Effects of Delayed Mating on Mating Performance and Reproductive Fitness of the Willow Leaf Beetle (Coleoptera: Chrysomelidae) under Laboratory Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Experimental Design
2.3. Measurements of Reproductive Fitness
2.4. Statistical Analysis
3. Results
3.1. Time from Pairing to Successful Mating and Mating Duration
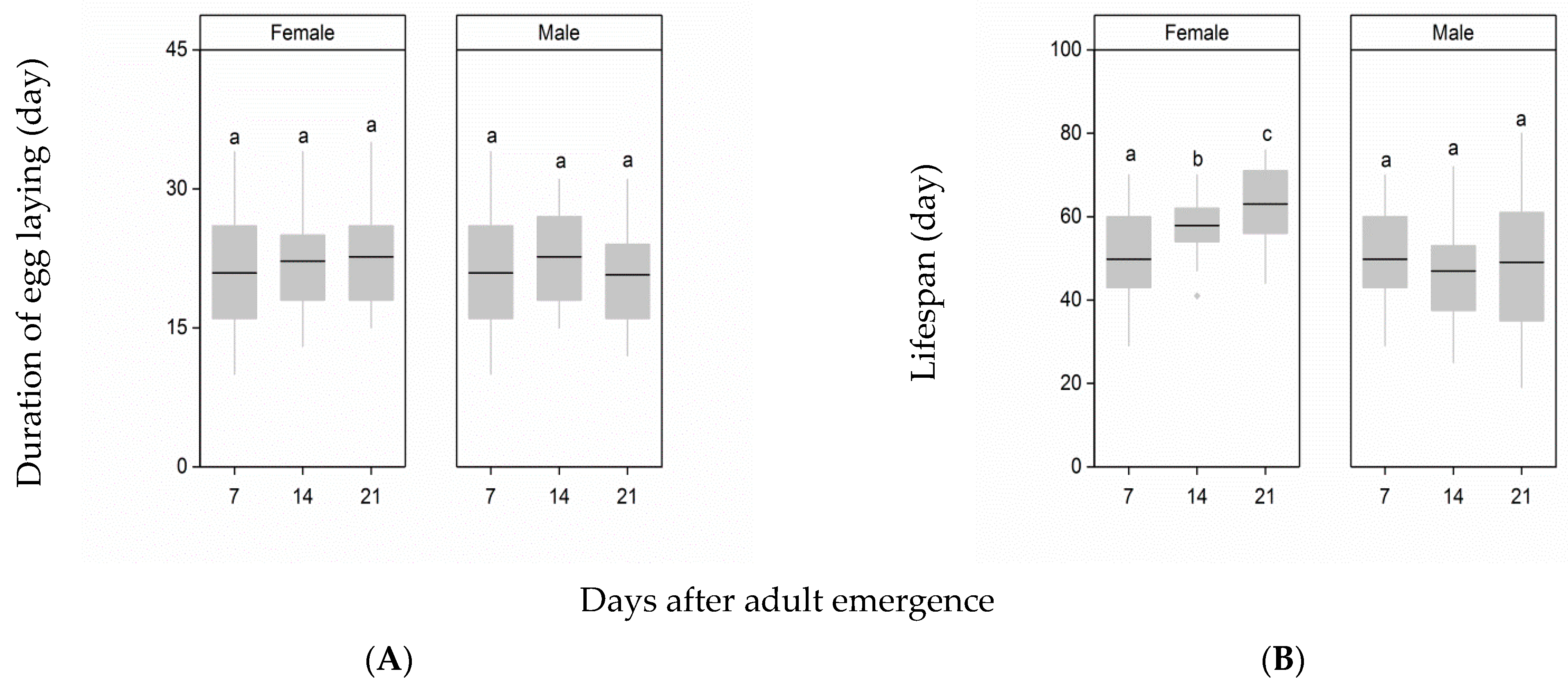
3.2. Duration of the Egg-Laying Period and Female Lifespan
3.3. Fecundity and Egg Hatching Rate
3.4. Number of Egg Clutches per Female and Number of Eggs per Clutch
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.-P.; Fang, Y.-L.; Zhang, Z.-N. Effects of delayed mating on the fecundity, fertility and longevity of females of diamondback moth, Plutella xylostella. Insect Sci. 2010, 18, 305–310. [Google Scholar] [CrossRef]
- Waqas, M.S.; Shoaib, A.A.Z.; ElAbasy, A.S.S.; Cheng, X.; Zhang, Q.; Shi, Z. Effects of delayed mating on male mating success and female reproductive performance of Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Crop Prot. 2020, 132, 105135. [Google Scholar] [CrossRef]
- Pervez, A.; Richmond, A.S. The influence of age on reproductive performance of the predatory ladybird beetle, Propylea dissecta. J. Insect Sci. 2004, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.D. The causes and evolutionary consequences of variation in female mate choice in insects: The effects of individual state, genotypes and environments. Curr. Opin. Insect Sci. 2018, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Zhang, S.; Zhang, Y.; Liu, X. Male age affects female mating preference but not fitness in the monandrous moth Dendrolimus punctatus Walker (Lepidoptera: Lasiocampidae). Physiol. Entomol. 2019, 45, 22–29. [Google Scholar] [CrossRef]
- Jiménez-Pérez, A.; Wang, Q. Effect of mating delay on the reproductive performance of Cnephasia jactatana (Lepidoptera: Tortricidae). J. Econ. Entomol. 2003, 96, 592–598. [Google Scholar] [CrossRef]
- Nandy, B.; Joshi, A.; Ali, Z.S.; Sen, S.; Prasad, N.G. Degree of adaptive male mate choice is positively correlated with female quality variance. Sci. Rep. 2012, 2, 447. [Google Scholar] [CrossRef] [Green Version]
- Mphosi, M.S. Effect of female sunflower moth, Homoeosoma electellum (Hulst) (Lepidoptera: Pyralidae) age at first mating on fecundity, fertility, longevity and egg laying pattern. Res. Crops 2019, 20, 169–173. [Google Scholar]
- Beck, C.W.; Powell, L.A. Evolution of female choice based on male age: Are older males better mates? Evol. Ecol. Res. 2000, 2, 107–118. [Google Scholar]
- Beck, C.W.; Promislow, D.E.L. Evolution of Female Preference for Younger Males. PLoS ONE 2007, 2, e939. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Averill, A.L. Effects of delayed mating on reproductive output of female oriental beetle Anomala orientalis (Coleoptera: Scarabaeidae). Agric. For. Entomol. 2006, 8, 221–231. [Google Scholar] [CrossRef]
