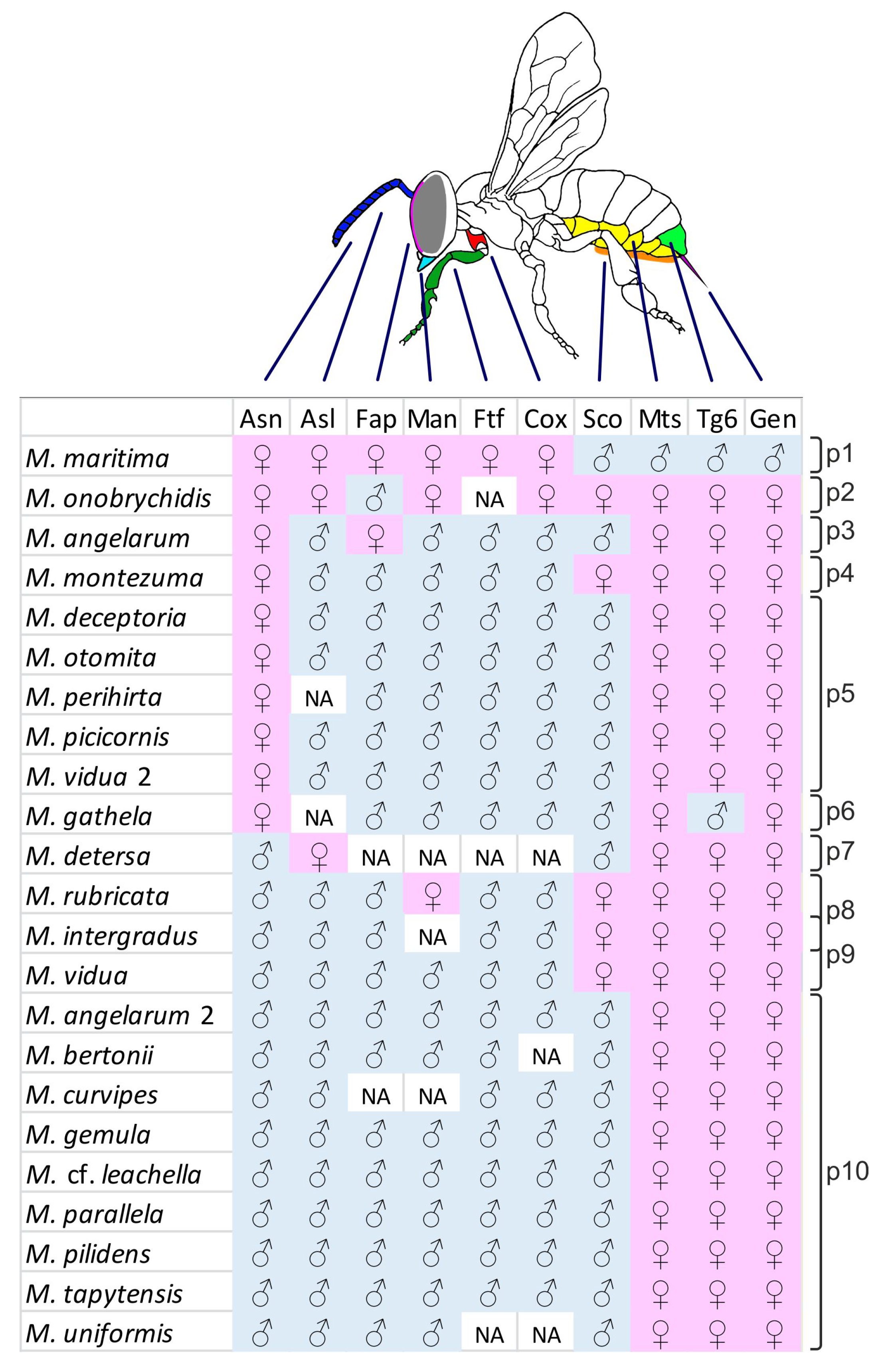
2.1. The Dataset
2.2. New Gynandromorphs in Megachile pilidens
3. Results
3.1. Description of the New M. pilidens Gynandromorphs
3.2. Transverse Gynandromorph Patterns in Megachile
3.3. Frequency Analysis
4. Discussion
4.1. New Megachile Gynandromorphs
4.2. Topographic Considerations
4.3. Developmental Interpretation
4.3.1. Sex Determination and Sexual Differentiation in the Honeybee
4.3.2. The Phylogenetic Background—Apis vs. Megachile
4.3.3. Tentative Interpretation of Megachile Gynandromorphs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Janning, W. Gynandromorph Fate Maps in Drosophila. In Genetic Mosaics and Cell Differentiation; Gehring, W.J., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1978; pp. 1–28. [Google Scholar] [CrossRef]
- Narita, S.; Pereira, R.A.S.; Kjellberg, F.; Kageyama, D. Gynandromorphs and intersexes: Potential to understand the mechanism of sex determination in arthropods. Terr. Arthropod Rev. 2010, 3, 63–96. [Google Scholar] [CrossRef] [Green Version]
- Fusco, G.; Minelli, A. The Biology of Reproduction; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar] [CrossRef]
- Minelli, A.; Fusco, G. Gynandromorphism and intersexuality: Two sides of the same coin? 2021; [In preparation]. [Google Scholar]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Danforth, B.N.; Cardinal, S.; Praz, C.; Almeida, E.A.B.; Michez, D. The impact of molecular data on our understanding of bee phylogeny and evolution. Annu. Rev. Entomol. 2013, 58, 57–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michez, D.; Rasmont, P.; Terzo, M.; Vereecken, N.J. A synthesis of gynandromorphy among wild bees (Hymenoptera: Apoidea), with an annotated description of several new cases. Ann. Soc. Entom. Fr. 2009, 45, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Hinojosa–Díaz, I.A.; Gonzalez, V.H.; Ayala, R.; Mérida, J.; Sagot, P.; Engel, M.S. New orchid and leaf-cutter bee gynandromorphs, with an updated review (Hymenoptera, Apoidea). Zoosyst. Evol. 2012, 88, 205–214. [Google Scholar] [CrossRef]
- Fateryga, A.V.; Ivanov, S.P.; Filatov, M.A. Gynandromorphs of Megachile picicornis (Morawitz, 1877) and Megachile deceptoria (Pérez, 1890) (Hymenoptera, Megachilidae) and their evolutionary interpretation. Russ. Entom. J. 2011, 20, 261–264. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World, 2nd ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 2007; ISBN 0801885736. [Google Scholar]
- Ascher, J.S.; Pickering, J. Discover Life Bee Species Guide and World Checklist (Hymenoptera: Apoidea: Anthophila). 2016. Available online: http://www.discoverlife.org/20/q?search=Apoidea (accessed on 10 March 2021).
