Pinyon Engraver Beetle Acoustics: Stridulation Apparatus, Sound Production and Behavioral Response to Vibroacoustic Treatments in Logs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Insects and Tree Materials
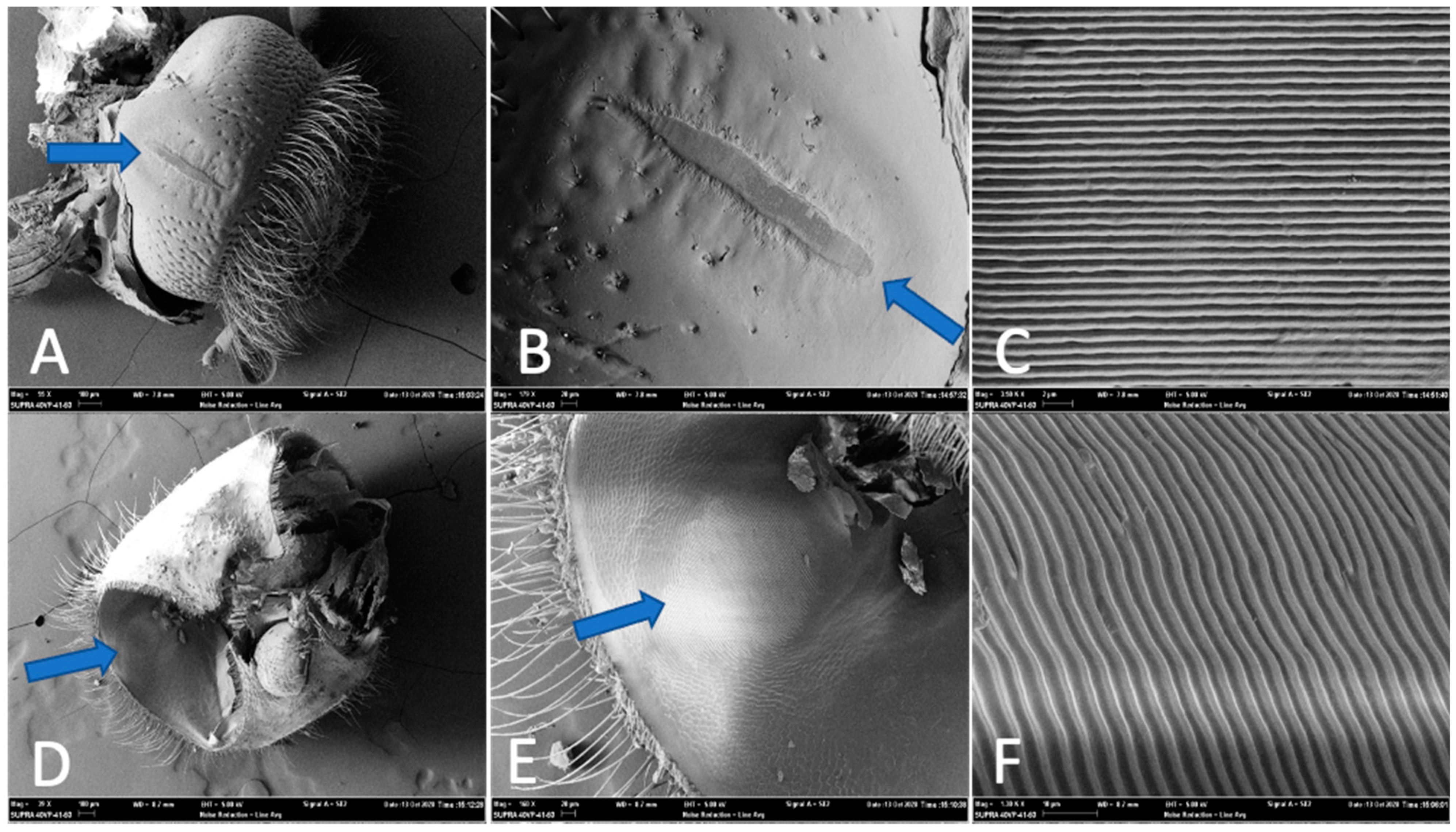
2.2. Stridulation Apparatus Anatomy
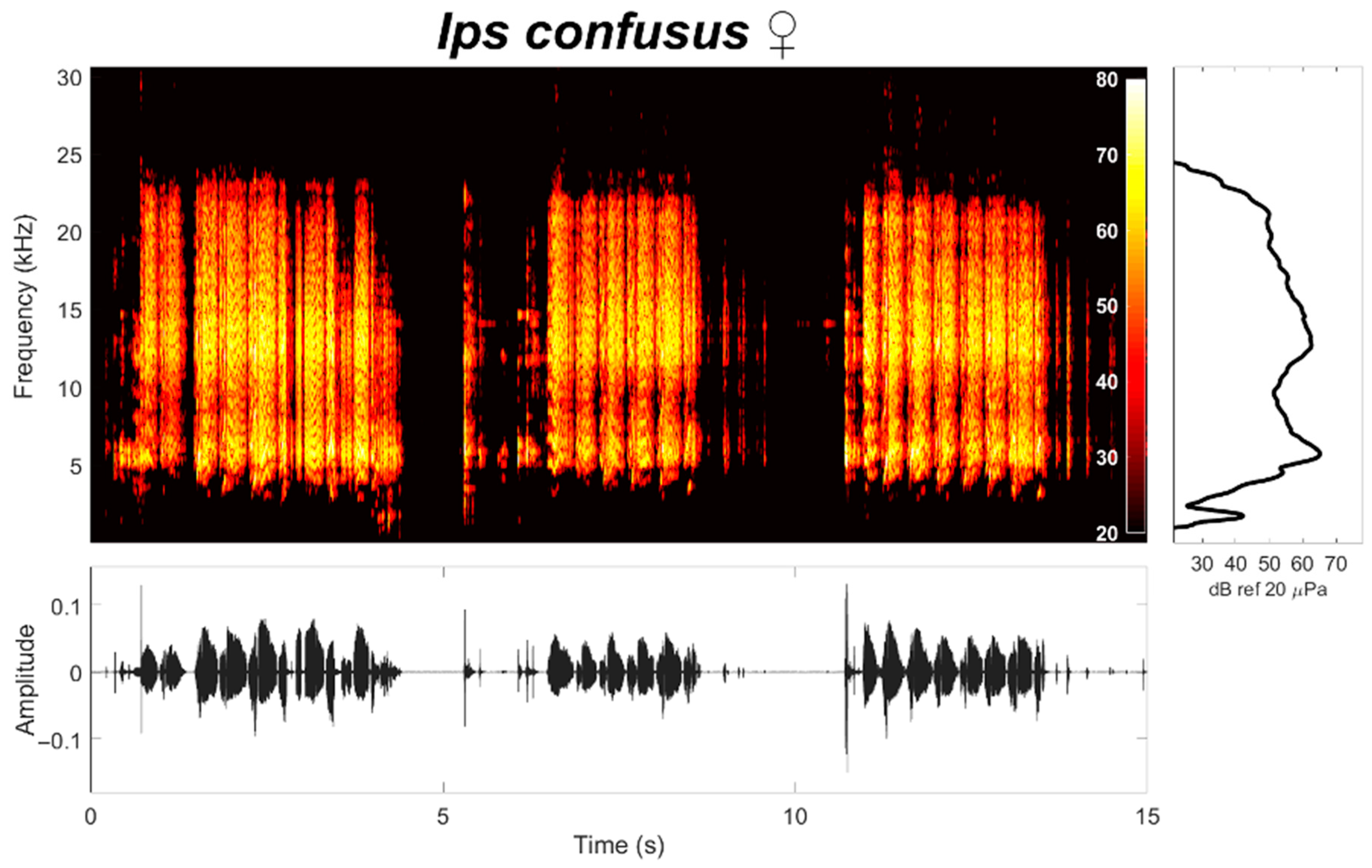
2.3. Airborne Acoustic Signals
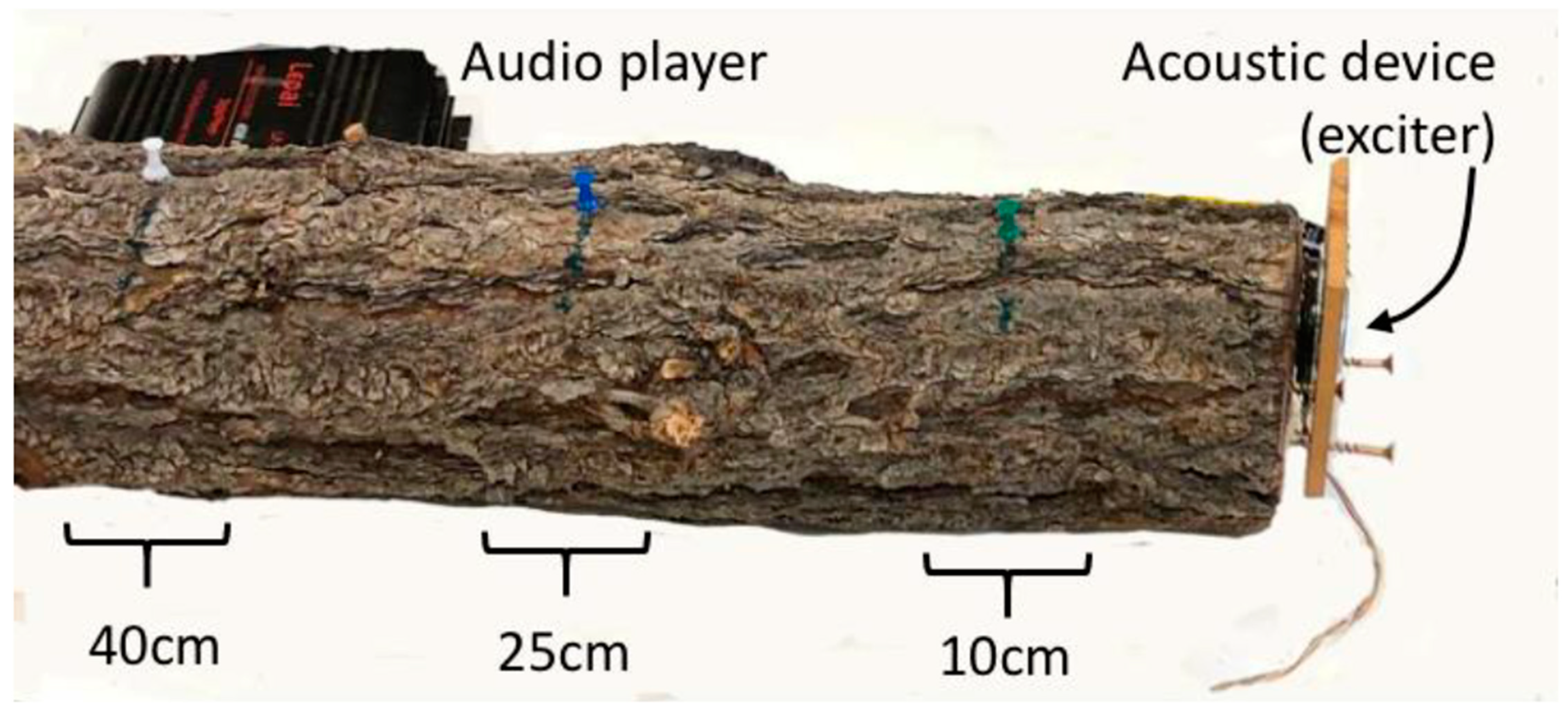
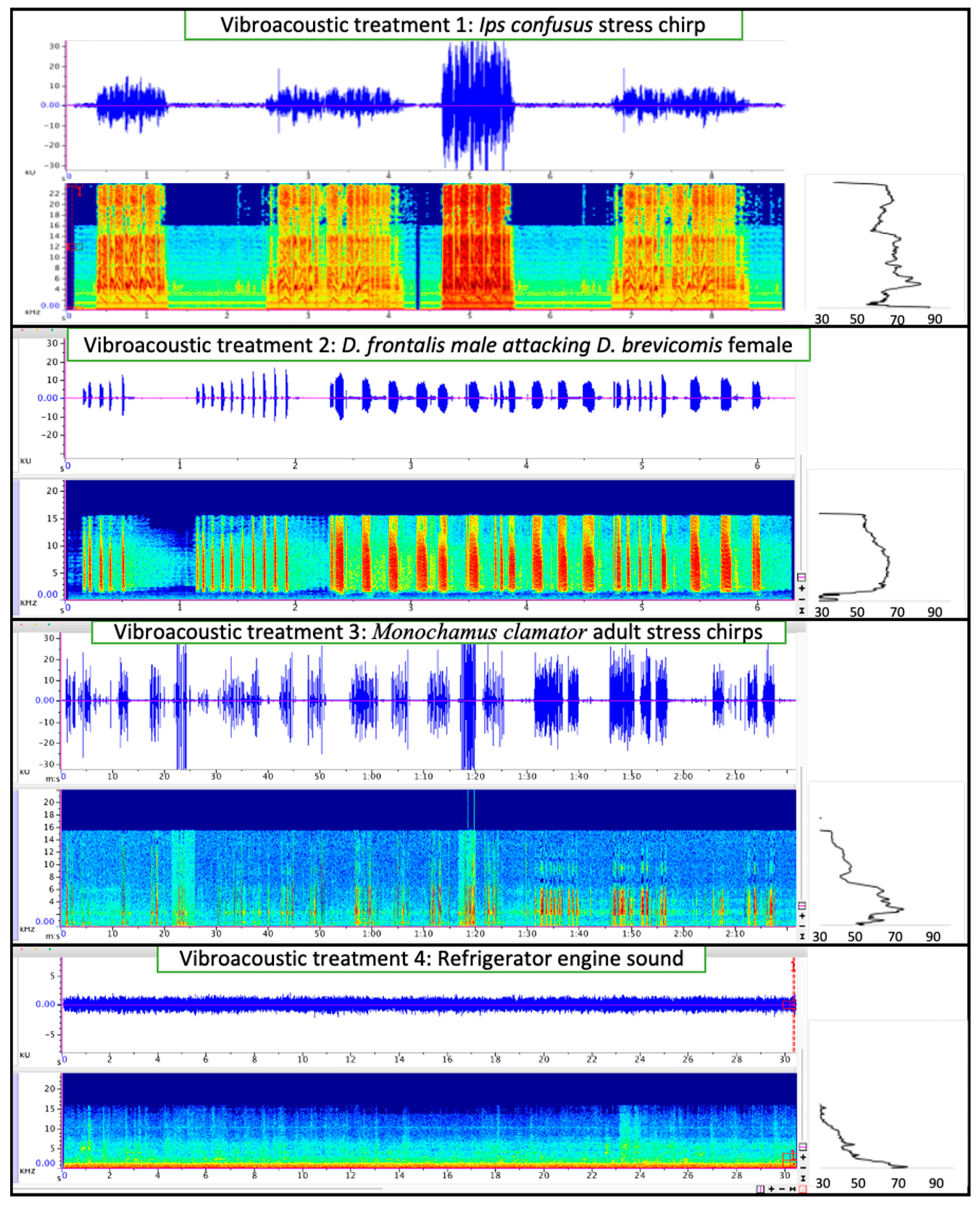
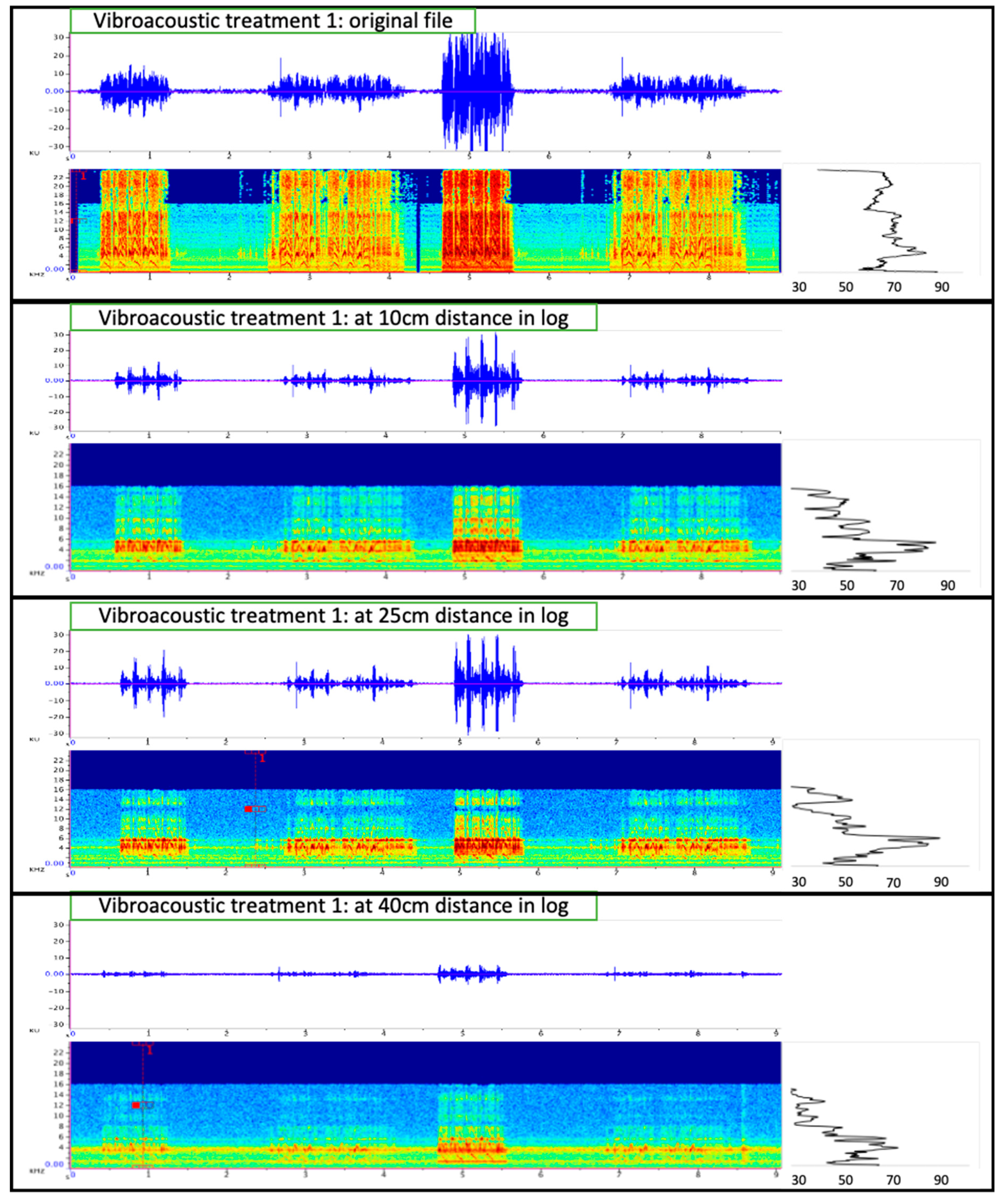
2.4. Vibroacoustic Treatments in Logs
2.4.1. Assay 1 Effects of Vibroacoustic Treatments on Male I. confusus Entry into Logs
2.4.2. Assay 2 Effects of Vibroacoustic Treatments on I. confusus Mating, Tunneling, and Fecundity
2.5. Statistical Analysis
3. Results
3.1. Stridulation Apparatus Anatomy
3.2. Acoustic Characteristics of Female Ips confusus
3.3. Behavioral Response of Vibroacoustic Treatments in Logs
3.3.1. Assay 1: Effects of Vibroacoustic Treatments on Male I. confusus Entry into Logs
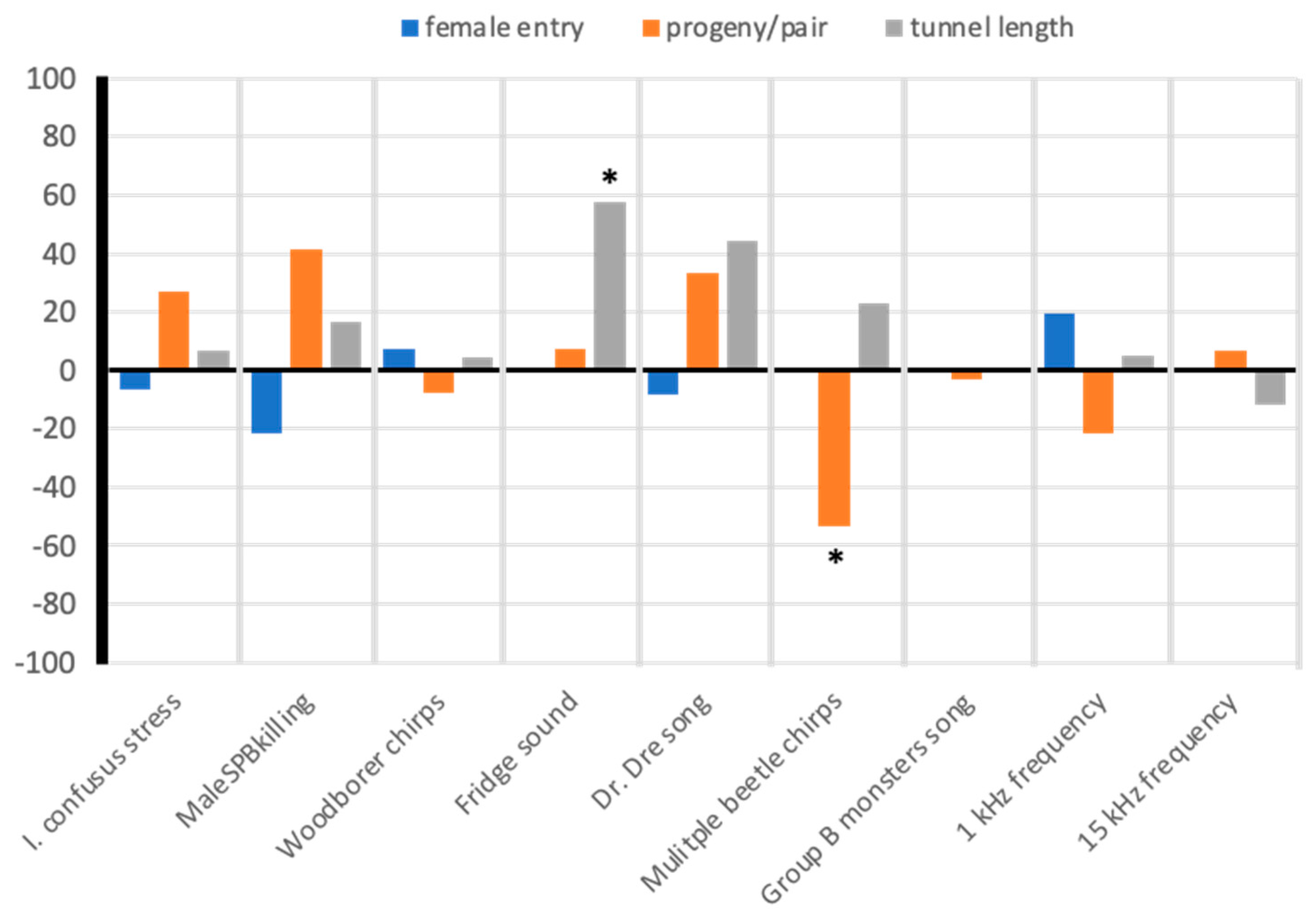
3.3.2. Assay 2: Effects of Vibroacoustic Treatments on I. confusus Mating, Tunneling, and Fecundity
4. Discussion
4.1. Stridulatory Structures
4.2. Stridulation in Response to Disturbance
4.3. Effects of Vibroacoustic Treatments on Beetle Entry, Gallery Length, and Progeny Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Acoustic Treatment | Measure Type | Mean ± SD |
|---|---|---|
| Ips confusus stress chirps produced by female beetles ~10 cm | Female tunnel lengths | 25.35 ± 12.86 a |
| Number of progeny per beetle pair | 9.50 ± 0.75 a | |
| Ips confusus stress chirps produced by female beetles ~25 cm | Female tunnel lengths | 36.75 ± 7.80 a |
| Number of progeny per beetle pair | 9.90 ± 1.46 a | |
| Ips confusus stress chirps produced by female beetles ~40 cm | Female tunnel lengths | 20.60 ± 19.03 a |
| Number of progeny per beetle pair | 9.12 ± 3.11 a | |
| Dendroctonus frontalis Hopkins (southern pine beetle) aggression call produced when confronting female D. brevicomis in gallery ~10 cm | Female tunnel lengths | 30.60 ± 9.07 a |
| Number of progeny per beetle pair | 10.04 ± 1.88 a | |
| Dendroctonus frontalis Hopkins (southern pine beetle) aggression call produced when confronting female D. brevicomis in gallery ~25 cm | Female tunnel lengths | 30.33 ± 7.23 a |