- Zheng, X.L.; Liu, J.Y.; Lu, W.; He, X.Z.; Wang, Q. Mating delay reduces reproductive performance but not longevity in a mo-nandrous moth. J. Insect Sci. 2020, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Kawazu, K.; Shintani, Y.; Tatsuki, S. Effect of increased male and female age at mating on the reproductive performance of Cnaphalocrocis medinalis (Crambidae: Lepidoptera). J. Econ. Entomol. 2014, 107, 1434–1439. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Zhang, Y.; Zhang, L.; Long, Y. Delayed Mating Impacts on the Reproductive Performance of Ectropis obliqua (Lepidoptera: Geometridae). J. Entomol. Sci. 2017, 52, 52–59. [Google Scholar] [CrossRef]
- Dhillon, M.K.; Tanwar, A.K.; Hasan, F. Fitness consequences of delayed mating on reproductive performance of Chilo partellus (Swinhoe). J. Exp. Zool. Ecol. Integr. Physiol. 2019, 331, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Cloonan, K.R.; Andreadis, S.S.; Baker, T.C. Little effect of delayed mating on fecundity or fertility of female fungus gnats Lycoriella ingenua. Physiol. Entomol. 2019, 44, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Lentini, A.; Mura, A.; Muscas, E.; Nuvoli, M.; Cocco, A. Effects of delayed mating on the reproductive biology of the vine mealybug, Planococcus ficus (Hemiptera: Pseudococcidae). Bull. Entomol. Res. 2017, 108, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, L.; Li, S.; Zhang, J. Bacteria-Mediated RNA Interference for Management of Plagiodera versicolora (Coleoptera: Chrysomelidae). Insects 2019, 10, 415. [Google Scholar] [CrossRef] [Green Version]
- Demirci, M.; Sevim, E.; Demir, İ.; Sevim, A. Culturable bacterial microbiota of Plagiodera versicolora (L.) (Coleoptera: Chrysomelidae) and virulence of the isolated strains. Folia Microbiol. 2013, 58, 201–210. [Google Scholar] [CrossRef]
- Dong, Y.; Du, S.; Zhang, J.; Yang, M.; Wang, J. Differential expression of dual Bt genes in transgene poplar Juba (Populus deltoides cv. ‘Juba’) transformed by two different transformation vectors. Can. J. For. Res. 2015, 45, 60–67. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, J.; Dong, Y.; Zhang, X.; Yang, M.; Gao, B. Genetic transformation and expression of Cry1Ac–Cry3A–NTHK1 genes in Populus × euramericana “Neva”. Acta Physiol. Plant. 2016, 38, 177. [Google Scholar] [CrossRef]
- Yang, R.; Wang, A.; Zhang, J.; Dong, Y.; Yang, M.; Wang, J. Genetic transformation and expression of transgenic lines of Populus × euramericana with insect-resistance and salt-tolerance genes. Genet. Mol. Res. 2016, 15, 15028635. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Qiu, Y.; Shi, X.; Wang, W.; Liu, S. Female Mating Frequency and Reproductive Fitness in the Willow Leaf Beetle (Coleoptera: Chrysomelidae). J. Insect Sci. 2019, 19, 14. [Google Scholar] [CrossRef]
