Insecticidal Effect of Entomopathogenic Nematodes and the Cell-Free Supernatant from Their Symbiotic Bacteria against Philaenus spumarius (Hemiptera: Aphrophoridae) Nymphs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collecting and Rearing of Organisms
2.2. Production of Cell-Free Supernatant from the Symbiotic Bacteria of Entomopathogenic Nematodes
2.3. Evaluation of Entomopathogenic Nematode Virulence after Exposure to Foam Produced by Philaenus spumarius
2.4. Evaluation of Entomopathogenic Nematode Virulence and Bacterial Cell-Free Supernatant Toxicity against Philaenus spumarius
2.5. Statistical Analyses
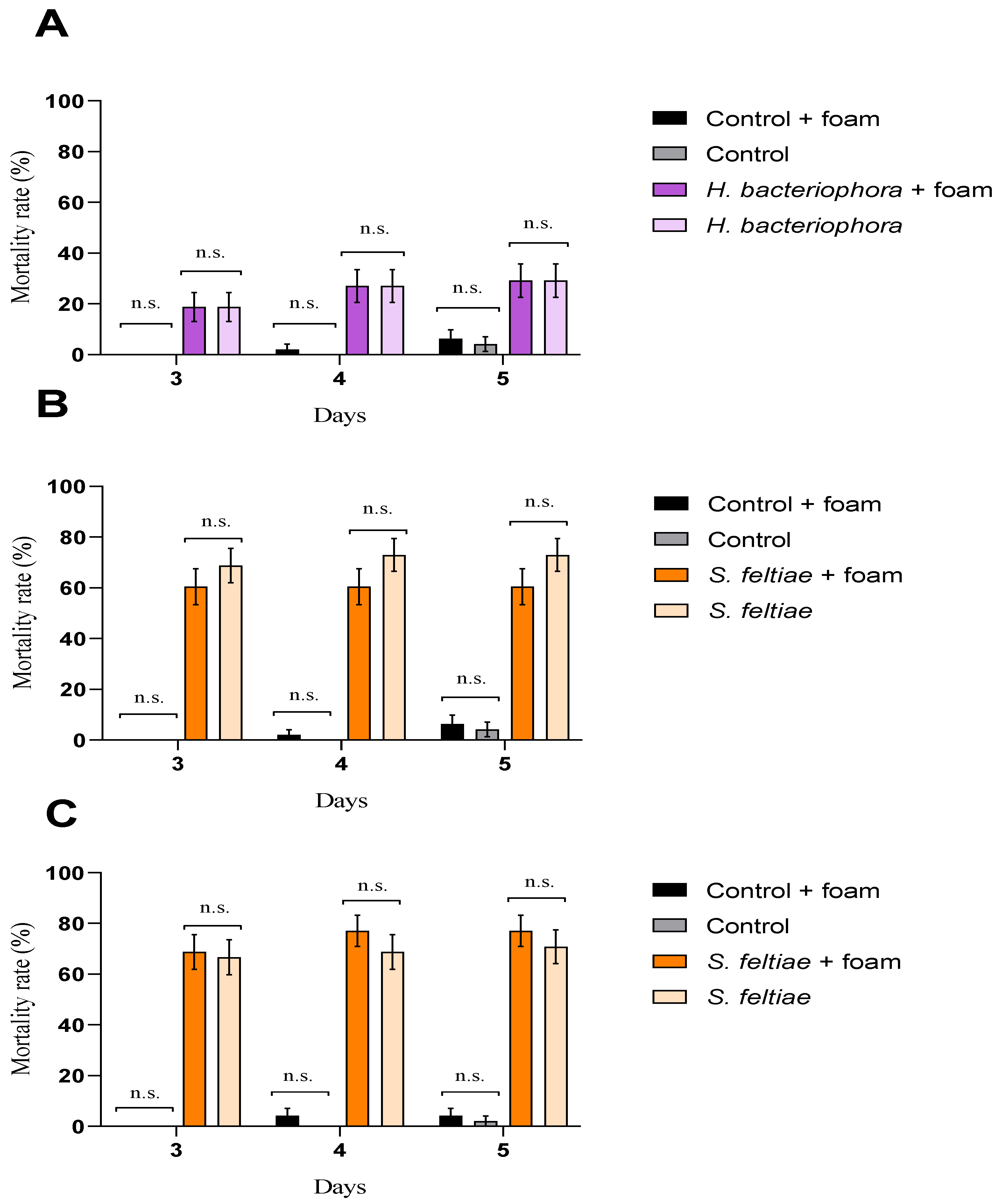