- Trunz, V.; Packer, L.; Vieu, J.; Arrigo, N.; Praz, C.J. Comprehensive phylogeny, biogeography and new classification of the diverse bee tribe Megachilini: Can we use DNA barcodes in phylogenies of large genera? Mol. Phylogenet. Evol. 2016, 103, 245–259. [Google Scholar] [CrossRef] [Green Version]
- Dalla Torre, K.W.; Friese, H. Die ermaphroditen und gynandromorphen Hymenopteren. Ber. Naturwiss. Med. Ver. Innsbruck 1899, 24, 1–96. [Google Scholar]
- Mitchell, T.B. Sex anomalies in the genus Megachile, with descriptions of new species (Hymenoptera: Megachilidae). Trans. Amer. Entomol. Soc. 1929, 54, 321–383. [Google Scholar]
- Mitchell, T.B. Some additional intersexes in Megachile (Hymenoptera: Megachilidae). Pan-Pac. Entomol. 1941, 17, 165–168. [Google Scholar]
- Stenton, R. Gynandromorphism of Megachile willughbiella. Entomol. Month. Mag. 1909, 20, 188. [Google Scholar]
- Mitchell, T.B. A gynandromorph of Megachile latimanus Say. J. Elisha Mitchell Sci. Soc. 1932, 47, 52–54. [Google Scholar]
- Benno, P. Aantekeningen over bijen en wespen I twee gynandromorphic bijen (Hym. Apidae). Entom. Ber. 1948, 12, 250–251. [Google Scholar]
- Wolf, H. Zwitter von Andrena fulva (Müller), Lasioglossum lissonotum (Noskiewicz) und Osmia bicolor (Schrank) (Hym., Apidae). Linz. Biol. Beitr. 1990, 22, 287–290. [Google Scholar]
- Akre, R.D.; Paul, C.; Zack, R.S.; Klostermeyer, X.C. Gynandromorphs of Megachile rotundata (Fab.) (Hymenoptera: Megachilidae). Entom. News 1982, 93, 85–94. [Google Scholar]
- Goulet, H.; Huber, J. Hymenoptera of the World, an Identification Guide to Families; Agriculture Canada: Ottawa, ON, Canada, 1993. [Google Scholar]
- Cockerell, T.D.A. Descriptions and records of bees.—XXXV. Ann. Mag. Nat. Hist. 1911, 7, 310–319. [Google Scholar] [CrossRef]
- Wolf, H. Zwitter von Chrysis pseudodichroa Linsenmaier 1959 und Megachile pilidens Alfken 1924 (Hymenoptera, Chrysididae und Apidae). Linz. Biol. Beiträge 2004, 36, 525–526. [Google Scholar]
- Sommaggio, D. The hoverfly fauna of the Berici Hills: An area of rich biodiversity in north-eastern Italy. Bull. Insectol. 2017, 70, 101–110. [Google Scholar]
- Pesarini, F.; Sommaggio, D. Ecology of the sawfly coenosis of Berici Hills (Veneto, NE Italy), with notes on taxonomy and distributional data of selected species (Hymenoptera). Quad. Mus. Civ. St. Nat. Ferrara 2020, 8, 45–66. [Google Scholar]
- Wolf, H. Zwitter von Arachnospila anceps (Wesmael) (Hym., Pompilidae), Andrena fulva (Müller) und Megachile maritima (Kirby) (Hym., Apidae). Linz. Biol. Beitr. 1993, 25, 123–125. [Google Scholar]
- Gonzalez, V.H. A gynandromorph of Megachile (Austromegachile) montezuma Cresson (Hymenoptera: Apoidea, Megachilidae). Entomotropica 2004, 19, 155–156. [Google Scholar]
- Gupta, R.V. A gynandromorph of Megachile (Eutricharaea) gathela Cameron (Insecta, Hymenoptera, Megachilidae). J. Bombay Nat. Hist. Soc. 2004, 101, 471–472. [Google Scholar]