- Ishihara, M.; Hayashi, T. Photoperiodic induction and termination of adult diapause in the willow leaf beetle, Plagiodera versicolora (Coleoptera: Chrysomelidae). Entomol. Sci. 2000, 3, 439–441. [Google Scholar]
- Wu, C.-X.; Liu, J.-F.; Di, X.-Y.; Yang, M.-F. Delay in Mating Reduces Reproductivity but Increases Life Span in Tobacco Cutworm, Spodoptera litura Fabricius (Lepidoptera: Noctuidae). J. Econ. Entomol. 2018, 111, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Himuro, C.; Fujisaki, K. The effects of male harassment on mating duration in the seed bug, Togo hemipterus. Entomol. Exp. Appl. 2011, 142, 53–59. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zeng, J.P.; Wu, X.F.; Peng, L.H.; Liu, X.P. A meta analysis of the effect of delayed mating on female reproductive fitness in moths. Acta Agric. Univ. Jiangxiensis 2016, 38, 113–123. [Google Scholar]
- Zhao, L.Q.; Wang, X.M.; Zhan, J.N. Effects of female and male density on their mating performance and female post-mating reproductive fitness in the willow leaf beetle (Coleoptera: Chrysomelidae). Unpublished work.
- Cocco, A.; Muscas, E.; Mura, A.; Iodice, A.; Savino, F.; Lentini, A. Influence of mating disruption on the reproductive biology of the vine mealybug, Planococcus ficus (Hemiptera: Pseudococcidae), under field conditions. Pest Manag. Sci. 2018, 74, 2806–2816. [Google Scholar] [CrossRef]
- Stockel, J. Fonctionnement de l’appareil reproducteur de la femelle de Sitotroga cerealella (Lep.: Gelechiidae). Ann. Soc. Entomol. Fr. 1973, 9, 627–645. [Google Scholar]
- Pivnick, K.A.; McNeil, J.N. Puddling in butterflies: Sodiumaffects reproductive success in Thymelicus lineola. Physiol. Entomol. 1987, 12, 461–472. [Google Scholar] [CrossRef]
- Hale, J.M.; Elgar, M.; Jones, T.M. Sperm Quantity Explains Age-Related Variation in Fertilization Success in the Hide Beetle. Ethology 2008, 114, 797–807. [Google Scholar] [CrossRef]
- Mariana, H.C.; Solana, A.; Nicolas, N.B. Male age and strain affect ejaculate quality in the Mexican fruit fly. Insect Sci. 2018, 25, 703–711. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Liu, Z.; Lin, Y.; Liu, S. Effects of Delayed Mating on Mating Performance and Reproductive Fitness of the Willow Leaf Beetle (Coleoptera: Chrysomelidae) under Laboratory Conditions. Insects 2021, 12, 481. https://doi.org/10.3390/insects12060481
Zhao L, Liu Z, Lin Y, Liu S. Effects of Delayed Mating on Mating Performance and Reproductive Fitness of the Willow Leaf Beetle (Coleoptera: Chrysomelidae) under Laboratory Conditions. Insects. 2021; 12(6):481. https://doi.org/10.3390/insects12060481
Chicago/Turabian StyleZhao, Lvquan, Zheng Liu, Yuqun Lin, and Shouzhu Liu. 2021. "Effects of Delayed Mating on Mating Performance and Reproductive Fitness of the Willow Leaf Beetle (Coleoptera: Chrysomelidae) under Laboratory Conditions" Insects 12, no. 6: 481. https://doi.org/10.3390/insects12060481