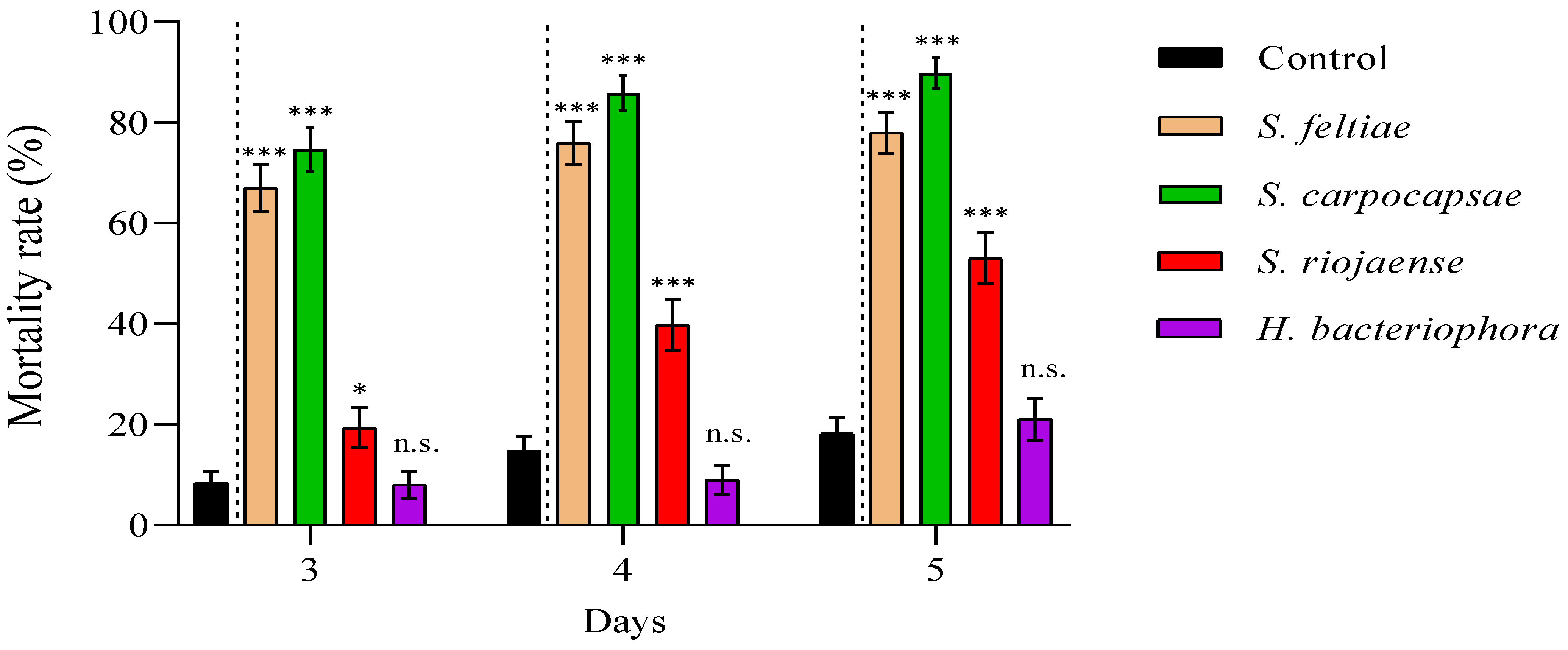
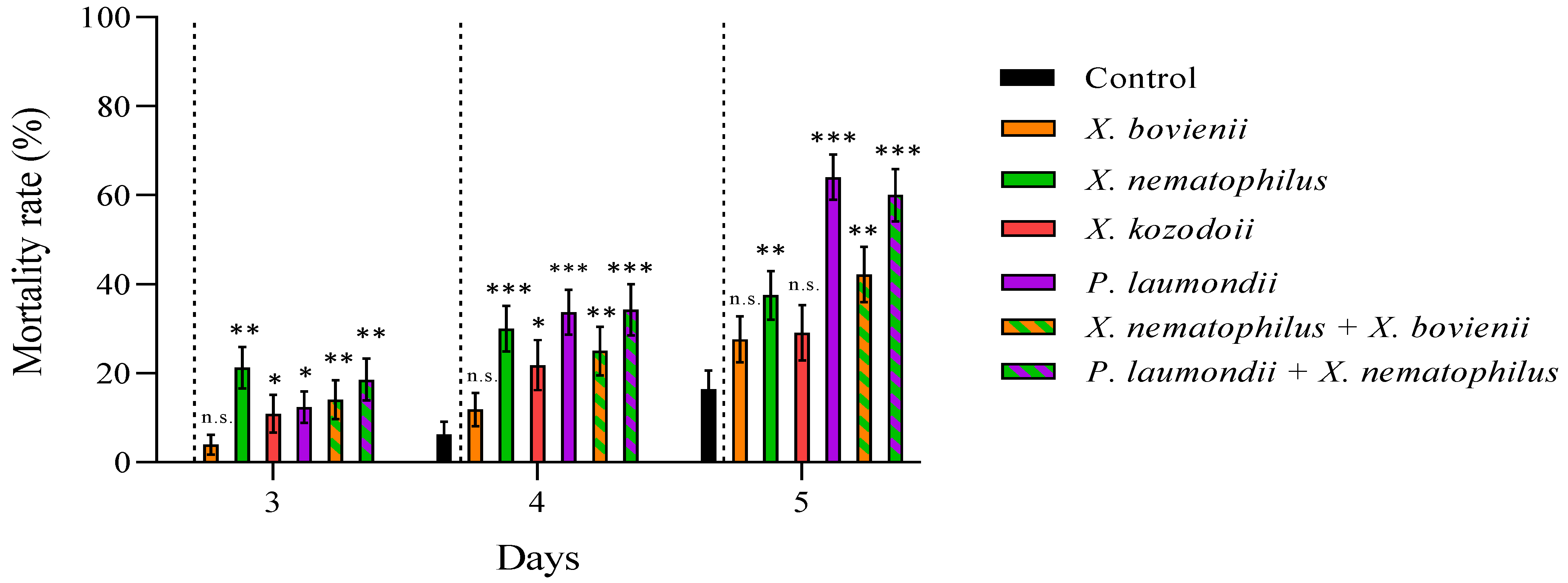
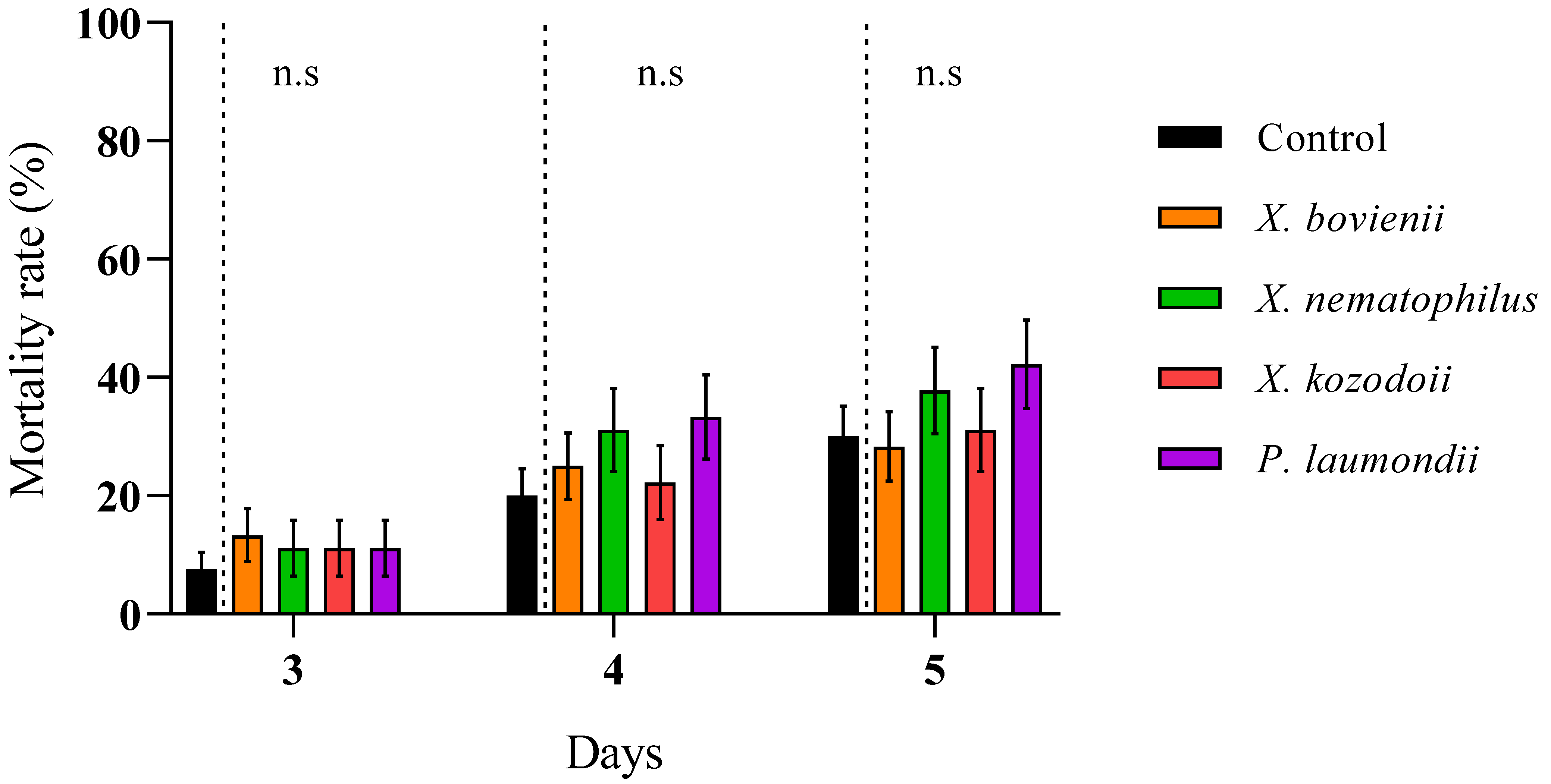
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
- -
- I.V.-D. funded by ADER I + D + i (2019) fellowship by the Rioja Agency of Economic Development (La Rioja, Spain) and currently is supported by an FPU-UR-2020 fellowship.
- -
- R.B.-P. was supported by the pre-doctoral contracts CAR-2018 (Department of Economic Development and Innovation of the Government of La Rioja).
- -
- M.d.M.G.-T. is supported by Program JAE-Intro CSIC call 2020 (JAEINT20_EX_0939).
- -
- R.C.-H. is supported by Ramón y Cajal contract award from the Government of Spain (RYC-2016-19939).