| Number of progeny per beetle pair | 9.56 ± 1.33 a | |
| Dendroctonus frontalis Hopkins (southern pine beetle) aggression call produced when confronting female D. brevicomis in gallery ~40 cm | Female tunnel lengths | 35.00 ± 10.39 a |
| Number of progeny per beetle pair | 11.76 ± 1.20 a | |
| Monochamus clamator LeConte 1852 (spotted pine sawyer woodborer) adult distress call ~10 cm | Female tunnel lengths | 18.80 ± 12.65 a |
| Number of progeny per beetle pair | 8.94 ± 3.74 a | |
| Monochamus clamator LeConte 1852 (spotted pine sawyer woodborer) adult distress call ~25 cm | Female tunnel lengths | 15.00 ± 6.00 a |
| Number of progeny per beetle pair | 7.42 ± 1.26 a | |
| Monochamus clamator LeConte 1852 (spotted pine sawyer woodborer) adult distress call ~40 cm | Female tunnel lengths | 28.20 ± 14.35 a |
| Number of progeny per beetle pair | 11.52 ± 4.13 a | |
| Control ~10 cm (no vibroacoustic treatment) | Female tunnel lengths | 21.40 ± 11.10 a |
| Number of progeny per beetle pair | 8.64 ± 3.77 a | |
| Control ~25 cm (no vibroacoustic treatment) | Female tunnel lengths | 17.40 ± 12.28 a |
| Number of progeny per beetle pair | 7.50 ± 1.97 a | |
| Control ~40 cm (no vibroacoustic treatment) | Female tunnel lengths | 30.00 ± 14.49 a |
| Number of progeny per beetle pair | 10.90 ± 0.72 a |
| Acoustic Treatment | Measure Type | Mean ± SD |
|---|---|---|
| Audio recording of refrigerator engine ~10 cm | Female tunnel lengths | 22.75 ± 9.21 a |
| Number of progeny per beetle pair | 10.05 ± 2.34 a | |
| Audio recording of refrigerator engine ~25 cm | Female tunnel lengths | 49.75 ± 18.80 a |
| Number of progeny per beetle pair | 15.60 ± 4.14 a | |
| Audio recording of refrigerator engine ~40 cm | Female tunnel lengths | 40.25 ± 29.60 a |
| Number of progeny per beetle pair | 11.80 ± 4.61 a | |
| Musical song ‘Dr. Dre ft. Snoop Dogg-Still D.R.E.’ ~10 cm | Female tunnel lengths | 50.00 ± 9.82 a |
| Number of progeny per beetle pair | 12.86 ± 2.83 a | |
| Musical song ‘Dr. Dre ft. Snoop Dogg-Still D.R.E.’ ~25 cm | Female tunnel lengths | 48.00 ± 5.29 a |
| Number of progeny per beetle pair | 11.06 ± 0.80 a | |
| Musical song ‘Dr. Dre ft. Snoop Dogg-Still D.R.E.’ ~40 cm | Female tunnel lengths | 42.00 ± 33.53 a |
| Number of progeny per beetle pair | 10.07 ± 3.87 a | |