- Coelho, I.R.; Zama, P.C.; Ferrari, R.R. First record of gynandromorphism in Megachile (Pseudocentron) rubricata Smith, 1853 (Hymenoptera: Megachilidae). Pan-Pac. Entomol. 2016, 92, 104–107. [Google Scholar] [CrossRef]
- Minelli, A. Holomeric vs. meromeric segmentation: A tale of centipedes, leeches, and rhombomeres. Evol. Dev. 2000, 2, 35–48. [Google Scholar] [CrossRef]
- Owen, R.E.; Packer, L. Estimation of the proportion of diploid males in populations of Hymenoptera. Heredity 1994, 72, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Whiting, P.W. The evolution of male haploidy. Q. Rev. Biol. 1945, 20, 231–260. [Google Scholar] [CrossRef]
- Whiting, P.W. Multiple alleles in complementary sex determination of Habrobracon. Genetics 1943, 28, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.W. Selective fertilization and sex determination in Hymenoptera. Science 1933, 78, 537–538. [Google Scholar] [CrossRef]
- Heimpel, G.E.; de Boer, J.G. Sex determination in the Hymenoptera. Annu. Rev. Entomol. 2008, 53, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Asplen, M.K.; Whitfield, J.B.; de Boer, J.G.; Heimpel, G.E. Ancestral state reconstruction analysis of hymenopteran sex determination mechanisms. J. Evol. Biol. 2009, 22, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Beye, M.; Moritz, R.F.A.; Epplen, C. Sex linkage in the honeybee Apis mellifera detected by multilocus DNA fingerprinting. Naturwissenschaften 1994, 81, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Beye, M.; Hunt, G.J.; Page, R.E.; Fondrk, M.K.; Grohmann, L.; Moritz, R.F.A. Unusually high recombination rate detected in the sex locus region of the honey bee (Apis mellifera). Genetics 1999, 153, 1701–1708. [Google Scholar] [CrossRef]
- Hunt, G.J.; Page, R.E., Jr. Linkage analysis of sex determination in the honey bee (Apis mellifera). Mol. Gen. Genet. 1994, 244, 512–518. [Google Scholar] [CrossRef]
- Hunt, G.J.; Page, R.E., Jr. Linkage map of the honey bee, Apis mellifera, based on RAPD markers. Genetics 1995, 139, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Hasselmann, M.; Beye, M. Signatures of selection among sex-determining alleles of the honey bee. Proc. Natl. Acad. Sci. USA 2004, 101, 4888–4893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasselmann, M.; Vekemans, X.; Pflugfelder, J.; Koeniger, N.; Koeniger, G.; Tingek, S.; Beye, M. Evidence for convergent nucleotide evolution and high allelic turnover rates at the complementary sex determiner gene of Western and Asian honeybees. Mol. Biol. Evol. 2008, 25, 696–708. [Google Scholar] [CrossRef] [Green Version]
- Beye, M.; Seelmann, C.; Gempe, T.; Hasselmann, M.; Vekemans, X.; Fondrk, M.K.; Page, R.E., Jr. Gradual molecular evolution of a sex determination switch through incomplete penetrance of femaleness. Curr. Biol. 2013, 23, 2559–2564. [Google Scholar] [CrossRef] [Green Version]