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, K.; van der Werf, W.; Cendoya, M.; Mourits, M.; Navas-Cortés, J.A.; Vicent, A.; Lansink, A.O. Impact of Xylella fastidiosa subspecies pauca in European olives. Proc. Natl. Acad. Sci. USA 2020, 117, 9250–9259. [Google Scholar] [CrossRef] [Green Version]
- Bucci, E.M. Xylella fastidiosa, a new plant pathogen that threatens global farming: Ecology, molecular biology, search for remedies. Biochem. Biophys. Res. Commun. 2018, 502, 173–182. [Google Scholar] [CrossRef]
- Godefroid, M.; Cruaud, A.; Streito, J.C.; Rasplus, J.Y.; Rossi, J.P. Climate change and the potential distribution of Xylella fastidiosa in Europe. BioRxiv 2018. [Google Scholar] [CrossRef] [Green Version]
- EPPO. PM 7/24 (3) Xylella fastidiosa. EPPO Bull. 2016, 46, 175–218. [Google Scholar] [CrossRef] [Green Version]
- EFSA PLH Panel. Scientific Opinion on the updated pest categorisation of Xylella fastidiosa. EFSA J. 2018, 16, 5357. [Google Scholar]
- Almeida, R.P.; Blua, M.J.; Lopes, J.R.S.; Purcell, A.H. Vector transmission of Xylella fastidiosa: Applying fundamental knowledge to generate disease management strategies. Ann. Entomol. Soc. Am. 2005, 98, 775–786. [Google Scholar] [CrossRef]
- Candresse, T.; Chatzivassiliou, E.; Dehnen-schmutz, K. Updated pest categorisation of Xylella fastidiosa. EFSA J. 2018. [Google Scholar] [CrossRef] [Green Version]
- Saponari, M.; Loconsole, G.; Cornara, D.; Yokomi, R.K.; De Stradis, A.; Boscia, D.; Bosco, D.; Martelli, G.P.; Krugner, R.; Porcelli, F. Infectivity and transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. J. Econ. Entomol. 2014, 107, 1316–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, C.; King, D. Meadow spittlebug, Philaenus leucophthalmus (L.). Ohio Agric. Exp. Station. Res. Bull. 1954, 741, 1–99. [Google Scholar]
- Beckett, K.I.S.; Robertson, A.B.; Matthews, P.G.D. Studies on gas exchange in the meadow spittlebug, Philaenus spumarius: The metabolic cost of feeding on, and living in, xylem sap. J. Exp. Biol. 2019, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morente, M.; Cornara, D.; Plaza, M.; Durán, J.M.; Capiscol, C.; Trillo, R.; Ruiz, M.; Ruz, C.; Sanjuan, S.; Pereira, J.A.; et al. Distribution and relative abundance of insect vectors of Xylella fastidiosa in olive groves of the Iberian peninsula. Insects 2018, 9, 175. [Google Scholar] [CrossRef] [Green Version]
- Ponti, L.; Gutierrez, A.P.; Boggia, A.; Neteler, M. Analysis of grape production in the face of climate change. Climate 2018, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, A.P.; Ponti, L.; Hoddle, M.; Almeida, R.P.P.; Irvin, N.A. Geographic distribution and relative abundance of the invasive glassy-winged sharpshooter: Effects of temperature and egg parasitoids. Environ. Entomol. 2011, 40, 755–769. [Google Scholar] [CrossRef] [Green Version]
- Morente, M.; Fereres, A. Enfermedades causadas por Xylella fastidiosa; Cajamar: Valencia, Spain, 2017; pp. 81–101. [Google Scholar]
- Whittaker, J.B. Cercopid spittle as a microhabitat. Oikos 1970, 21, 59–64. [Google Scholar] [CrossRef]
- del Campo, M.L.; King, J.T.; Gronquist, M.R. Defensive and chemical characterization of the froth produced by the cercopid Aphrophora cribrata. Chemoecology 2011, 21, 1–8. [Google Scholar] [CrossRef]
- Dongiovanni, C.; Altamura, G.; Di Carolo, M.; Fumarola, G.; Saponari, M.; Cavalieri, V. Evaluation of efficacy of different insecticides against Philaenus spumarius L., vector of Xylella fastidiosa in olive orchards in Southern Italy, 2015–2017. Arthropod Manag. Tests 2018, 43. [Google Scholar] [CrossRef] [Green Version]