| Blend of bark beetle sounds that include attraction, distress and aggression chirps from three Dendroctonus species ~10 cm | Female tunnel lengths | 22.50 ± 8.81 a |
| Number of progeny per beetle pair | 10.82 ± 1.06 a | |
| Blend of bark beetle sounds that include attraction, distress and aggression chirps from three Dendroctonus species ~25 cm | Female tunnel lengths | 18.50 ± 7.18 a |
| Number of progeny per beetle pair | 10.50 ± 3.60 a | |
| Blend of bark beetle sounds that include attraction, distress and aggression chirps from three Dendroctonus species ~40 cm | Female tunnel lengths | 11.00 ± 10.14 a |
| Number of progeny per beetle pair | 8.96 ± 3.07 | |
| Control ~10 cm (no vibroacoustic treatment) | Female tunnel lengths | 43.50 ± 3.53 a |
| Number of progeny per beetle pair | 14.00 ± 1.13 | |
| Control ~25 cm (no vibroacoustic treatment) | Female tunnel lengths | 29.75 ± 24.68 a |
| Number of progeny per beetle pair | 9.90 ± 3.36 a | |
| Control ~40 cm (no vibroacoustic treatment) | Female tunnel lengths | 4.80 ± 10.73 a |
| Number of progeny per beetle pair | 8.50 ± 4.53 a |
| Acoustic Treatment | Measure Type | Mean ± SD |
|---|---|---|
| Musical song ‘Group B monsters—with pure engine sounds’ ~10 cm | Female tunnel lengths | 26.00 ± 16.91 a |
| Number of progeny per beetle pair | 10.82 ± 4.35 a | |
| Musical song ‘Group B monsters—with pure engine sounds’ ~25 cm | Female tunnel lengths | 27.00 ± 5.29 a |
| Number of progeny per beetle pair | 8.20 ± 1.15 a | |
| Musical song ‘Group B monsters—with pure engine sounds’ ~40 cm | Female tunnel lengths | 18.00 ± 3.60 a |
| Number of progeny per beetle pair | 9.20 ± 2.56 a | |
| 1 kHz sin wave ~10 cm | Female tunnel lengths | 21.20 ± 12.27 a |
| Number of progeny per beetle pair | 11.26 ± 5.20 a | |
| 1 kHz sin wave ~25 cm | Female tunnel lengths | 15.00 ± 6.24 a |
| Number of progeny per beetle pair | 10.00 ± 1.73 a | |
| 1 kHz sin wave ~40 cm | Female tunnel lengths | 12.20 ± 10.96 a |
| Number of progeny per beetle pair | 7.40 ± 2.22 a | |
| 15 kHz sin wave ~10 cm | Female tunnel lengths | 16.40 ± 10.99 a |
| Number of progeny per beetle pair | 6.94 ± 2.84 a | |
| 15 kHz sin wave ~25 cm | Female tunnel lengths | 31.00 ± 18.86 a |
| Number of progeny per beetle pair | 9.80 ± 2.92 a | |
| 15 kHz sin wave ~40 cm | Female tunnel lengths | 0.00 ± 0.00 a |