- Marsh, J.L.; Wieschaus, E. Is sex determination in germ line and soma controlled by separate genetic mechanisms? Nature 1978, 272, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Cline, T.W.; Meyer, B.J. Vive la difference: Males vs females in flies vs worms. Annu. Rev. Genet. 1996, 30, 637–702. [Google Scholar] [CrossRef] [PubMed]
- Schütt, C.; Nöthiger, R. Structure, function and evolution of sex-determining systems in Dipteran insects. Development 2000, 127, 667–677. [Google Scholar] [CrossRef]
- Schmieder, S.; Colinet, D.; Poirie, M. Tracing back the nascence of a new sex-determination pathway to the ancestor of bees and ants. Nat. Commun. 2012, 3, 895. [Google Scholar] [CrossRef] [Green Version]
- Gempe, T.; Hasselmann, M.; Schiøtt, M.; Hause, G.; Otte, M.; Beye, M. Sex determination in honeybees: Two separate mechanisms induce and maintain the female pathway. PLoS Biol. 2009, 7, e1000222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wilgenburg, E.; Driessen, G.; Beukeboom, L.W. Single locus complementary sex determination in Hymenoptera: An ‘unintelligent’ design? Front. Zool. 2006, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Sann, M.; Niehuis, O.; Peters, R.S.; Mayer, C.; Kozlov, A.; Podsiadlowski, L.; Bank, S.; Meusemann, K.; Misof, B.; Bleidorn, C.; et al. Phylogenomic analysis of Apoidea sheds new light on the sister group of bees. BMC Evol. Biol. 2018, 18, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, B.; Su, T.; Wu, Y.; Xu, J.; Huang, D. Phylogenetic analysis of the mitochondrial genomes in bees (Hymenoptera: Apoidea: Anthophila). PLoS ONE 2018, 13, e0202187. [Google Scholar] [CrossRef]
- Zheng, B.Y.; Cao, L.J.; Tang, P.; van Achterberg, K.; Hoffmann, A.A.; Chen, H.Y.; Chen, X.X.; Wei, S.J. Gene arrangement and sequence of mitochondrial genomes yield insights into the phylogeny and evolution of bees and sphecid wasps (Hymenoptera: Apoidea). Mol. Phylogenet. Evol. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Bossert, S.; Murray, E.A.; Almeida, E.A.B.; Brady, S.G.; Blaimer, B.B.; Danforth, B.N. Combining transcriptomes and ultraconserved elements to illuminate the phylogeny of Apidae. Mol. Phylogenet. Evol. 2019, 130, 121–131. [Google Scholar] [CrossRef]
- Boveri, T. Über die Entstehung der Eugsterschen Zwitterbienen. Arch. Entwicklungsmech. Org. 1915, 41, 264–311. [Google Scholar] [CrossRef] [Green Version]
- Morgan, T.H. The Eugster gynandromorph bees. Am. Nat. 1916, 50, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Morgan, T.H.; Bridges, C.B. The Origin of Gynandromorphs. In Contributions to the Study of Drosophila; Carnegie Institution of Washington Publications: Washigton, DC, USA, 1919; Volume 278, pp. 1–122. [Google Scholar]
- Rothenbuhler, W.C. Progress and problems in the analysis of gynandromorphic honey bees. Proc. Tenth Int. Congr. Entomol. 1958, 2, 867–873. [Google Scholar]