- Kanga, L.H.B.; Jones, W.A.; Humber, R.A.; Boyd, D.W. Fungal pathogens of the glassy-winged sharpshooter Homalodisca coagulata (Homoptera: Cicadellidae). Florida Entomol. 2004, 87, 225–228. [Google Scholar] [CrossRef]
- Morgan, D.J.W.; Triapitsyn, S.V.; Redak, R.A.; Bezark, L.G.; Hoddle, M.S. Biological control of the glassy-winged sharpshooter: Current status and future potential. In California Conference on Biological Control II, The Historic Mission Inn Riverside, California, USA, 11–12 July 2000; Center for Biological Control, College of Natural Resources, University of California: Riverside, CA, USA, 2000; pp. 167–171. [Google Scholar]
- Delcour, I.; Spanoghe, P.; Uyttendaele, M. Literature review: Impact of climate change on pesticide use. Food Res. Int. 2015, 68, 7–15. [Google Scholar] [CrossRef]
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Ciancio, A.; Pieterse, C.M.J.; Mercado-Blanco, J. Editorial: Harnessing useful rhizosphere microorganisms for pathogen and pest biocontrol. Front. Microbiol. 2016, 7, 1–5. [Google Scholar] [CrossRef]
- Dillman, A.R.; Chaston, J.M.; Adams, B.J.; Ciche, T.A.; Goodrich-Blair, H.; Stock, S.P.; Sternberg, P.W. An entomopathogenic nematode by any other name. PLoS Pathog. 2012, 8, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Boemare, N.; Givaudan, A.; Brehelin, M.; Laumond, C. Symbiosis and pathogenicity of nematode-bacterium complexes. Symbiosis 1997, 22, 21–45. [Google Scholar]
- Forst, S.; Nealson, K. Molecular biology of the symbiotic-pathogenic bacteria Xenorhabdus spp. and Photorhabdus spp. Microbiol. Rev. 1996, 60, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Owuama, C.I. Entomopathogenic symbiotic bacteria, Xenorhabdus and Photorhabdus of nematodes. World J. Microbiol. Biotechnol. 2001, 17, 505–515. [Google Scholar] [CrossRef]
- Boemare, N.E. Biology, taxonomy and systematics of Xenorhabdus and Photorhabdus. In Entomopathogenic Nematology; CABI Publishing: Wallingford, UK, 2002; pp. 35–56. [Google Scholar]
- Adams, B.J.; Fodor, A.; Koppenhöfer, H.S.; Stackebrandt, E.; Patricia Stock, S.; Klein, M.G. Biodiversity and systematics of nematode-bacterium entomopathogens. Biol. Control 2006, 37, 32–49. [Google Scholar] [CrossRef]
- Blanco-Pérez, R.; Bueno-Pallero, F.Á.; Vicente-Díez, I.; Marco-Mancebón, V.S.; Pérez-Moreno, I.; Campos-Herrera, R. Scavenging behavior and interspecific competition decrease off spring fitness of the entomopathogenic nematode Steinernema feltiae. J. Invertebr. Pathol. 2019, 164, 5–15. [Google Scholar] [CrossRef]
- Bode, H.B. Entomopathogenic bacteria as a source of secondary metabolites. Curr. Opin. Chem. Biol. 2009, 13, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Waterfield, N.R.; Ciche, T.; Clarke, D. Photorhabdus and a host of hosts. Annu. Rev. Microbiol. 2009, 63, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Bussaman, P.; Sa-Uth, C.; Rattanasena, P.; Chandrapatya, A. Acaricidal activities of whole cell suspension, cell-free supernatant, and crude cell extract of Xenorhabdus stokiae against mushroom mite (Luciaphorus sp.). J. Zhejiang Univ. Sci. B 2012, 13, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Dlamini, T.M.; Allsopp, E.; Malan, A.P. Application of Steinernema yirgalemense to control Frankliniella occidentalis (Thysanoptera: Thripidae) on blueberries. Crop Prot. 2020, 128, 105016. [Google Scholar] [CrossRef]