| Number of progeny per beetle pair | 6.15 ± 0.35 a | |
| Control ~10 cm (no vibroacoustic treatment) | Female tunnel lengths | 19.20 ± 23.46 a |
| Number of progeny per beetle pair | 9.58 ± 6.70 a | |
| Control ~25 cm (no vibroacoustic treatment) | Female tunnel lengths | 21.60 ± 22.56 a |
| Number of progeny per beetle pair | 7.90 ± 2.98 a | |
| Control ~40 cm (no vibroacoustic treatment) | Female tunnel lengths | 21.00 ± 4.24 a |
| Number of progeny per beetle pair | 9.00 ± 5.65 a |
References
- Lieutier, F.; Day, K.R.; Battisti, A.; Grégoire, J.C.; Evans, H.F. Bark and Wood Boring Insects in Living Trees in Europe: A Synthesis; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; p. 569. [Google Scholar]
- Vega, F.E.; Hofstetter, R.W. Bark Beetles: Biology and Ecology of Native and Invasive Species; Academic Press: Amsterdam, The Netherlands, 2014; p. 620. [Google Scholar]
- Biedermann, P.H.; Müller, J.; Grégoire, J.C.; Gruppe, A.; Hagge, J.; Hammerbacher, A.; Hofstetter, R.W.; Dineshkumar, K.; Kolarik, M.; Kostovcik, M.; et al. Bark beetle population dynamics in the Anthropocene: Challenges and solutions. Trends Ecol. Evol. 2019, 34, 914–924. [Google Scholar] [CrossRef]
- Hlásny, T.; Krokene, P.; Liebhold, A.; Montagné-Huck, C.; Müller, J.; Qin, H.; Raffa, K.; Schelhaas, M.J.; Seidl, R.; Svoboda, M.; et al. Living with Bark Beetles: Impacts, Outlook and Management Options. From Science to Policy 8; European Forest Institute: Joensuu, Finland, 2019. [Google Scholar]
- Raffa, K.F.; Gregoire, J.C.; Lindgren, B.S. Natural history and ecology of bark beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 1–40. [Google Scholar]
- Renwick, J.A.A.; Vité, J.P.; Vit, J.P. Bark Beetle attractants: Mechanism of colonization by Dendroctonus frontalis. Nat. Cell Biol. 1969, 224, 1222–1223. [Google Scholar] [CrossRef]
- Raffa, K.F.; Berryman, A.A. Physiological differences between lodgepole pines resistant and susceptible to the mountain pine beetle 1 and associated microorganisms. Environ. Entomol. 1982, 11, 486–492. [Google Scholar] [CrossRef]
- Sauvard, D. General biology of Bark Beetles. In Bark and Wood Boring Insects in Living Trees in Europe: A Synthesis; Lieutier, F., Day, K.R., Battisti, A., Grégoire, J.C., Evans, H.F., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 63–88. [Google Scholar]