- Cline, T.W. Autoregulatory functioning of a Drosophila gene product that establishes and maintains the sexually determined state. Genetics 1984, 107, 231–277. [Google Scholar] [CrossRef] [PubMed]
- Crick, F.H.C.; Lawrence, P.A. Compartments and polyclones in insect development. Science 1975, 189, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Kettle, C.; Arthur, W.; Jowett, T.; Minelli, A. Homeotic transformation in a centipede. Trends Genet. 1999, 15, 393. [Google Scholar] [CrossRef]
- Di, Z.; Edgecombe, G.D.; Sharma, P.P. Homeosis in a scorpion supports a telopodal origin of pectines and components of the book lungs. BMC Evol. Biol. 2018, 18, 73. [Google Scholar] [CrossRef] [Green Version]
- Lesniewska, M.; Bonato, L.; Minelli, A.; Fusco, G. Trunk anomalies in the centipede Stigmatogaster subterranea provide insight into late-embryonic segmentation. Arthropod Struct. Dev. 2009, 38, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Akkari, N.; Enghoff, H.; Minelli, A. Segmentation of the millipede trunk as suggested by a homeotic mutant with six extra pairs of gonopods. Front. Zool. 2014, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- Minelli, A.; Munari, L. An ectopic macrochaeta in the middle of a compound eye of a field-collected anthomyiid fly. Dev. Genes Evol. 2013, 223, 195–197. [Google Scholar] [CrossRef]
- Yang, A.S.; Abouheif, E. Gynandromorphs as indicators of modularity and evolvability in ants. J. Exp. Zool. 2011, 316, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bellido, A.; Merriam, J.R. Cell lineage of the imaginal discs in Drosophila gynandromorphs. J. Exp. Zool. 1969, 170, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Held, L.I., Jr. Animal Anomalies; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Wolf, H. Ein Zwitter von cf. Megachile leachella Curtis (Hymenoptera, Apidae). Linz. Biol. Beitr. 1998, 30, 613. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommaggio, D.; Fusco, G.; Uliana, M.; Minelli, A. Possible Epigenetic Origin of a Recurrent Gynandromorph Pattern in Megachile Wild Bees. Insects 2021, 12, 437. https://doi.org/10.3390/insects12050437
Sommaggio D, Fusco G, Uliana M, Minelli A. Possible Epigenetic Origin of a Recurrent Gynandromorph Pattern in Megachile Wild Bees. Insects. 2021; 12(5):437. https://doi.org/10.3390/insects12050437
Chicago/Turabian StyleSommaggio, Daniele, Giuseppe Fusco, Marco Uliana, and Alessandro Minelli. 2021. "Possible Epigenetic Origin of a Recurrent Gynandromorph Pattern in Megachile Wild Bees" Insects 12, no. 5: 437. https://doi.org/10.3390/insects12050437
APA StyleSommaggio, D., Fusco, G., Uliana, M., & Minelli, A. (2021). Possible Epigenetic Origin of a Recurrent Gynandromorph Pattern in Megachile Wild Bees. Insects, 12(5), 437. https://doi.org/10.3390/insects12050437