- Cevizci, D.; Ulug, D.; Cimen, H.; Touray, M.; Hazir, S.; Cakmak, I. Mode of entry of secondary metabolites of the bacteria Xenorhabdus szentirmaii and X. nematophila into Tetranychus urticae, and their toxicity to the predatory mites Phytoseiulus persimilis and Neoseiulus californicus. J. Invertebr. Pathol. 2020, 174, 107418. [Google Scholar] [CrossRef]
- Van Niekerk, S.; Malan, A.P. Adjuvants to improve aerial control of the citrus mealybug Planococcus citri (Hemiptera: Pseudococcidae) using entomopathogenic nematodes. J. Helminthol. 2015, 89, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Grifaldo-Alcántara, P.F.; Alatorre-Rosas, R.; Villanueva-Jiménez, J.A.; Hernández-Rosas, F.; Stock, S.P.; Ramírez-Valverde, G. Evaluación de dos cepas de nematodos entomopatógenos (Steinernematidae, Heterorhabditidae) para el control del salivazo (Hemiptera: Cercopidae) en caña de azúcar. Nematropica 2019, 49, 83–90. [Google Scholar]
- Eroglu, C.; Cimen, H.; Ulug, D.; Karagoz, M.; Hazir, S.; Cakmak, I. Acaricidal effect of cell-free supernatants from Xenorhabdus and Photorhabdus bacteria against Tetranychus urticae (Acari: Tetranychidae). J. Invertebr. Pathol. 2019, 160, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Morente, M.; Cornara, D.; Moreno, A.; Fereres, A. Continuous indoor rearing of Philaenus spumarius, the main European vector of Xylella fastidiosa. J. Appl. Entomol. 2018, 142, 901–904. [Google Scholar] [CrossRef]
- Woodring, J.L.; Kaya, H.K. Steinernematid and Heterorhabditid Nematodes: A Handbook of Biology and Techniques; Southern Cooperative Series Bulletin 331; Arkansas Agricultural Experiment Station: Fayetteville, AR, USA, 1988. [Google Scholar]
- Blanco-Pérez, R.; Sáenz-Romo, M.G.; Vicente-Díez, I.; Ibáñez-Pascual, S.; Martínez-Villar, E.; Marco-Mancebón, V.S.; Pérez-Moreno, I.; Campos-Herrera, R. Impact of vineyard ground cover management on the occurrence and activity of entomopathogenic nematodes and associated soil organisms. Agric. Ecosyst. Environ. 2020, 301, 107028. [Google Scholar] [CrossRef]
- Campos-Herrera, R.; Jaffuel, G.; Chiriboga, X.; Blanco-Pérez, R.; Fesselet, M.; Půža, V.; Mascher, F.; Turlings, T.C.J. Traditional and molecular detection methods reveal intense interguild competition and other multitrophic interactions associated with native entomopathogenic nematodes in Swiss tillage soils. Plant Soil 2015, 389, 237–255. [Google Scholar] [CrossRef]
- Wang, Y.; Xiangling, F.; An, F.; Wang, G.; Zhang, X. Improvement of antibiotic activity of Xenorhabdus bovienii by medium optimization using response surface methodology. Microb. Cell Fact. 2011, 10, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boemare, N.E.; Akhurst, R.J. Biochemical and physiological characterization of colony form variants in Xenorhabdus spp. (Enterobacteriaceae). J. Gen. Microbiol. 1988, 134, 751–761. [Google Scholar] [CrossRef] [Green Version]
- Enright, M.E.; McInerney, J.O.; Griffin, C.T. Characterization of endospore-forming bacteria associated with entomopathogenic nematodes, Heterorhabditis spp., and description of Paenibacillus nematophilus sp. nov. Int. J. Syst. Evol. Microbiol. 2003, 53, 435–441. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Jackson, M.; Reilly, C.C.; Hotchkiss, M.W. Effects of combining an entomopathogenic fungi or bacterium with entomopathogenic nematodes on mortality of Curculio caryae (Coleoptera: Curculionidae). Biol. Control 2004, 30, 119–126. [Google Scholar] [CrossRef]