- Byers, J.A. Host-tree chemistry affecting colonization in bark beetles. In Chemical Ecology of Insects 2; Carde, R.T., Bell, W.J., Eds.; Springer: Boston, MA, USA, 1995; pp. 154–213. [Google Scholar]
- Kirkendall, L.R.; Biedermann, P.H.W.; Jordal, B.H. Evolution and diversity of Bark and Ambrosia Beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 85–156. [Google Scholar]
- Barr, B.A. Sound production in Scolytidae (Coleoptera) with emphasis on the genus Ips. Can. Entomol. 1969, 101, 636–672. [Google Scholar] [CrossRef]
- Bedoya, C.L.; Hofstetter, R.W.; Nelson, X.J.; Hayes, M.; Miller, D.R.; Brockerhoff, E.G. Sound production in bark and ambrosia beetles. Bioacoustics 2021, 30, 58–73. [Google Scholar] [CrossRef]
- Wilkinson, R.C.; McClelland, W.T.; Murillo, R.M.; Ostmark, E.O. Stridulation and behavior in two Southeastern Ips Bark Beetles (Coleoptera: Scolytidae). Fla. Entomol. 1967, 50, 185. [Google Scholar] [CrossRef]
- Ryker, L.C.; Rudinsky, J.A. Sound production in Scolytidae: Aggressive and mating behavior of the Mountain Pine Beetle. Ann. Entomol. Soc. Am. 1976, 69, 677–680. [Google Scholar] [CrossRef]
- Swaby, J.A.; Rudinsky, J.A. Acoustic and olfactory behaviour of Ips pini (Say) (Coleoptera: Scolytidae) during host invasion and colonisation. Z. Angew. Entomol. 2009, 81, 421–432. [Google Scholar] [CrossRef]
- Ryker, L.C. Acoustic studies of Dendroctonus Bark Beetles. Fla. Entomol. 1988, 71, 447. [Google Scholar] [CrossRef]
- Sivalinghem, S. Acoustic Communication in the Pine Engraver Bark Beetle, Ips pini (Coleoptera: Scolytinae). Ph.D. Thesis, Carleton University, Ottawa, ON, Canada, 2011. [Google Scholar]
- Fleming, A.J.; Lindeman, A.A.; Carroll, A.L.; Yack, J.E. Acoustics of the mountain pine beetle (Dendroctonus pondero-sae) (Curculionidae, Scolytinae): Sonic, ultrasonic, and vibration characteristics. Can. J. Zool. 2013, 91, 235–244. [Google Scholar] [CrossRef]
- Yturralde, K.M.; Hofstetter, R.W. Characterization of stridulatory structures and sounds of the larger Mexican Pine Beetle, Dendroctonus approximates (Coleoptera: Curculionidae: Scolytinae). Fla. Entomol. 2015, 98, 516–527. [Google Scholar] [CrossRef]
- Ryker, L.C.; Rudinsky, J.A. Sound production in Scolytidae: Acoustic signals of male and female Dendroctonus valens LeConte. Z. Angew. Entomol. 1976, 80, 113–118. [Google Scholar] [CrossRef]