- Ansari, M.A.; Shah, F.A.; Butt, T.M. The entomopathogenic nematode Steinernema kraussei and Metarhizium anisopliae work synergistically in controlling overwintering larvae of the black vine weevil, Otiorhynchus sulcatus, in strawberry growbags. Biocontrol Sci. Technol. 2010, 20, 99–105. [Google Scholar] [CrossRef]
- Carvalho, G.S.; Webb, M.D. Cercopid Spittle Bugs of the New World (Hemiptera, Auchenorrhyncha, Cercopidae); Pensoft Publishers: Sofia, Bulgaria, 2005; pp. 13–16. [Google Scholar]
- Grewal, P.S.; Bornstein-Forst, S.; Burnell, A.M.; Glazer, I.; Jagdale, G.B. Physiological, genetic, and molecular mechanisms of chemoreception, thermobiosis, and anhydrobiosis in entomopathogenic nematodes. Biol. Control 2006, 38, 54–65. [Google Scholar] [CrossRef]
- Shrestha, Y.K.; Lee, K.Y. Oral toxicity of Photorhabdus culture media on gene expression of the adult sweetpotato whitefly, Bemisia tabaci. J. Invertebr. Pathol. 2012, 109, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, M.; Jarrett, P.; Ousley, M.; Morgan, J.A.W. Interactions of insecticidal toxin gene products from Xenorhabdus nematophilus PMFI296. Appl. Environ. Microbiol. 2003, 69, 3344–3349. [Google Scholar] [CrossRef] [Green Version]
- Ffrench-Constant, R.H.; Dowling, A.; Waterfield, N.R. Insecticidal toxins from Photorhabdus bacteria and their potential use in agriculture. Toxicon 2007, 49, 436–451. [Google Scholar] [CrossRef]
- Rodou, A.; Ankrah, D.O.; Stathopoulos, C. Toxins and secretion systems of Photorhabdus luminescens. Toxins 2010, 2, 1250–1264. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| EPN Species | Population | ITS-GenBank Accession | Bacterial Species | ITS-GenBank Accession |
|---|---|---|---|---|
| Steinernema feltiae | RM-107 | MW480131 | Xenorhabdus bovienii | MW467374 |
| Steinernema carpocapsae | All | MW574913 | Xenorhabdus nematophilus | MW574906 |
| Steinernema riojaense | RM-30 | MK503133 | Xenorhabdus kozodoii | MW467375 |
| Heterorhabditis bacteriophora | RM-102 | MW480132 | Photorabhdus laumondii subsp. laumondii | MW574908 |
| Combinations | Observed Mortality (%) | Expected Mortality (%) | χ2 | Interaction |
|---|---|---|---|---|
| X. nematophilus + X. bovienii | 42 | 43 | 3.20 | Additive |
| P. laumondii + X. nematophilus | 60 | 68 | 2.33 | Additive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente-Díez, I.; Blanco-Pérez, R.; González-Trujillo, M.d.M.; Pou, A.; Campos-Herrera, R. Insecticidal Effect of Entomopathogenic Nematodes and the Cell-Free Supernatant from Their Symbiotic Bacteria against Philaenus spumarius (Hemiptera: Aphrophoridae) Nymphs. Insects 2021, 12, 448. https://doi.org/10.3390/insects12050448
Vicente-Díez I, Blanco-Pérez R, González-Trujillo MdM, Pou A, Campos-Herrera R. Insecticidal Effect of Entomopathogenic Nematodes and the Cell-Free Supernatant from Their Symbiotic Bacteria against Philaenus spumarius (Hemiptera: Aphrophoridae) Nymphs. Insects. 2021; 12(5):448. https://doi.org/10.3390/insects12050448
Chicago/Turabian StyleVicente-Díez, Ignacio, Rubén Blanco-Pérez, María del Mar González-Trujillo, Alicia Pou, and Raquel Campos-Herrera. 2021. "Insecticidal Effect of Entomopathogenic Nematodes and the Cell-Free Supernatant from Their Symbiotic Bacteria against Philaenus spumarius (Hemiptera: Aphrophoridae) Nymphs" Insects 12, no. 5: 448. https://doi.org/10.3390/insects12050448