- Rudinsky, J.A.; Michael, R.R. Sound production in Scolytidae: Chemostimulus of sonic signal by the Douglas-Fir Beetle. Science 1972, 175, 1386–1390. [Google Scholar] [CrossRef]
- Rudinsky, J.; Michael, R. Sound production in Scolytidae: Stridulation by female Dendroctonus beetles. J. Insect Physiol. 1973, 19, 689–705. [Google Scholar] [CrossRef]
- Rudinsky, J.A.; Michael, R.R. Sound production in Scolytidae: ‘Rivalry’ behaviour of male Dendroctonus beetles. J. Insect Physiol. 1974, 20, 1219–1230. [Google Scholar] [CrossRef]
- Rudinsky, J.; Ryker, L.; Michael, R.; Libbey, L.; Morgan, M. Sound production in Scolytidae: Female sonic stimulus of male pheromone release in two Dendroctonus beetles. J. Insect Physiol. 1976, 22, 1675–1681. [Google Scholar] [CrossRef]
- Lindeman, A.A.; Yack, J.E. What is the password? Female bark beetles (Scolytinae) grant males access to their galleries based on courtship song. Behav. Process. 2015, 115, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Dobai, A.; Sivalinghem, S.; Guedes, R.N.; Yack, J.E. Acoustic communication in the pine engraver bark beetle: Do signals vary between behavioural contexts? Physiol. Entomol. 2018, 43, 30–41. [Google Scholar] [CrossRef]
- Liu, Z.; Mi, G.; Raffa, K.F.; Sun, J. Physical contact, volatiles, and acoustic signals contribute to monogamy in an invasive aggregating bark beetle. Insect Sci. 2020, 27, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Rudinsky, J.A. Pheromone-mask by female Dendroctonus pseudotsugae Hopk an attraction regulator (Coleoptera-Scolytidae). Pan-Pac. Entomol. 1968, 44, 248. [Google Scholar]
- Ryker, L.C. Acoustic and chemical signals in the life cycle of a Beetle. Sci. Am. 1984, 250, 112–123. [Google Scholar] [CrossRef]
- Goyer, R.A.; Wagner, M.R.; Schowalter, T.D. Current and proposed technologies for bark beetle management. J. For. 1998, 96, 29–33. [Google Scholar]
- Wermelinger, B. Ecology and management of the spruce bark beetle Ips typographus—A review of recent research. For. Ecol. Manag. 2004, 202, 67–82. [Google Scholar] [CrossRef]
- Fettig, C.J.; Hilszczański, J. Management strategies for bark beetles in conifer forests. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 555–584. [Google Scholar]
- Furness, R.L.; Carolin, V.W. Western Forest Insects; U.S. Department of Agriculture, Forest Service, Miscellaneous Publication: Washington, DC, USA, 1977; Volume 1339, 654p.
- Borden, J.H. Semiochemicals and bark beetle populations: Exploitation of natural phenomena by pest management strategists. Ecography 1989, 12, 501–510. [Google Scholar] [CrossRef]
- Bakke, A. Using pheromones in the management of bark beetle outbreaks. In Forest Insect Guilds: Patterns of Interaction with Host Trees; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1991; pp. 371–377. [Google Scholar]
- Gillette, N.E.; Hansen, E.M.; Mehmel, C.J.; Mori, S.R.; Webster, J.N.; Erbilgin, N.; Wood, D.L. Area-wide application of verbenone-releasing flakes reduces mortality of Whitebark pine Pinus albicaulis caused by the mountain pine beetle Dendroctonus ponderosae. Agric. For. Entomol. 2012, 14, 367–375. [Google Scholar] [CrossRef]
- Fettig, C.J.; Munson, A.S. Efficacy of verbenone and a blend of verbenone and nonhost volatiles for protecting lodgepole pine from mountain pine beetle (Coleoptera: Curculionidae). Agric. For. Entomol. 2020, 22, 373–378. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Rodin, J.O.; Wood, D.L. Sex attractants in frass produced by male Ips confusus in Ponderosa pine. Science 1966, 154, 509–510. [Google Scholar]
- Bakke, A. Deploying pheromone-baited traps for monitoring Ips typographus populations. Z. Angew. Entomol. 2009, 99, 33–39. [Google Scholar] [CrossRef]
- Symonds, M.R.E.; Elgar, M.A. The mode of pheromone evolution: Evidence from bark beetles. Proc. R. Soc. B Boil. Sci. 2004, 271, 839–846. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Figueroa-Teran, R.; Aw, M.; Song, M.; Gorzalski, A.; Abbott, N.L.; Chang, E.; Tittiger, C. Pheromone production in bark beetles. Insect Biochem. Mol. Biol. 2010, 40, 699–712. [Google Scholar] [CrossRef]
- Sullivan, B.T. Semiochemicals in the natural history of southern pine beetle Dendroctonus frontalis Zimmermann and their role in pest management. In Pine Bark Beetles; Tittiger, C., Blomquist, G.J., Eds.; Academic Press: Amsterdam, The Netherlands, 2016; pp. 1–304. [Google Scholar]
- Bark Beetle Trap Lures. Available online: https://www.forestrydistributing.com/bark-beetle-pheromone-tree-trap-lures-baits-attractants (accessed on 25 November 2020).
- Hofstetter, R.W.; Dunn, D.D.; McGuire, R.; Potter, K.A. Using acoustic technology to reduce bark beetle reproduction. Pest Manag. Sci. 2013, 70, 24–27. [Google Scholar] [CrossRef]
- Hofstetter, R.W.; Aflitto, N.; Bedoya, C.L.; Yturralde, K.; Dunn, D.D. Vibrational behavior in bark beetles: Applied aspects. In Biotremology: Studying Vibrational Behavior; Hill, P.S.M., Lakes-Harlan, R., Mazzoni, V., Narins, P.M., Virant-Doberlet, M., Wessel, A., Eds.; Springer: London, UK, 2019; pp. 415–435. [Google Scholar]
- Aflitto, N.C.; Hofstetter, R.W. Use of acoustics to deter bark beetles from entering tree material. Pest Manag. Sci. 2014, 70, 1808–1814. [Google Scholar] [CrossRef]
- Dobai, A. “Can You Hear Me?” Investigating the Acoustic Communication Signals and Receptor Organs of Bark Beetles. Ph.D. Thesis, Carleton University, Ottawa, ON, Canada, 2017. [Google Scholar]
- Wichmann, H.E. Ipidae. In Biologie der Tiere Deutschlands; Schulze, P., Ed.; Gebrüder Bornträger: Berlin, Germany, 1927; pp. 347–381. (In German) [Google Scholar]
- Lewis, E.E.; Cane, J.H. Stridulation as a primary anti-predator defence of a beetle. Anim. Behav. 1990, 40, 1003–1004. [Google Scholar] [CrossRef]
- Allen, D.G.; Michael RRStone, S.A. Sounds of Douglas Fir Beetle Activity: Recorded and Interpreted, Equipment Techniques; Research Note No. 36; Oregon Forest Lands Research Center: Portland, OR, USA, 1958; pp. 1–19. [Google Scholar]
- Oester, P.T.; Rudinsky, J.A. Acoustic behaviour of three sympatric species of Ips (Coleoptera: Scolytidae) co-inhabiting Sitka spruce. Z. Angew. Entomol. 2009, 87, 398–412. [Google Scholar] [CrossRef]
- Lyal, C.; King, T. Elytro-tergal stridulation in weevils (Insecta: Coleoptera: Curculionoidea). J. Nat. Hist. 1996, 30, 703–773. [Google Scholar] [CrossRef]
- Hill, M.P.S.; Lakes-Harlan, R.; Mazzoni, V.; Narins, P.M.; Virant-Doberlet, M.; Wessel, A. Biotremology: Studying Vibrational Behavior; Springer: New York, NY, USA, 2014; 526p. [Google Scholar]
- Caldwall, M.S. Chapter 6: Interactions between airborne sound and substrate vibration in animal communication. In Studying Vibrational Communication; Cocroft, R.B., Gogala, M., Hill, P.S.M., Wessel, A., Eds.; Animal Signals and Communication; Springer: New York, NY, USA, 2014; Volume 3, pp. 65–92. [Google Scholar]
- Wood, S.L. The Bark and Ambrosia Beetles of North and Central America (Coleoptera: Scolytidae), a Taxonomic Monograph; Brigham Young University: Provo, UT, USA, 1982; p. 1359. [Google Scholar]
- Yandell, K.L. Sound production of Dendroctonus ponderosae Hopkins (Coleoptera, Scolytidae): A comparison of populations from three host pines in Oregon. Z. Angew. Entomol. 2009, 97, 180–187. [Google Scholar] [CrossRef]
- Kerchev, I.A. Context-dependent acoustic signals in the Four-Eyed Fir Bark Beetle, Polygraphus proximus (Coleoptera: Curculionidae: Scolytinae). Environ. Entomol. 2018, 48, 181–188. [Google Scholar] [CrossRef]
- Dunn, D.D. Microphones, Hydrophones, Vibration Transducers: Rolling Your Own. Available online: https://www.zachpoff.com/site/wp-content/uploads/David-Dunn-Microphones_Hydrophones_Vibration-Transducers__Rolling_Your_Own__Dunn2007.pdf (accessed on 25 November 2020).
- Fujioka, E.; Mantani, S.; Hiryu, S.; Riquimaroux, H.; Watanabe, Y. Echolocation and flight strategy of Japanese house bats during natural foraging, revealed by a microphone array system. J. Acoust. Soc. Am. 2011, 129, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, B.; Shi, Y.Q. MP3 bit rate quality detection through frequency spectrum analysis. In Proceedings of the 11th ACM Workshop on Multimedia and Security, Princeton, NJ, USA, 7–8 September 2009; pp. 57–62. [Google Scholar]
- Sterne, J. MP3: The Meaning of a Format; Duke University Press: Durham, NC, USA, 2012. [Google Scholar]
- Hofstetter, R.W.; Copp, B.E.; Lukic, I. Acoustic noise of refrigerators promote increased growth rate of the gray mold Botrytis cinerea. J. Food Saf. 2020, 40. [Google Scholar] [CrossRef]
- Wilkinson, R.C. Stridulating organs in three Southeastern Ips Bark Beetles. Fla. Entomol. 1962, 45, 43. [Google Scholar] [CrossRef]
- Oester, P.T. Sound Production in Three Sympatric Ips (Coleoptera: Scolytidae) Species Co-Inhabiting Sitka spruce (Picea sitchensis). Master’s Thesis, Oregon State University, Corvallis, OR, USA, 1977. [Google Scholar]
- Swaby, J.A. Acoustic and Olfactory Behavior of Ips pini (Say) (Coleoptera: Scolytidae) during Hots Invasion and Colonization. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 1975; p. 83. [Google Scholar]
- Eriksson, A.; Anfora, G.; Lucchi, A.; Lanzo, F.; Virant-Doberlet, M.; Mazzoni, V. Exploitation of insect vibrational signals reveals a new method of pest management. PLoS ONE 2012, 7, e32954. [Google Scholar] [CrossRef] [PubMed]
- Polajnar, J.; Eriksson, A.; Virant-Doberlet, M.; Mazzoni, V. Mating disruption of a grapevine pest using mechanical vibrations: From laboratory to the field. J. Pest Sci. 2016, 89, 909–921. [Google Scholar] [CrossRef]
- Fleming, A.J. Acoustic Communication in the Mountain Pine Beetle, Dendroctonus ponderosae Hopk. (Coleoptera: Scolytidae): Characterization of Signals and Anatomy. Master’s Thesis, Carleton University, Ottawa, ON, Canada, 2009. [Google Scholar]
- Page, M.; Nelson, L.J.; Blomquist, G.J.; Seybold, S.J. Cuticular hydrocarbons as chemotaxonomic characters of Pine Engraver Beetles (Ips spp.) in the Grandicollis subgeneric group. J. Chem. Ecol. 1997, 23, 1053–1099. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukic, I.; Bedoya, C.L.; Hofstetter, E.M.; Hofstetter, R.W. Pinyon Engraver Beetle Acoustics: Stridulation Apparatus, Sound Production and Behavioral Response to Vibroacoustic Treatments in Logs. Insects 2021, 12, 496. https://doi.org/10.3390/insects12060496
Lukic I, Bedoya CL, Hofstetter EM, Hofstetter RW. Pinyon Engraver Beetle Acoustics: Stridulation Apparatus, Sound Production and Behavioral Response to Vibroacoustic Treatments in Logs. Insects. 2021; 12(6):496. https://doi.org/10.3390/insects12060496
Chicago/Turabian StyleLukic, Ivan, Carol L. Bedoya, Evan M. Hofstetter, and Richard W. Hofstetter. 2021. "Pinyon Engraver Beetle Acoustics: Stridulation Apparatus, Sound Production and Behavioral Response to Vibroacoustic Treatments in Logs" Insects 12, no. 6: 496. https://doi.org/10.3390/insects12060496
APA StyleLukic, I., Bedoya, C. L., Hofstetter, E. M., & Hofstetter, R. W. (2021). Pinyon Engraver Beetle Acoustics: Stridulation Apparatus, Sound Production and Behavioral Response to Vibroacoustic Treatments in Logs. Insects, 12(6), 496. https://doi.org/10.3390/insects12060496







